ABSTRACT
Objective: To summarize the clinical characteristics of acquired pure red cell aplasia (PRCA) patients diagnosed in our hospital in the last 10 years.
Method: The clinical features, immune state and treatment response of acquired PRCA patients diagnosed in our hospital from January 2007 to January 2017 were retrospectively analyzed.
Results: The results showed that thymoma (13.21%) and parvovirus B19 (11.32%) were the most common causes for secondary PRCA. Ferritin (Fer) levels and erythropoietin (EPO) levels were increased in PRCA patients. The total CR and PR rate of immunosuppressive therapy in our studies was 68.29% and 12.20%, respectively. Patients with EPO level >400 U/L and Fer level >200 ng/ml had significantly lower CR rate than others. The patients with EPO level >400 U/L also had longer hemoglobin recovery time than patients with EPO level ≤400 U/L. Patients treated with corticosteroids (CS) + cyclosporine A (CsA) had lower relapse rate compared to the CS group (29.17% vs. 80.00%, P < .05).
Conclusion: Our data showed that patients with PRCA had high EPO and Fer levels. Thymoma and viral infections are the most common causes for secondary PRCA. The CS+ CsA group had lower relapse rate than CS group although response rate was similar. Increased EPO and Fer levels might be the negative factors for prognosis of acquired PRCA.
Short description
Pure red cell aplasia (PRCA) is a rare hematopoietic disorder characterized by normocytic and normochromic anemia, reticulocytopenia and absence of erythroid precursors in bone marrow. The clinical characteristics of patients with acquired PRCA in China were poorly understood at present. The clinical features, immune state and treatment response of acquired PRCA patients diagnosed in our hospital from January 2007 to January 2017 were retrospectively analyzed in this study. The results showed that thymoma (13.21%) and parvovirus B19 infection (11.32%) were the most common causes for secondary PRCA. Ferritin (Fer) levels (784.02 ± 643.03 ng/mL) and erythropoietin (EPO) levels (10 of 34 patients were 20–400U/L, 20 of 34 patients were >400 U/L) were increased in PRCA patients. The total CR and PR rate of immunosuppressive therapy (IST) were 68.29% and 12.20%, respectively. Patients with EPO level >400 U/L and Fer level >200 ng/ml had significantly lower CR rate than others (57.14% vs. 88.98%, and 57.10% vs. 91.67%, respectively). The patients with EPO level >400 U/L also had longer hemoglobin recovery time (45.57 ± 12.75 days). Patients treated with corticosteroids (CS) + cyclosporine A (CsA) had lower relapse rate compared to the CS group (29.17% vs. 80.00%). In conclusion, patients with PRCA had high EPO and Fer levels. The immune pathogenesis was heterogeneity in acquired PRCA. Thymoma and viral infections are the most common causes for secondary PRCA. Secondary PRCA has more abnormal immune and longer recovery time than idiopathic PRCA. IST is effective for both idiopathic and acquired PRCA, but with high relapse rate. Therapy of CS + CsA could reduce the relapse rate of PRCA. Increased EPO and Fer levels might be the negative factors for prognosis of acquired PRCA. The most common complication of acquired PRCA is infection during treatments.
1. Introduction
Pure red cell aplasia (PRCA) is a rare hematopoietic disorder characterized by normocytic and normochromic anemia, reticulocytopenia and absence of erythroid precursors in bone marrow. It is first described by Kaznelson in 1922 [Citation1]. According to pathogenesis, PRCA can be divided into Diamond–Blackfan anemia and acquired PRCA. Acquired PRCA may occur as a primary disease without any other diseases (idiopathic PRCA), or secondary to thymoma and hematological malignant diseases (such as lymphoproliferative disorders, viral or bacterial infection, autoimmune diseases, pregnancy, neoplastic disease, ABO blood group-incompatible hematopoietic stem cell transplantation and exposure to various drugs or chemicals) (secondary PRCA). Immune mechanisms play a key role in the pathogenesis of idiopathic PRCA and secondary PRCA. Therefore, IST is the main measure to treat acquired PRCA [Citation2].
Due to low morbidity of PRCA, few clinical studies with large sample were seen in China currently, and most studies only focus on therapy. We retrospectively analyzed the clinical features, immune state, therapeutic response and prognosis of acquired PRCA patients diagnosed in our hospital in the last 10 years to understand the characteristics of patients with acquired PRCA in China.
2. Patients and methods
2.1. Patients
A total of 53 acquired PRCA patients diagnosed in the General Hospital of Tianjin Medical University from January 2007 to January 2017 were enrolled in this study, including 24 males and 29 females with the median age of 60 years (range 12–87). Among them, 30 patients were idiopathic PRCA (56.60%, 13 males and 17 females, median age was 60 years, range 12–85), and 23 patients were diagnosed as secondary PRCA (43.40%, 11 males and 12 females, median age was 60 years, range 26–87).
The patients diagnosed as acquired PRCA according to the following criterions: hemoglobin (Hb) concentration < 120 g/L (males) or 110 g/L (females); the percentage of reticulocyte (Ret) < 1%; white blood cell count and platelet (PLT) count within the normal range; the percentage of erythroid precursors in bone marrow <5% in bone marrow. All patients were taken bone marrow biopsy and aspiration before treatments. Patients were detected for paroxysmal nocturnal hemoglobinuria clone and Coombs test, and no positive results had been found.
Our protocol was approved by the Ethics Committee of General Hospital of Tianjin Medical University, and all patients enrolled in this study signed informed consent by themselves or their guardians in accordance with the Declaration of Helsinki.
2.2. Therapy
A total of 41 patients were treated in our hospital. Thirty-two of them received combination therapy by corticosteroids (CS, 0.5–1 mg/kg×d−1) and cyclosporine A (CsA, 3–5 mg/kg×d−1). Nine patients used single CS treatment because of renal inadequacy or hepatitis virus infection. Patients with virus infections were given intravenous immunoglobulin (IVIG, 10 g per week) additionally. Erythropoietin (EPO), androgen, thymectomy, transfusion and anti-infection therapy were given according to the condition of diseases. CS was discontinued gradually after hemoglobin recovered to normal (halved every 2 weeks). After hemoglobin recovered to normal, CsA (1–2 mg/kg*d) was given as maintenance therapy and lasted for at least 1 year.
The therapeutic response was evaluated with below criterions [Citation3]: (1) complete response (CR): hemoglobin concentration rises to normal range (120 g/L for males and 110 g/L for females), bone marrow recovers to normal; (2) partial response (PR): no dependence for transfusion. Hemoglobin concentration increases more than 30 g/L; (3) no response: transfusion dependence. No improvement in anemia. (4) Relapse: new dependence for transfusion.
2.3. Statistical analysis
The data in our study were collected from medical records carefully and analyzed by SPSS software (version 19.0). Normal distribution data were presented as mean ± SD and analyzed using unpaired t-test. Skewed distribution data were presented as Median, and analyzed using nonparametric test. Comparison of categorical data analyzed with the chi-square test. Statistically different was defined as P-value <.05.
3. Results
3.1. The clinical features of acquired pure red cell aplasia
3.1.1. Symptoms and signs
In our study, all of 53 patients had the symptoms of anemia, including dizziness, fatigue, tinnitus, palpitation and pale. Among them, 8 patients (15.09%) had symptoms of infection, including 6 (75%) with respiratory infection and the rest (25%) with fever without any other symptoms. Three patients (5.66%) had mild splenomegaly.
Among secondary PRCA patients, 7 cases (30.43%) had the history of thymoma. Thirteen cases were secondary to other diseases, including T-LGL (n = 1), myelodysplastic syndrome (MDS, n = 2), tumor (n = 2), connective tissue disease (CTD, n = 3), renal dysfunction (n = 3) and hypothyroidism (n = 2). Sixteen cases (69.57%) had virus infection, including 6 patients with parvovirus B19 infection, 2 patients with parvovirus B19 and an additional virus infection (1 of parvovirus B19 and hepatitis B virus co-infection, 1 of parvovirus B19, hepatitis B and Epstein–Barr virus co-infection) and 8 patients with other virus infection (4 with hepatitis B virus infection, 1 with hepatitis A virus infection, 2 with cytomegalovirus infection, 1 with Epstein–Barr virus infection).
3.1.2. Peripheral blood and bone marrow analysis
The hemoglobin level, erythrocyte and reticulocyte counts decreased obviously in all patients with acquired PRCA, while leukocytes, PLT, mean corpuscular volume and mean corpuscular hemoglobin concentration were normal. Although most patients had hyperplasia marrow (sternum: 86.36% of patients, iliac: 78.69% of patients), erythroid progenitor was absence in all patients. These data were lower in secondary PRCA compared with idiopathic PRCA (). Bone marrow biopsies showed the same results as the bone marrow smears. One case had mild fiber hyperplasia. Chromosome karyotype analysis was performed in 40 patients, and all of results were normal except one patient who developed to 5q-syndrome after a year. The 20/30 patients (66.7%) had iron overload (extracellular iron grade ≥2+) examined by bone marrow iron stain. Ringed sideroblasts were found in 1 patient. No positive finding in staining for glycogen (PAS) of nucleated red blood cells.
Table 1. Peripheral blood and bone marrow teats of acquired PRCA patients.
3.1.3. Iron measurement
Data of serum ferritin (Fer), serum iron (SI), transferring (TRF), total iron binding capacity (TIBC) and unsaturated iron-binding capacity (UIBC) were collected from patients with acquired PRCA (). The 37/49 of patients (75.51%) had increased Fer load. The secondary PRCA patients had higher Fer levels than that in idiopathic PRCA patients (P < .05). The elevated SI levels were found in 25/32 patients (78.13%) with reduced UIBC and TRF levels. Decreased TIBC appeared in 9 of these 25 patients. Eighteen patients had blood transfusion before diagnosis. The median number of transfusion was 4 U (2-10U). There was no significant difference in Fer level between transfusion and non-transfusion group.
Table 2. Iron tests of acquired PRCA patients.
3.1.4. Erythropoietin level
A total of 34 patients tested for serum EPO level. Ten of them (29.41%) had mild/moderate increased EPO levels (≤400U/L), and the rest (70.59%) had severe increased EPO levels (>400 U/L). There was no significant difference between idiopathic and secondary PRCA group.
3.1.5. Other laboratory examinations
In our study, a small part of patients with acquired PRCA had liver and kidney dysfunction. The 18/53 patients (33.96%) had an abnormal liver function, including 9 cases of idiopathic PRCA (50%) and 9 cases of secondary PRCA (50%). Among these 18 cases, 17 of them (94.44%) had mild liver dysfunction, and 1 patient with hepatitis B virus infection had moderate liver dysfunction. The 11/53 patients (20.75%) had an abnormal renal function, including 5 cases of idiopathic PRCA (45.45%) and 6 cases of secondary PRCA (54.54%). Among these 11 cases, 10 patients (90.91%) were in the compensatory stage of renal dysfunction, while 1 patient had renal failure.
3.2. T cell and B cell subsets of patients with acquired pure red cell aplasia
In order to evaluate the subset of T cell and B cell in acquired PRCA, the ratio of CD4+/CD8+ T cells and CD5+CD19+/CD19+ B cells was investigated in 46 patients. The CD5+CD19+ B cells were defined as activated B cells. The results showed that the ratio of CD4+/CD8+ T cells and the percentage of CD5+CD19+ B cells was higher in idiopathic PRCA patients than that in secondary PRCA patients, but the difference was not statistically significant (1.78 ± 1.52 vs. 1.28 ± 0.89, P = .15; 19.56 ± 11.32% vs. 13.89 ± 8.01%, P = .64, respectively).
3.3. Therapy
3.3.1. Response of therapy
Response rate of IST in 41 patients with acquired PRCA was evaluated. The median follow-up time was 31 months (range 6–120 months). The rate of CR and PR in all patients was 68.29% and 12.20%, respectively. Patients with high EPO level (>400 U/L) and Fer level (>200 ng/ml) had significantly lower response rate (CR + PR) than others (P < .05). The 17/23 secondary PRCA patients were given the IST, including thymoma (n = 5), T-LGL (n = 1), MDS (n = 1), CTD (n = 3), renal dysfunction (n = 3), hypothyroidism (n = 1) and virus infection (2 patients with parvovirus B19 infection, 1 patients with hepatitis B virus infection). IST was given to the patients because IST was needed to primary disease or hemoglobin level had no improvement after primary diseased remission. The response rate had no significant difference between idiopathic PRCA and secondary PRCA group, or in CS group and CsA + CS group ().
Table 3. The response rate of therapy in acquired PRCA patients.
3.3.2. Recovery time
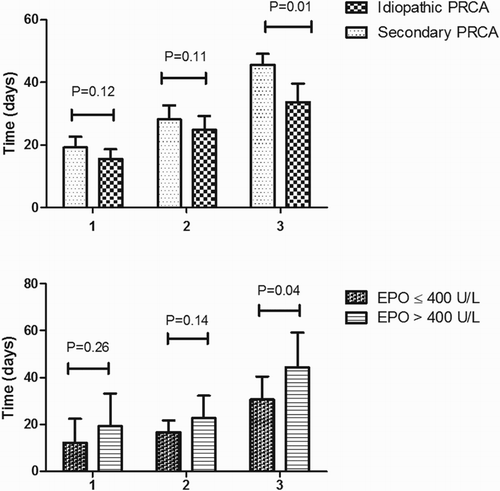
After IST, mean recovery time of PR was 24.07 ± 12.50 days, mean recovery time of CR was 38.43 ± 13.45 days, and mean time of reticulocyte recovered to normal was 15.85 ± 11.25 days in all patients. The mean recovery time of PR, CR and reticulocyte recovered to normal had no significant difference between CS and CS + CsA group (19.00 ± 8.92 vs. 25.39 ± 13.12 days, 34.50 ± 10.38 vs. 38.74 ± 14.22 days, and 15.25 ± 2.41 vs. 16.04 ± 12.51 days, respectively, all P > .05). The recovery time of CR was longer in patients with secondary PRCA than in idiopathic PRCA (45.62 ± 12.47 vs. 28.10 ± 6.84 days, P = .01). The patients with high EPO level (EPO > 400 U/L) also had longer hemoglobin recovery time (45.57 ± 12.75 vs. 30.67 ± 9.69 days, P = .04). The recovery time of PR and reticulocyte recovered to normal was also longer in these two groups; however, the difference was not statistically significant ().
Figure 1. The recovery time of hemoglobin and reticulocyte in acquired PRCA patients. (A) Recovery time of idiopathic and secondary PRCA group. Groups 1, 2 and 3 were termed as time of reticulocyte recovered to normal, PR and CR, respectively. (B) Recovery time of patients with different EPO level. Groups 1, 2 and 3 were termed as time of reticulocyte recovered to normal, PR and CR, respectively.
3.3.3. Relapse rate of therapy
In our study, a total relapse rate of IST was 26.83% (11/41 patients). The median time of relapse was 12 months (range 3–116 months). The 1-year and 2-year relapse rate were 17.07% (7/41 patients) and 19.51% (8/41 patients), respectively. Patients treated with CS + CsA had lower relapse rate compared to the CS group (29.17% vs. 80.00%, P = .05). Patients with low Fer level also had lower relapse rate (16.67% vs. 56.25%, P = .05). EPO level had no effect on the relapse rate (12.50% vs. 33.33% in EPO ≤400U/L and >400 U/L groups respectively). Most patients (72.73%) relapsed once, while few patients (27.27%) relapsed more than twice. The 42.86% of patients relapsed within 12 months after first remission.
3.3.4. Complications of treatments
In our study, the total incidence rate of complications was 30.14%. The most frequent complication was infection (22.64%) during treatments, followed by hyperglycemia (18.87%), renal dysfunction (15.09%), liver dysfunction (9.43%) and hypertension (3.77%). Pulmonary infection was the most common infection (58.33% of all patients with infection). All of patients with complications were relieved after treatments, except one patient died of serious pulmonary infection.
4. Discussion
Acquired PRCA is defined as an autoimmune disease with low morbidity. Acquired PRCA can be divided into idiopathic PRCA and secondary PRCA according to whether secondary to a primary disease. Thymoma is considered as the most common factor of secondary PRCA, accounted for 7–10% in all acquired PRCA [Citation3,Citation4]. In our study, the proportion of secondary PRCA was 43.40%, and there was no difference in age and gender compared with idiopathic PRCA group. Moreover, thymoma- associated PRCA had highest incidence rate (13.21%), followed by B19 parvovirus-associated PRCA (11.32%). These results suggested that patients with acquired PRCA should accept a careful examination to eliminate primary diseases.
The major symptom of acquired PRCA was anemia, with the signs of dizziness, fatigue, tinnitus, palpitation or pale. Most patients had moderate anemia, and mean hemoglobin concentration was 63.96 ± 16.91 g/L. The patents rarely had other symptoms unless combined with other complications. Abnormal cells in bone marrow or abnormal karyotype indicated other diseases. For laboratory examinations, the main findings were iron overload and increased EPO levels in acquired PRCA patients. In addition, the viral infections occurred frequently in PRCA. In the present study, 30.19% of total PRCA patients had a recent virus infection, including hepatitis B virus, hepatitis A virus, cytomegalovirus, parvovirus B19 and Epstein–Barr virus infection. Thus, it is necessary to screen viruses infection in patients with acquired PRCA [Citation5,Citation6].
In previous reports, humoral and cellular immunity are both altered in patients with acquired PRCA. The patients with acquired PRCA had autoantibodies targeting to erythroblast nuclei and EPO [Citation7,Citation8]. The abnormal quantity and function of cytotoxic T lymphocytes, helper T cells (Ths), regulatory T cells (Tregs) and γ/δ T cells have been reported in many studies [Citation9–12].The clonal expansion of T cells was referred in many researches [Citation13–15]. In this study, we found increased IgG and IgM on the surface of erythroid precursors in parts of acquired PRCA patients (32.65%). Meanwhile, the immune states were obviously different in different PRCA patients. These results implied that the immune pathogenesis was heterogeneity in acquired PRCA. The humoral and cellular immunity might play different roles in PRCA induced by different causes.
Acquired PRCA has a favorable prognosis. IST could make a remarkable improvement in hematopoietic function in approximately two-thirds of patients with acquired PRCA [Citation16]. The efficacy of CS and CsA reported in past years is 30–62% and 65–87% in PRCA treatments [Citation17]. However, the relapse rate of acquired PRCA is very high. And the response rate of IST after relapse is lower than initial treatment. Infection and organ failure are the main causes of death in PRCA [Citation3].Consistent with previous reports, the rate of CR and PR of total patients in our studies was 68.29% and 12.20% respectively. The total relapse rate of IST was 26.83%. The CS + CsA group had similar response rate, but lower relapse rate compared with CS group. Infection was the most common complication in PRCA during treatments. In our study, we found that iron overload and increased EPO levels were common in acquired PRCA patients. EPO and Fer levels might suggest poor prognosis (lower CR rate, longer recovery time and higher relapse rate). The patients with acquired PRCA had autoantibodies targeting to erythroblast nuclei and EPO [Citation7,Citation8]. Recently, many reports supported that PRCA could be induced by the use of recombinant EPO because of the appearance of EPO antibody [Citation8]. In addition, Ah Ram Kim reported that a severe anemia could result from a homozygous mutation in the EPO (R150Q). The mutation could alter the binding of EPO to its receptor and EPO downstream signaling leading to disease [Citation18]. Therefore, we speculate that the high-level EPO associated with poor prognosis in PRCA might be caused by the presence of EPO antibodies or mutation.
Due to long-term transfusion and inflammatory, iron overload often occurs in patients with PRCA. Growth differentiation factor-15 (GDF-15) is a primary regulator of systemic iron homeostasis and iron availability for erythropoiesis. It can inhibit hepcidin secretion and then lead to iron overload. GDF-15 is increased by stimulation of EPO. Previous research approved that GDF-15 increased in severe aplastic anemia (SAA) patients, and might play an important role in iron metabolism in SAA [Citation19]. In our article, an abnormal increase in EPO level is found in some PRCA patients. We speculate that elevated EPO might stimulate the elevation of GDF-15, then aggravate iron overload in patients with PRCA. Iron overload can increase the production of reactive oxygen species, which has a significant inhibition on hematopoiesis [Citation20,Citation21]. Hirokawa reported that treatment of post-transfusion iron overload may contribute to the improvement of the outcome of acquired PRCA [Citation3]. Kojima reported that iron chelation therapy improved hematopoiesis and led to CR in one PRCA patient [Citation22]. Our research is consistent with previous studies, confirms that iron overload is associated with poor prognosis of PRCA. Acquired PRCA can be divided into idiopathic PRCA and secondary PRCA since different pathogenesis. Secondary PRCA is equally sensitive to IST compared with idiopathic PRCA. Japanese researchers conducted nationwide cohort study for adult acquired chronic PRCA in 2004 and 2006, including idiopathic PRCA [Citation23], thymoma-associated PRCA [Citation24], and large granular lymphocyte (LGL) leukemia-associated PRCA [Citation25]. Those studies demonstrated that IST could induce remission and prevent relapse in secondary PRCA effectively. But survival time was not different among idiopathic PRCA and secondary PRCA [Citation3]. The high response rate of secondary PRCA induced by B19 parvovirus infection was reported in a recent study [Citation6]. In our study, we compared clinical features and response rate of idiopathic PRCA and secondary PRCA. The results showed that secondary PRCA had more serious erythrocytopenia and higher Fer level. The recovery time of hemoglobin and reticulocyte after therapy was longer in secondary PRCA. It implied that abnormal immune might be more severe in secondary PRCA. However, the CR rate and relapse rate had no difference between idiopathic and secondary PRCA. For those patients whom primary diseases could be removed, the CR rate of IST was significantly increased, especially in patients with thymoma or parvovirus B19 infection. These results suggested that IST was more effective in secondary PRCA patients.
In conclusion, most acquired PRCA patients had high EPO and Fer levels. Increased EPO and Fer levels might be the negative factors for prognosis of acquired PRCA. The immune pathogenesis was heterogeneity in acquired PRCA. Thymoma and viral infection are the most common causes for secondary PRCA. Secondary PRCA patients have longer recovery time than idiopathic PRCA patients. IST is effective in both idiopathic and acquired PRCA, but with high relapse rate. Therapy of CS + CsA could reduce the relapse rate of PRCA. The most common complication of acquired PRCA is infection during treatments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Rong Fu
Dr Rong Fu is M.D., Ph.D., professor in Tianjin Medical University, Tianjin, People’s Republic of China.
Tian Zhang
Dr Tian Zhang is attending doctor in Department of Hematology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China.
Bingnan Liu
Bingnan Liu is attending doctor in Department of Hematology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China.
Jia Song
Dr Jia Song is M.D., associate chief physician in Department of Hematology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China.
Guojin Wang
Guojin Wang is associate chief physician in Department of Hematology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China.
Lijuan Li
Dr Lijuan Li is M.D., associate professor in Tianjin Medical University, Tianjin, People’s Republic of China.
Huaquan Wang
Dr Huaquan Wang is M.D., associate professor in Tianjin Medical University, Tianjin, People’s Republic of China.
Limin Xing
Dr Liming Xing is M.D., associate professor in Tianjin Medical University, Tianjin, People’s Republic of China.
Yuhong Wu
Yuhong Wu is associate chief physician in Department of Hematology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China.
Jing Guan
Jing Guan is associate chief physician in Department of Hematology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China.
Hong Liu is associate chief physician in Department of Hematology, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China.
Wen Qu is associate professor in Tianjin Medical University, Tianjin, People’s Republic of China.
Zonghong Shao
Dr Zonghong Shao is M.D., Ph.D., professor in Tianjin Medical University, Tianjin, People’s Republic of China.
References
- Yoshida Y. Historical review. The light and shadow of Paul Kaznelson: his life and contribution to hematology. Ann Hematol. 2008;87(11):877–879. doi: https://doi.org/10.1007/s00277-008-0553-1
- Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: updated review of treatment. Br J Haematol. 2008;142(4):505–514. doi: https://doi.org/10.1111/j.1365-2141.2008.07216.x
- Hirokawa M, Sawada K, Fujishima N, et al. Long-term outcome of patients with acquired chronic pure red cell aplasia (PRCA) following immunosuppressive therapy: a final report of the nationwide cohort study in 2004/2006 by the Japan PRCA collaborative study group. Br J Haematol. 2015;169(6):879–886. doi: https://doi.org/10.1111/bjh.13376
- Dessypris EN. Aplastic anemia and other bone marrow failure syndromes: pure red cell aplasia. New York: Springer Press; 1990.
- Frickhofen N, Chen ZJ, Yong NS, et al. Parvovirus B19 as a cause of acquired chronic pure red cell aplasia. Br J Haematol. 1994;87(4):818–824. doi: https://doi.org/10.1111/j.1365-2141.1994.tb06743.x
- Crabol Y, Terrier B, Rozenberg F, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis. 2013;56(7):968–977. doi: https://doi.org/10.1093/cid/cis1046
- Krantz SB, Kao V. Studies on red cellaplasia. I. Demonstration of a plasma inhibitor to heme synthesis and an antibody to erythroblast nuclei. Proc Natl Acad Sci USA. 1967;58(2):493–500. doi: https://doi.org/10.1073/pnas.58.2.493
- Casadevall N, Nataf J, Viron B, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346(7):469–475. doi: https://doi.org/10.1056/NEJMoa011931
- Masuda M, Arai Y, Okamura T, et al. Pure red cell aplasia with thymoma: evidence of T-cell clonal disorder. Am J Hematol. 1997;54(4):324–328. doi: https://doi.org/10.1002/(SICI)1096-8652(199704)54:4<324::AID-AJH12>3.0.CO;2-B
- Fujisao S, Tsuda H. Th1/Th2 balance alteration in the clinical course of a patient with pure red cell aplasia and thymoma. Br J Haematol. 1998;103(2):308–310. doi: https://doi.org/10.1046/j.1365-2141.1998.01011.x
- Higuchi M, Osaki K, Yamano Y. Relapse of autoimmune hemolytic anemia in Evans syndrome after thymectomy for pure red cell aplasia. Rinsho Ketsueki. 2007;48(6):501–504.
- Hara T, Mizuno Y, Nagata M, et al. Human gamma delta T-cell receptor-positive cell-mediated inhibition of erythropoiesis in vitro in a patient with type autoimmune polyglandular syndrome and pure red blood cell aplasia. Blood. 1990;75(4):941–950.
- Handgretinger R, Geiselhart A, Moris A, et al. Pure Red-cell aplasia associated with clonal expansion of granular lymphocytes expressing killer-cell inhibitory receptors. N Eng J Med. 1999;340(4):278–284. doi: https://doi.org/10.1056/NEJM199901283400405
- Sivakumaran M, Bhavnani M, Stewart A, et al. Is pure red cell aplasia (PRCA) a clonal disorder? Clin Lab Haematol. 1993;15(1):1–5. doi: https://doi.org/10.1111/j.1365-2257.1993.tb00115.x
- Masuda M, Saitoh H, Mizoguchi H. Clonality of acquired primary pure red cell aplasia. Am J Hematol. 1999;62(3):193–195. doi: https://doi.org/10.1002/(SICI)1096-8652(199911)62:3<193::AID-AJH10>3.0.CO;2-D
- Means RJ. Pure red cell aplasia. Blood. 2016;128(21):2504–2509. doi: https://doi.org/10.1182/blood-2016-05-717140
- Sawada K, Hirokawa M, Fujishima N. Diagnosis and management of acquired pure red cell aplasia. Hematol Oncol Clin N Am. 2009;23(2):249–259. doi: https://doi.org/10.1016/j.hoc.2009.01.009
- Kim AR, Ulirsch JC, Wilmes S, et al. Functional selectivity in cytokine signaling revealed through a pathogenic EPO mutation. Cell. 2017;168(6):1053–1064. doi: https://doi.org/10.1016/j.cell.2017.02.026
- Shao Y, Wang H, Liu C, et al. Transforming growth factor 15 increased in severe aplastic anemia patients. Hematology. 2017;22(9):548–553. doi: https://doi.org/10.1080/10245332.2017.1311462
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: https://doi.org/10.1038/nature08313
- Kops GJ, Dansen TB, Polderman PE, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: https://doi.org/10.1038/nature01036
- Kojima M, Machida S, Sato A, et al. Deferasirox treatment improved hematopoiesis and led to complete remission in a patient with pure red cell aplasia. Int J Hematol. 2013;98(6):719–722. doi: https://doi.org/10.1007/s12185-013-1455-0
- Sawada K, Hirokawa M, Fujishima N, et al. Long-term outcome of patients with acquired primary idiopathic pure red cell aplasia receiving cyclosporine A. A nationwide cohort study in Japan for the PRCA Collaborative Study Group. Haematologica. 2007;92(8):1021–1028. doi: https://doi.org/10.3324/haematol.11192
- Hirokawa M, Sawada K, Fujishima N, et al. Long-term response and outcome following immunosuppressive therapy in thymoma-associated pure red cell aplasia: a nationwide cohort study in Japan by the PRCA collaborative study group. Haematologica. 2008;93(1):27–33. doi: https://doi.org/10.3324/haematol.11655
- Fujishima N, Sawada K, Hirokawa M, et al. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: a nationwide cohort study in Japan for the PRCA collaborative study group. Haematologica. 2008;93(10):1555–1559. doi: https://doi.org/10.3324/haematol.12871
