ABSTRACT
Objectives: To evaluate the association of interleukin 6 (IL-6) levels with deep vein thrombosis (DVT) and to assess the impact of IL-6 promoter polymorphisms (−174G > C, −572G > C and −597G > A) on its plasma levels and their influence in the development of DVT in India.
Methods: One hundred DVT patients and 100 age and sex-matched healthy controls were study subjects. IL-6 polymorphisms were identified by polymerase chain reaction-restriction fragment length polymorphism. IL-6 levels were detected by enzyme-linked immunosorbent assay.
Results: Significantly raised IL-6 levels were observed in patients as compared to controls. (Patients: 13.73 ± 6.30 pg/ml, Controls: 11.83 ± 4.47 pg/ml, p = 0.014). The prevalence of C allele of −572G > C polymorphism was significantly higher in patients than controls (Patients: 39.5%, Controls: 27.5%, p = 0.011, χ2=6.463). Subjects with GC and CC genotype had significantly higher IL-6 levels than GG genotype (p=<0.001). Patients with GC and CC genotype increased the DVT risk by 1.39 fold (ORa: 1.39, CI: 0.74–2.62) and 2.69 fold (ORa: 2.42, CI: 1.08–6.70), respectively. IL-6 −174G > C and −597G > A polymorphisms were not associated with raised IL-6 levels and nor with thrombotic risk (−174G > C: p = 0.823 χ2=0.369; −597G > A: p = 0.678 χ2=1.08).
Conclusion: Our study emphasizes the importance of −572G > C polymorphism in increasing IL-6 levels, thereby showing its significant role in DVT in India. IL-6 −174G > C and −597G > A were neither associated with raised plasma IL-6 levels nor with thrombotic risk. Thus −572G > C polymorphism detection may be one of the connecting links between IL-6 and thrombotic risk in Indian DVT patients.
Introduction
Deep vein thrombosis (DVT) including venous thromboembolism (VTE) is a common thrombotic vascular condition that affects about 1/1000 people per year [Citation1]. Clinically, the DVT patients manifest with four cardinal signs of inflammation i.e. heat, redness, pain and swelling. Systemic inflammation plays a crucial role in thrombogenesis by upregulating the procoagulant factors, downregulating the natural anticoagulants and by inhibiting the fibrinolytic activity. Inflammatory molecular markers such as Interleukin-6 (IL-6), Interleukin-8 (IL-8) and C reactive protein (CRP) may be the key mediators in the development of DVT.
The IL-6, a protein with molecular weight ∼26 kDA, is the major stimulus for the synthesis of acute phase reactants and plays a major role in the development of thrombosis. The role of raised IL-6 levels in arterial thrombosis and its clinical manifestations (atherosclerosis, myocardial infarction (MI)) is well documented [Citation2,Citation3]. However, there are arguments and mixed opinions about the association of IL-6 levels with venous thrombosis. Some clinical studies have confirmed a significant association of raised IL-6 levels with initial and recurrent venous thromboembolic disorders [Citation1,Citation4]. Also, as per Leiden Thrombophilia Study (LETS), raised IL-6 levels were associated with increased the risk of venous thrombosis [Citation5]. In contrast to above studies, the association of IL-6 and other inflammatory cytokines with venous thrombosis was not recognized by some research studies [Citation6,Citation7]. Although the exact role of raised IL-6 levels in DVT pathogenesis is not fully understood, one of the various mechanisms which may play a crucial role in the regulation of IL-6 levels is promoter polymorphisms. These promoter polymorphisms are not fully elucidated and explored till date.
As per our knowledge, the association of IL-6 levels and its polymorphisms with DVT has been sparsely reported in Indian population. Considering this, we conducted this study to evaluate the role of IL-6 levels in DVT risk and to assess the impact of IL-6 promoter polymorphisms (−174G > C, −572G > C and −597G > A) on its plasma levels and their influence in the development of DVT.
Methods
Patients and controls
Total 100 patients with DVT (age: 21–60 years) were recruited from the Department of Hematology, All India Institute of medical sciences, New Delhi, India. DVT was diagnosed by compression ultrasonography and magnetic resonance imaging. Patients with pregnancy, malignancy, liver disorder, inflammatory bowel disease, antiphospholipid syndrome and cardiovascular disease were excluded from the study. A total of 100 patients were included in the study, of whom 93 had DVT of the lower limbs and 7 patients had DVT of upper limbs. Fourteen patients out of 100 had pulmonary thromboembolism.
The control group comprised of 100 healthy age and sex-matched subjects recruited from acquaintances or partners of patients. Controls had no medical history of thrombosis, cardiovascular disorders, malignancies, rheumatic and inflammatory bowel diseases and chronic renal or liver diseases. Patients and controls were from the same geographic region and had the similar ethnic background. Written informed consent was obtained from both patients and controls and ethical clearance was obtained from the institutional review board.
Sample collection and laboratory investigations
Five millilitres venous blood was collected in siliconized glass containers, containing 3.2% trisodium citrate solution. Plasma was separated by centrifugation for 10 min at 2000 × g at room temperature and stored at −70°C. The blood sample was collected after 3 months of DVT diagnosis and at least 12 weeks after the discontinuation of oral anticoagulant therapy. Plasma levels of IL-6 were detected by enzyme-linked immunosorbent assay using commercially available kits (Quantikine, R&D system, Minneapolis, USA). Genomic DNA was extracted from whole blood collected from patients and controls using Bioserve DNA isolation kit. IL-6 gene polymorphisms were identified by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).
For IL-6 −174G > C single nucleotide polymorphism, a 190 bp fragment was amplified by PCR using the following primers; sense 5′-TGACTTCAGCTTTACTCTTGT-3′ and anti-sense 5′-CTGATTGGAACCCTTATTAAG-3′[Citation8]. PCR condition follows: 1 cycle of 95°C for 4 minutes, followed by 35 cycles of 95°C for 30 seconds, 50°C for 30 seconds and 72°C for 90 seconds, followed by 1 cycle of 72°C for 10 minutes. Digestion with NlaIII enzyme (New England of Biolab) at 37°C for overnight yielded two DNA fragments when C allele was present (143 and 47 bp) and one fragments when G allele was present (190 bp).
The −572G > C and −597G > A polymorphisms genotypes were determined by using primers 5′-GGAGACGCCTTGAAGTAACTGC-3′ and 5′-GAGTTTCCTCTGACTCCATCGCAG-3′ to generate a PCR fragment of 163 bp. PCR condition follows: 1 cycle of 94°C for 4 minutes, followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds and 72°C for 90 seconds, followed by 1 cycle of 72°C for 10 minutes. Genotypes were resolved by using restriction endonuclease digestion. The rare −597A allele has a FokI restriction cutting site and the −572C allele lacks the digestion site for BsrBI. Positive and negative controls were used to ensure accuracy [Citation9].
Statistical analysis
Descriptive and frequency statistical analysis were obtained, and comparisons were performed by use of the Stata 11. P values were two-tailed, and statistical significance was set at p < 0.05. Comparison between categorical variables was performed with chi-square testing. Student t-test was used for the comparison of continuous variables which were normally distributed. Coefficient of variation (CV) was calculated to determine the variability of the IL-6 levels between patients and controls. Allele frequencies were derived from genotypic data. Deviations from the Hardy–Weinberg equilibrium were also tested. Odds ratios (ORs) for DVT associated with IL-6 polymorphisms and corresponding 95% confidence intervals (CIs) were calculated using logistic regression, adjusted for age, body mass index (BMI), blood pressure (BP), smoking status and prevalence of type 2 diabetes mellitus (DM).
Results
A summary of basic characteristics of the study subjects is presented in . Mean age of the patients and controls were 45.5 ± 5.5 and 44.6 ± 5.2 years, respectively (p = 0.235). The male to female ratio was 0.92:1. There was no significant difference between patients and controls regarding the distribution of hypertension, DM and current smoking. Patients had significantly higher mean BMI as compared to controls (p = 0.023, ).
Table 1. Characteristics of study subjects.
Plasma IL-6 levels
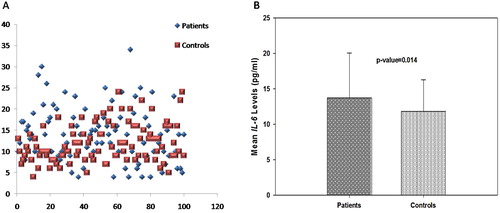
The mean plasma IL-6 level in patients was 13.73 ± 6.30 pg/ml, compared to 11.83 ± 4.47 pg/ml in controls. Patients had higher mean plasma IL-6 levels than controls and this difference was found to be statistically significant (p = 0.014, (A,B)). The CV was 37.8% for control and 45.9% for the DVT patients.
Promoter polymorphisms of IL-6 gene
shows the distributions of IL-6 genotypes and allele frequencies of the patients and controls. The frequencies of the IL-6 −174G > C genotypes (GG, GC and CC) were 65, 34 and 1% for patients and 69, 30 and 1% for control, respectively (p = 0.823, χ2 = 0.369). The derived allele frequencies of G and C alleles for IL-6 −174G > C were 82 and 18% in patients and 84 and 16% in the controls, respectively (p = 0.191, χ2 = 1.71) ().
Table 2. Alleles frequencies and genotypes frequencies of IL-6 promoter polymorphisms.
The frequencies of the IL-6 −572G > C genotypes (GG, GC and CC) were 41, 39 and 20% for patients and 54, 37 and 9% for control, respectively (p = 0.050, χ2 = 6.004). The derived allele frequencies of G and C alleles for IL-6 −572G > C were 60.5 and 39.5% in patients and 72.5 and 27.5% in the controls and this difference was statistically significant (p = 0.011, χ2 = 6.463).
The frequencies of the IL-6 −597G > A genotypes (GG, GA and AA) were 75, 24 and 1% for patients and 72, 25 and 3% for control, respectively (p = 0.678, χ2 = 1.08). The derived allele frequencies of G and A alleles for IL-6 −597G > A were 87 and 13% in patients and 84.5 and 15.5% in the controls, respectively (p = 0.531, χ2 = 0.392).
Relative risk of DVT according to IL-6 genotypes
The relative risk of DVT according to IL-6 genotypes is summarized in . Patients with −174G > C polymorphism had OR of 1.20 (95% CI: 0.66–2.18) in GC genotype and 1.06 (95% CI: 0.06–17.32) in CC genotype (). After adjusting for age, BMI, BP, current smoking status and DM type 2 the OR (ORa) was 1.16 (CI: 0.62–2.17) for GC genotype and 1.25 (CI: 0.06–23.22) for CC genotype ().
Table 3. Relative risk of DVT according to IL-6 genotypes.
Patients with IL-6 −572G > C polymorphism had increased ORs of 1.38 (95% CI: 0.75–2.54) and 2.92 (95% CI: 1.20–7.09) in GC and CC genotypes than OR for GG genotype. Similar results were obtained after adjusting for BMI, BP, current smoking status and DM type 2, adjusted ORa = GG: 1.0, GC = 1.39 (95% CI: 0.74–2.62), CC = 2.69 (95% CI: 1.08–6.70, []).
Patients with IL-6 −597G > A polymorphism had decreased ORs of 0.921 (95% CI: 0.48–1.75) in GA genotype and 0.32 (95% CI: 0.03–3.14) in AA genotypes. After adjusting for age, BMI, BP, current smoking status and DM type 2 the OR (ORa) was 0.892 (CI: 0.45–1.74) for GA genotype and 0.412 (CI: 0.040–4.16) for CC genotype ([]).
Influence of IL-6 polymorphisms on plasma IL-6 levels
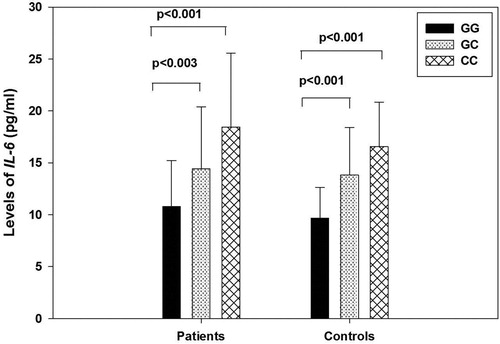
The distribution of IL-6 levels according to genotypes in patients and controls are shown in []. Patients and controls with GC and CC genotypes of −572G > C polymorphism had significantly higher plasma IL-6 levels than GG genotype (Patients: GC: 14.41 ± 5.99 pg/ml, CC: 18.45 ± 7.12pg/ml, GG: 10.78 ± 4.43 pg/ml, p = <0.001, Controls: GC: 13.83 ± 4.56 pg/ml, CC: 16.55 ± 4.30 pg/ml, GG: 9.66 ± 2.99 pg/ml, p = <0.001, []). As compared to mean IL-6 levels in GG genotype of −174G > C polymorphism, values were not significantly higher in genotypes with GC and CC in patients (GG: 13.44 ± 6.61 pg/ml, GC: 14.20 ± 5.81 pg/ml, CC: 16.00 pg/ml, p = 0.590) and controls (GG: 11.15 ± 3.99 pg/ml, GC: 13.33 ± 5.23 pg/ml, CC: 13.00 pg/ml, p = 0.156, []). Patients and controls with GA and AA genotypes of −597G > C polymorphism had lower plasma IL-6 levels than GG genotype but this difference was not statistically significant (Patients: GG: 14.06 ± 6.06 pg/ml, GA: 12.83 ± 7.14 pg/ml, AA: 10.00 pg/ml, p = <0.425, Controls: GG: 11.88 ± 4.38 pg/ml, GA: 11.72 ± 4.93 pg/ml, AA: 11.33 ± 3.78 pg/ml, p = 0.981, []).
Table 4. Plasma levels of IL-6 by genotypes of IL-6 promoter polymorphisms in subjects.
Discussion
Of late, there has been a great deal of research interest in understanding the pathophysiology of thrombosis and evaluating the association between inflammatory mediators and thrombogenesis. Numerous studies have reported that increased IL-6 and CRP levels are associated with raised arterial thrombotic events e.g. increased cardiovascular disease/MI and atherosclerosis [Citation10–12]. Apart from the studies as mentioned above, whose work was primarily centered on investigating the role of these inflammatory mediators in arterial thrombosis, few research aspirates also focused on their possible role in venous thrombosis.
Roumen-Klappe et al (1998) measured the baseline levels of IL-6, IL-8, CRP and their levels post-heparin administration in DVT patients. Plasma IL-6, IL-8 and CRP levels reduced during heparin treatment which is known to possess anti-inflammatory properties. These findings strengthen the possible role of the inflammatory cytokines like IL-6 in DVT [Citation13]. Later, Van Aken (2000) observed the elevated levels of IL-6 in patients with VTE [Citation1]. Furthermore, two studies observed similar findings in European population [Citation4,Citation5]. In our study we observed significantly raised IL-6 levels in DVT patients as compared to controls (p = 0.010), thus suggesting its role in increasing the risk of DVT. These are comparable to abovementioned studies [Citation1,Citation4,Citation5].
Though research work has been done in exploring the association of IL-6 levels with thrombosis, studies investigating the role of IL-6 polymorphisms in DVT patients in Asian population especially in India are sparsely mentioned in the literature. So, we evaluated the correlation of IL-6 promoter polymorphisms with its plasma levels and their influence on DVT risk in India.
Among IL-6 polymorphisms, we observed that C allele of −572G > C polymorphism was significantly higher in patients as compared to controls (patients: 39.5%, controls: 27.5%, p = 0.011). This polymorphism was also found to be associated with significantly higher IL-6 levels (p = <0.001). In our study population −572G > C polymorphism was seen to confer an almost 2.6 fold risk for thrombosis after adjusted for BMI, BP, current smoking and type 2 DM. A study had earlier shown the cooperative influence of the genetic polymorphisms on IL-6 transcriptional regulation [Citation13]. Two previous studies conducted by Brull (2001) and Bennermo et al. (Citation2004) have revealed that −572G > C polymorphism is one of the strong predictors of raised IL-6 levels and is associated with higher mortality and morbidity in coronary artery disease (CAD) patients [Citation9,Citation14]. In addition to above, One more study conducted on VTE patients in Chinese population showed that −572G > C polymorphism is an independent risk factor for VTE [Citation15]. In contrast to these studies, two recent studies reported that this polymorphism is not associated with increased risk of CAD and recurrent pregnancy loss (RPL), respectively [Citation16,Citation17]. Our results are similar to Brull and Bennermo findings [Citation9,Citation14]. We did not find any significant association of −597G > C polymorphism with high IL-6 levels and the risk of DVT in the present study. Our findings are in agreement with two previous studies conducted on CAD and RPL patients, respectively [Citation16,Citation17]. Despite extensive review of the literature, we did not find data pertaining to −597G > C polymorphisms, its impact on IL-6 levels and association with DVT.
The prevalence of −174G > C polymorphism was higher in patients than controls but the difference was not statistically significant in our study [p = 0.823]. However, there are unequivocal opinions regarding the role of this polymorphism with thrombosis. Our results are in line with two previous studies where they have reported no association of this polymorphism with the risk of ischemic stroke in South Korean and DVT in European population, respectively [Citation6,Citation18]. In contrary to these findings, one recent study suggested marginal association of −174 G > C polymorphism with thrombotic risk in venous and arterial thrombotic disorders in Asian population [Citation19].
Regarding the association of −174G > C polymorphism with IL-6 levels in DVT patients; there are different schools of thought. Fishman et al. (Citation1998) reported reduced IL-6 levels in patients with −174G > C polymorphism and thus pointed towards its protective nature, whereas two studies have shown the association of this polymorphism with high IL-6 levels and thus a causal role in the development of DVT [Citation8,Citation19]. Another European study revealed no association of −174G > C polymorphism and IL-6 levels with the risk of spontaneous VTE [Citation6]. In our study, we also did not find any association of this polymorphism with high IL-6 levels and risk of DVT. Therefore, we suggest further exploration of this polymorphism and its effect on IL-6 levels in a larger subset of DVT patients to evaluate its conclusive role in venous thrombosis.
In conclusion, our study highlights the importance of −572G > C polymorphism in increasing IL-6 levels, thereby showing significant association with thrombosis in Indian DVT patients. We observed 2.6 fold increased risk of thrombosis in patients as compared to controls, thus emphasizing causative role of this polymorphism in Indian DVT patients. However, −174G > C and −597G > A polymorphisms were not associated with raised IL-6 levels and thrombotic risk. Thus −572G > C polymorphism detection may be one of the connecting links between IL-6 and thrombotic risk, which will help to understand the complexity of mechanisms involved in DVT. These findings may play important role in overall management of DVT in Indian patients. However, a study in larger subset of patients is required to confirm our findings.
Authors contribution
AA designed the study, performed experimental studies and drafted the manuscript. KS compiled the data and contributed to writing. AB and RR helped in drafting the manuscript. KK and HRP reviewed the manuscript and helped in shaping the manuscript. MM and RS provided valuation suggestions and clinical inputs. All the authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Van Aken BE, den Heijer M, Bos GM, et al. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost. 2000;83:536–539. doi: 10.1055/s-0037-1613858
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207
- Lee WY, Allison MA, Kim DJ, et al. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernado Study). Am J Cardiol. 2007;99:99–102. doi: 10.1016/j.amjcard.2006.07.070
- Van Aken BE, Reitsma PH, Rosendaal FR. Interleukin 8 and venous thrombosis: evidence for a role of inflammation in thrombosis. Br J Haematol. 2002;116:173–177. doi: 10.1046/j.1365-2141.2002.03245.x
- Reitsma PH, Rosendaal FR. Activation of innate immunity in patients with venous thrombosis: the Leiden Thrombophilia Study. J Thromb Haemost. 2004;2:619–622. doi: 10.1111/j.1538-7836.2004.00689.x
- Vormittage R, Hsieh K, Kaider A, et al. Interleukin-6 and interleukin-6 promoter polymorphism (-174) G > C in patients with spontaneous venous thromboembolism. Thromb Haemost. 2006;95(5):802–806. doi: 10.1160/TH05-12-0816
- Christiansen SC, Naess IA, Cannegieter SC, et al. Inflammatory cytokines as risk factors for a first venous thrombosis: a prospective population-based study. PLoS Med. 2006;3:e334. doi: 10.1371/journal.pmed.0030334
- Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629
- Brull DJ, Montgomery HE, Sanders J, et al. Interleukin-6 gene -174G > C and -572G > C promoterpolymorphisms are strong predictors of plasmainterleukin6 levels after coronary artery bypass surger. Arterioscler Thromb Vasc Biol. 2001;21(9):1458–1463. doi: 10.1161/hq0901.094280
- Erren M, Reinecke H, Junker R, et al. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol. 1999;19:2355–2363. doi: 10.1161/01.ATV.19.10.2355
- Volpato S, Guralnik JM, Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation. 2001;103:947–953. doi: 10.1161/01.CIR.103.7.947
- Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.CIR.101.15.1767
- Roumen-Klappe EM, den Heijer M, van Uum SH, et al. Inflammatory response in the acute phase of deep vein thrombosis. J Vasc Surg. 2002;35:701–706. doi: 10.1067/mva.2002.121746
- Bennermo M, Held C, Green F, et al. Prognostic value of plasma interleukin-6 concentrations and the -174G > C and -572G > C promoter polymorphisms of the interleukin-6 gene in patients with acute myocardial infarction treated with thrombolysis. Atheroscierosis. 2004;174:157–163. doi: 10.1016/j.atherosclerosis.2004.01.019
- Mahemuti A, Abudureheman K, Aihemaiti X, et al. Association of interleukin-6 and C-reactive protein genetic polymorphisms levels with venous thromboembolism. Chin Med J. 2012;125(22):3997–4002.
- Sun GQ, Wu GD, Meng Y, et al. IL-6 gene promoter polymorphisms and risk of coronary artery disease in a Chinese population. Genet Mol Res. 2014;13(3):7718–7724. doi: 10.4238/2014.September.26.9
- Demirturk F, Ates O, Gunal O, et al. IL-6 gene promoter polymorphisms: genetic susceptibility to recurrent pregnancy loss. Bratist Lek Listy. 2014;115(8):479–482.
- Kim SK, Chung JH, Kwon OY. Promoter polymorphism (-174, G/C) of interleukin-6 and arterial thromboembolic events: a meta-analysis. Med Sci Monti. 2016;22:4345–4353. doi: 10.12659/MSM.901467
- Ren H, Zhang Y, Yao Y, et al. Association between the interleukin-6 genetic polymorphism 174 G/C and thrombosis disorder risk. Medicine (Baltimore). 2016;95(27):e4030. doi: 10.1097/MD.0000000000004030