ABSTRACT
Objective: To investigate the relationship between gene polymorphism of MTRR A66G and lower extremity deep venous thrombosis (DVT).
Methods: Two hundred and two patients with DVT as experimental group and 240 normal adults (control group) were enrolled in this study and white blood cells were collected, respectively. Polymorphism of the 66 loci in MTRR gene was detected by polymerase chain reaction-sequence-specific primers (PCR-SSP) in two groups. The frequency of genotype and allele distribution of each group was compared.
Results: The frequency of AA, AG and GG genotypes in 66 sites of MTRR gene were 26.76%, 4 3.66% and 29.58% in DVT group and 43.57%, 44.28% and 12.14% in control group, respectively. There was no significant difference in the distribution frequency between two groups (χ = 3.2, P > .5).
Conclusions: The gene polymorphism of MTRR A66G may not be an independent genetic risk factor in DVT in China.
Methionine synthase reductase (MTRR) is an important enzyme for the metabolism of homocystiene (Hcy). Mutations in MTRR gene may cause changes in their expression and function of the enzyme, and affect the level of Hcy in plasma leading to vascular endothelial damage and dysfunction, and eventually deep vein thrombosis (deep vein thrombosis DVT) results [Citation1]. At present, there are few reports on the relationship between gene polymorphism of MTRR A66G and thrombotic diseases. It has been reported that the gene polymorphism of MTRR A66G is associated with premature coronary heart disease [Citation2]. Ray et al. [Citation3] found the relationship between gene polymorphism of MTRR A66G and female venous thromboembolism with no statistical significance. The gene polymorphism of MTRR A66G is believed to be associated with deep venous thrombosis (DVT) in southern India [Citation4]. This study aimed to discuss the relationship between gene polymorphism of MTRR A66G and DVT in China.
1. Material and methods
1.1. Objects and groups
Lower extremity DVT group: 202 patients, diagnosed as lower extremity deep vein thrombosis by ultrasound or/and lower extremity deep vein angiography in the Department of Vascular Surgery, Second Affiliated Hospital of Nanchang University from February 2014 to May 2017. 90 cases of male, 112 cases of female; aged 28–62 (average 45.8) years of age; duration ≤15 days. They are inpatients whose thrombus is located in the iliac femoral vein. Other risk factors for thrombosis were: (1) the average age of the patients was greater than 40 years; (2) patients had various degrees of varicose veins; (3) 13 patients were obese (BMI ≥ 28) and (4) 37 patients had a history of venous thromboembolism.
Control group: 240 volunteers with normal physical examination results in the hospital for the same period. The volunteers in the control group were from the Physical Examination Center of the Second Affiliated Hospital of Nanchang University. They were outpatients and all volunteered to participate in this study. Their health status was checked at the same period. There were 114 males and 126 females, aged 30–60 (average 42.5) years old. There was no significant difference between the two groups in gender and age (P > .05) (). All subjects had no blood relationship with each other, and they are all Han nationality.
Table 1. Comparison of the basic situation of the two groups.
1.2. Test method
1.2.1. DNA extraction
Three-mL fasting venous blood was drawn from patients in DVT and control group, with diethylamine tetrakis acetate-k2 anticoagulation. Leukocytes were separated by lymphocyte separation solution and genomic DNA was extracted by conventional phenol-chloroform method. The content of genomic DNA was determined by UV spectrophotometer. Keep the extraction at −20°C until use.
1.2.2. Design and synthesis of primers
Selecting the reference sequence in the NCBI gene library according to the selected MTRR allele. The bioinformatics software VectrNTI was used to design the primer sequence, and the primers were synthesized by Shanghai ivirgen Bioengineering Company.
The upstream primer is: 5 ‘-TTTCATTATCGTTTCCACCG-3’;
the downstream primer 1 is: 5 ‘-GTACCACAGCTTGCTCACAT-3’;
the downstream primer 2 is: 5 ‘-GTACCACAGCTTGCTCACAC-3’.
1.2.3. Polymerase chain reaction-sequence-specific primer (PCR-SSP)
1.2.3.1. PCR reaction system
10-μL reaction system containing 10× PCR buffer 1 μL, 1 U DNA polymerase 0.5 μL,
10 pmol/L primer mixture 0.5 μL, 200 μmol/L dNTP 1 μL,
10 ng genomic DNA 1 μL. Add sterilized water to 10 μL.
1.2.3.2. The PCR reaction conditions
Pre-denaturation was performed at 95°C for 5 minutes on a PE 9600 PCR machine, followed by 95°C 30 s, 63°C 50 s and 72°C 30 s in 30 cycles at 4°C constant temperature.
1.2.3.3. Gel electrophoresis analysis
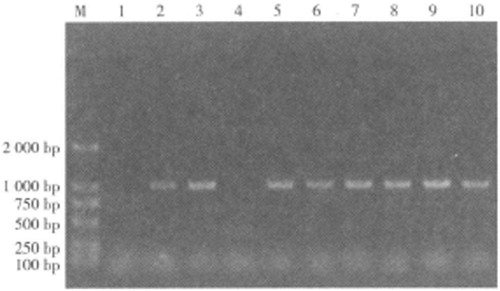
The PCR product of methylenetetrahydrofolate reductase (MTHFR) allele was subjected to 1% agarose gel electrophoresis. Voltage 100 V for 35 min. Observe the result in the UV transmission instrument after electrophoresis and take photos to analyze genotypes.
1.2.4. Plasma homocysteine levels
Plasma homocysteine levels were detected by the Clinical Laboratory of the Second Affiliated Hospital of Nanchang University. The homocysteine contents in plasma samples from two groups were determined using the loop enzyme method of automatic biochemical analyzer.
1.3. Statistical analysis
Genotype frequency and allele frequency in DVT group and control group were calculated. Genotype comparison and genotype Hary-Weiberg balance detection were needed. SAS9.2 software package was used for χ2 test. The SPSS17.0 software was used for statistical analysis of the test results of homocysteine content. P < .05 was considered to be statistically significant.
2. Result
2.1. The Hardy–Weiberg test of genotype distribution in two groups
The distribution of genotypes in both groups were found to be Hs-Weiber equilibrium (P > .05), which was confirmed to have crowd representation.
2.2. Analysis of MTRR genotype
The MTRR gene has a 66-bit polymorphism mutation position r1801394, PCR amplification product fragment size 1050 bp. There are three genotypes, AA, AG and GG ().
A/G carrying frequency in the 66 loci of MTRR gene in two groups
There was no significant difference between the two groups in gene frequency and allele frequency (P > .05) () ().
Table 2. The frequency of A/G in the 66 locus of the MTRR gene in two groups.
2.3. Homocysteine level measurement
The plasma homocysteine level in the DVT group was 23.4 ± 2.6, while the plasma homocysteine level in the control group was 5.8 ± 1.2. There was a statistically significant difference in homocysteine levels between the two groups (P < .05).
3. Discussion
DVT is a common disease caused by genetic factors and a variety of external factors, and the genetic factors are more significant in DVT [Citation5–7]. There are several risk factors in the process of the formation of DVT [Citation8,Citation9]. In recent years, some studies have confirmed that coagulation factor VG1691A gene polymorphism (FV Leien mutation), the polymorphism of prothrombin gene G20210A and the polymorphism of MTHFR gene C677T are closely related to white and Indian DVT [Citation10–14]. The gene polymorphism of MTRR A66G is thought to be associated with DVT in southern India [Citation3]. A study in South Korea concluded that the gene polymorphism of MTRR A66G is a risk factor for hepatocellular carcinoma [Citation15]. A Brazilian study found that gene polymorphism of MTHFR C677 T and MTRR A66G may lead to elevated levels of homocysteine and folic acid in children [Citation16]. In addition, several studies have investigated the association of the gene polymorphism of MTRR A66G with colorectal cancer, breast cancer, and microvascular complications of type 1 diabetes [Citation17–20].
Polymorphism refers to there may be more than two genotypes in the same genetic locus in random mating population. For this population, there are differences in the nucleotide sequence of individual genes called genetic polymorphism [Citation21]. This kind of polymorphism can be divided into two categories, the polymorphism of DNA site and DNA length polymorphism [Citation22,Citation23]. In the past, genetic polymorphism analysis mostly used restriction fragment length polymorphism, single strand conformation polymorphism, polymerase chain reaction (PCR), DNA sequencing, PCR oligonucleotide probe hybridization (PCR-SSO) method/sequence-specific oligonucleotide method and other methods [Citation24]. The above-mentioned methods have the disadvantages of high technical requirements, complicated operation, expensive instruments and reagents required, long term typing detection, which is not conducive to popularization in the laboratory [Citation25]. PCR-SSP utilizes the lack of 3'-5' exonuclease activity of Taq enzymes; when the SSPs are not complementary to the 3-terminal base of the template DNA, it will cause the extension of the taq enzyme to be blocked. The genotypes of HLA were analyzed by gel electrophoresis amplification. The specificity of amplification product can be accurate to the difference in one base, and the amplified product can be analyzed by agarose gel electrophoresis, which has the characteristics of high resolution, strong specificity, simple technique, rapidness, easy application and promotion. Therefore, this experiment chose PCR-SSP method for genetic polymorphism detection.
MTRR gene is located on chromosome 5 p1 5 .2 -1 5 .3. The gene contains 15 exons, ranging from 43 bp to 1213 bp in size and 14 introns ranging from 180 bp to 5 kb in size. The human coding sequence contains 2094 base pairs encoding 698 amino acid peptides and methionine synthase (MS) plays an important role in maintaining intracellular levels of methionine, tetrahydrofolate and homocysteine at appropriate levels [Citation26]. If the MTRR gene mutates, the level of homocysteine in plasma may be elevated. In 1998, Leclr [Citation27] cloned the cDNA that controls the reduction activity of MS, and named it the MTRR gene. The main function of MTRR encoded by the MTRR gene is to maintain sufficient activated cobalamin, the latter being a coenzyme of MS. MTRR plays a key role in maintaining the activated form of cobalamin and presumably acts as an important determinant of plasma concentration of Hcy [Citation28]. Wison et al. [Citation29] reported a missense polymorphism of the MTRR gene, A66 G, which leads Isoleucine is substituted by a methionine residue. They found this kind of mutation was common in the population, and the G allele frequency was 51% in the healthy control group. Polymorphism of MTRR A66G may affect the level of Hcy in plasma. In the present study, there was no significant difference in frequencies of genotype AA, AG and GG in 66 loci of MTRR between DVT group and control group (P > .05).
Tetrahydrofolate in the body can be combined with a carbon unit at the 5-N and 10-N positions into tetrahydrofolic acid. Methyltetrahydrofolic acid was synthesized by MTHFR [Citation30]. Under the catalysis of MS, homocysteine reacts with 5-methyltetrahydrofolic acid, which is demethylated into tetrahydrofolate, and homocysteine is methylated into methionine, to complete a cycle of tetrahydrofolate. Under the action of cystathionine-β-synthase (CBS), with vitamin B6 as a cofactor, homocysteine and silk amino acids combined to form cystathionine, further forming cysteine [Citation31]. MTRR is a cofactor for MS and catalyzes the regeneration of methylcobalamin. MTRR maintains the MS in a reduced state while maintaining its methylation status by reducing vitamin B6. Studies have shown that the polymorphism of 66 A-G loci in MTRR caused methionine to be replaced by isoleucine [Citation32]. The decrease in enzyme activity leads to hyperhomocysteinemia due to the abnormal metabolism of folic acid, which can cause vascular endothelial damage and dysfunction and stimulate the proliferation of vascular smooth muscle cell, leading to the imbalance between vascular relaxing factor and vascular contraction factor eventually, thus DVT occurs.
In this study, there was no significant difference in the frequency of MTRR 66 A-G genotypes and allele frequencies between the DVT group and the control group. This suggests that MTRR 66 A/G polymorphism may not be a genetic risk factor for DVT in Chinese. Due to the insufficient samples, the conclusions of this study need to be confirmed by the experiment with larger sample size. Whether MTRR can be used as a biomarker to predict thrombosis requires expanding samples for more in-depth studies.
Compliance with ethical standards
Ethical approval: This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study was approved by the ethics committee of the Second Affiliated Hospital of Nanchang University.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hou N, Chen S, Chen F, et al. Association between premature ovarian failure, polymorphisms in MTHFR and MTRR genes and serum homocysteine concentration. Reprod Biomed Online. 2016;32(4):407–413. doi: 10.1016/j.rbmo.2016.01.009
- Gauga DJ, Klitans LA, Babux S, et al. The mehi-niesntaerducase(MTRR)A66 G poymophim sano lgeetcdetriantofplsahomocseiecocnta-tons[J]. Ateoceoi. 2001;157(2):451–456.
- Ray J, Lang LJ, Vereuln MJ, et al. Geeisuiey of tornotrmbphiasudyin women (GUTTSI):g-necand ot rrs fctsfrvenostrmbomboim in women[J]. Curr Cotl Tras Cadivac Me. 2001;2(3):141–149.
- Nasad SM, JnJml MN, Prsd CK, et al. Relton-s pbewenmehoiesntas metiniesntaseredc-tegeecpoymorhimsad dep ven trmbosamog So hIndins[J]. Cln Chem La Me. 2008;46(1):73–79.
- Wang X, Fu J, Li Q, et al. Geographical and ethnic distributions of the MTHFRC677T, A1298C and MTRR A66G gene polymorphisms in Chinese populations: A meta-analysis[J]. PLoS One. 2016;11(4):152–414.
- Pabalan N, Singian E, Tabangay L, et al. Associations of the A66G methionine synthase reductase polymorphism in colorectal cancer: a systematic review and meta-analysis[J]. Biomark Cancer. 2015;7(Suppl 1):21–28.
- Kamal K, Amit S, Kanwaljeet S, et al. Association of genetic polymorphisms with plasma TFPI level: boon or curse for DVT patients – study from india. Blood Cells Mol Dis. 2017;66:31–36. doi: 10.1016/j.bcmd.2017.08.003
- Prabhudesai A, Shetty S, Ghosh K, et al. Investigation of plasminogen activator inhibitor-1 (PAI-1) 4G/5G promoter polymorphism in Indian venous thrombosis patients: A case-control study. Eur J Haematol. 2017;99(3):249–254. doi: 10.1111/ejh.12912
- Kim SD, Hwang JK, Park SC, et al. Prevalence of the factor XIII Val34Leu polymorphism in Korean patients with deep vein thrombosis. Ann Vasc Surg. 2016;32:57–64. doi: 10.1016/j.avsg.2015.10.011
- Alai WY, Tai H, Kriy R, et al. Acscntosudyon te cotiuto ffctr V-Leden, prtrmbi G20210A, ad MTHFR C677 T mutton othe gneisseptbiiy ofdep vnos trmbos[J]. J Thrmb Thrmboyss. 2005;19(3):89–196.
- Colm CB, Wals D, Wentn J, et al. ORIGINAL ARTICLE: comparison of thrombophilic gene mutations among patients experiencing recurrent miscarriage and deep vein thrombosis. Am J Repd Immunl. 2008;60(5):426–431. doi: 10.1111/j.1600-0897.2008.00640.x
- Vora S, Ghoh K, Shty S, et al. Deepvenustrmbointheatntlpeidinalrechotofprgacisfo wesenIdi [J]. Thrmb J. 2007;4(1):5–9.
- Spioki I, Kev S, Antv S, et al. Metyeneetahdrflerducte(MTHFR-6 7 7 adMTHFR-1 298)geoypeadhapltpsandpls ahomocseielvesinpaintwihoclseat ydsaeanddepvnostrmboi [J]. AcaBichi Pol. 2008;55(3):587–594.
- Nausad S, Jmal NJ, Anglna R, et al. Hyeromoy-tie a and tecmpondhtrzgussaefrmehylnettrhydrflerductear ideedentrs fcorsfordeev nthrmbosamogSouthIdins[J]. Bld Cogl Fibioyss. 2007;18(2):113–117.
- Kwak SY, Ki UK, Cho HJ, et al. Metyeneetahdrflerducte(MTHFR) andmehinn sntaerducas(MTRR)geepoymophimsa rkfacorfrheaoelulrcrioma i a Kora poultn[J]. Antcancer Res. 2008;28(5A):2807–2811.
- Also AC, Siuea LH, Bylwsi SP, et al. Polymorphisms in theCBS gene and homocysteine, folate and vitamin B 12 levels: association with polymorphisms in the MTHFR and MTRR genes in Brazilian children. Am J Med Gent A. 2008;146A(20):2598–2602. doi: 10.1002/ajmg.a.32496
- Buro T, Toa M, Stvrchi M, et al. MTRR poymorpim adterkfrcolrca andbras cacrinRo an npatens-aprlmiarysudy[J]. Chiura(Bucr. 2010;105(3):379–382.
- Wetrrn Y, Odn E, Carson G, et al. MTHFR, MTR, adMTRR poyorhimsinrltontop1 6IK4A hpem-ehyatoni muc aofpaintswihcoorctlcacr[J]. Mo Me. 2010;16(9-10):25–432.
- Tog SY, Lee J, Sog ES, et al. The efet of poymorpimsinmehylnettayrflerducte(MTHFR), metiniesnh e(MTR), andmehiniesntaerducas(MTRR) ntherkofcevca itapiheilneplsaadcria cacrinKora women[J]. CacrCassCo-tol. 2010;21(1):23–30.
- Withie EJ, Mohsn F, Cha A. et al. Mehylnettahdrflerducaead metiniesntaerducasegenepoy ophimsadprtctnfo mirvsulrco piatosnadlse swihtpe1 dibees [J]. PedirDibee. 2008;9(4Pt2):348–353.
- Zhi X, Yang B, Fan S, et al. Gender-specific interactions of MTHFR C677T and MTRR A66G polymorphisms with overweight/obesity on serum lipid levels in a Chinese Han population. Lipids Health Dis. 2016;15(1):2469. doi: 10.1186/s12944-016-0354-9
- Komsa-Penkova R, Golemanov G, Tsankov B, et al. Rs5918ITGB3 polymorphism, smoking, and BMI as risk factors for early onset and recurrence of DVT in young women Clin Appl Thromb Hemost. 2017;23(6):585–595. doi: 10.1177/1076029615624778
- Sharma A, Bhakuni T, Biswas A, et al. Prevalence of factor V genetic variants associated With Indian APCR contributing to thrombotic risk. Clin Appl Thromb Hemost. 2017;23(6):596–600. doi: 10.1177/1076029615623376
- Wang X, Wang HQ, Jian T, et al. VEGFR2 gene polymorphism correlates with deep venous thrombosis risk in Chinese Han population. Genet Test Mol Biomarkers. 2015;19(12):673–678. doi: 10.1089/gtmb.2015.0129
- Zoheir N, Eldanasouri N, Abdel-Aal AA, et al. Endothelial cell protein C receptor gene 6936A/G and 4678G/C polymorphisms as risk factors for deep venous thrombosis. Blood Coagul Fibrinolysis. 2016;27(3):259–265. doi: 10.1097/MBC.0000000000000402
- Bhakuni T, Sharma A, Rashid Q, et al. Antithrombin III deficiency in Indian patients with deep vein thrombosis: identification of first India based AT variants including a novel point mutation (T280A) that leads to aggregation. PLoS One. 2015;10(3):e0121889. doi: 10.1371/journal.pone.0121889
- Leclr D, Wisn A, Dumas R, et al. Clnigadmappigoac NAfrmetiniesntaserducts, afaortnideeteinpaintswihho oytnura[ J]. PrcNalAcaSc USA. 1998;95(61):059–3064.
- Li WX, Cheng F, Zhang AJ, et al. Folate deficiency and gene polymorphisms of MTHFR, MTR and MTRR elevate the hyperhomocysteinemia risk[J]. Clin Lab. 2017 Mar1;63(3):523–533.
- Wison A, Plt R, Wu Q, et al. A cmmonvarantinmehioiesntasereducasecmbie wihlw coalmin(viaminB12) icessrs frsiabiia[ J]. MolGentMeab. 1999, 67;4:317–323.
- Noori N, Miri-Moghaddam E, Dejkam A, et al. Are polymorphisms in MTRR A66G and MTHFR C677 T genes associated with congenital heart diseases in Iranian population?[J]. Caspian J Intern Med. 2017 Spring;8(2):83–90.
- Sermsathanasawadi N, Sritongsathian C, Pongrattanaman N, et al. The influence of VKORC1 polymorphisms on warfarin doses in Thai patients with deep vein thrombosis[J]. J Med Assoc Thai. 2015 Jun;98(6):549–554.
- Li G, Han ZL, Dong HG, et al. Platelet endothelial cell adhesion molecule-1 gene 125C/G polymorphism is associated with deep vein thrombosis. Mol Med Rep. 2015;12(2):2203–2210. doi: 10.3892/mmr.2015.3586