ABSTRACT
Objective: This research aimed to explore the significance of low activity of ADAMTS-13 in acute myeloid leukemia (AML) after bone morrow transplantation (BMT), and to evaluate the disease progress and prognosis of the patients with low or normal activity of ADAMTS-13 after BMT.
Methods: 46 AML patients were included in our research. ADAMTS-13 activity was measured before BMT. Their medical indicators were recorded one month after BMT. All the patients were followed up and their disease progression was evaluated afterwards. The medical indicators and prognosis situation were compared between Low ADAMTS13 Group (<481 ng/ml) and Normal Group (481–785 ng/ml) according to the reference range of local laboratory. ROC curves were used to evaluate the predictive value of ADAMTS13 for infection complications and survival.
Results: Low ADAMTS13 Group show extended APTT, PT and elevated CRP and D-Dimer levels, compared with Normal Group. Low ADAMTS13 Group suffered more BMT-related complications than Normal Group. In addition, Low ADAMTS13 Group underwent higher mortality than Normal Group in the one-year follow up after BMT and two-year follow up after onset of AML. ADAMTS13 has AUC of 0.7675, 0.7254, 0.8019 for lung infection, CMV infection and death within one year after BMT, suggesting that ADAMTS13 has predictive value for prognosis of AML patients after BMT.
Discussion: For patients with low ADAMTS13 activity, the prognosis was worse and the probability of serious complications and mortality was significantly higher than AML patients with normal ADAMTS13 activity, which suggest predictive role of ADAMTS13 activity for the prognosis of AML after BMT.
Conclusion: AML patients with low activity of ADAMTS-13 had worse prognosis after BMT.
Introduction
Von Willebrand Factor (VWF) is a subset of the circulating glycoprotein in the blood that mediates the adhesion of platelets to damaged blood vessels [Citation1,Citation2]. Mature VWF is a multimeric protein that can be cleaved by the ADAMTS13 metalloprotease of the ADAMTS family [Citation3]. ADAMTS13 is essential for maintaining the normal size distribution of VWF multimers. In the absence of ADAMTS13, large amounts of VWF accumulate and high concentrations of large VWF multimers lead to excessive platelet aggregation [Citation4,Citation5]. Low activity of ADAMTS-13 (<5% of normal plasma) is considered to be responsible for thrombotic thrombocytopenic purpura (TTP) [Citation4–6]. The decreased activity of ADAMTS13 can be seen not only in TTP, but also in metastatic malignancy or after surgery [Citation7,Citation8]. It has been reported that a complete lack of ADAMTS13 in mice results in a prothrombotic phenotype [Citation9,Citation10]. In addition, the decreased activity of ADAMTS13 in patients with acute systemic inflammation suggests a relationship between inflammation and ADAMTS13 deficiency [Citation11]. However, knowledge of the role of ADAMTS-13 in acute myeloid leukemia (AML) disease is still limited.
As cancer of myeloid lineage, AML is characterized by abnormal white blood cells accumulating in the bone marrow and disturbing the growth of normal blood cells [Citation12]. Bone marrow transplantation (BMT) is one of the choice of treatment for many patients with acute leukemia. The major problems after BMT include relapse, graft-versus-host disease (GVHD) and infection [Citation13,Citation14].
This research aimed to explore the significance of low activity of ADAMTS-13 in AML after BMT, evaluating prognosis of the patients with low or normal activity of ADAMTS-13 after BMT.
Materials and methods
Patients
46 AML patients admitted to the hospital from September 2016 to September 2017 were involved in our research. Everyone has carried out haploidentical allo-HSCT. The preconditioning consisted of cytarabine, busulfan, cyclophosphamide, simustine and rabbit antithymocyte globulin (ATG) to deplete T cells. The prophylaxis for graft-versus-host disease (GVHD) was the same for all patients, including cyclosporine (CsA), mycophenolate mofetil, and short-term methotrexate (MTX). ADAMTS13 activity was measured before pretreatment of BMT, and their followed-up medical records were collected with approval from institutional ethical review board of the Hospital. The research had been performed in accordance with the ethical standards of Declaration of Helsinki and all selected individuals had given informed consent. All these patients were without irrelevant complications, chronic diseases, or thromboembolic events before BMT and patients with TTP were excluded.
Blood sampling and analyze of ADAMTS13 activity
The citrated plasma samples were assayed for ADAMTS13 activity using recombinant VWF86-ALEXA FRET substrate. The activity of ADAMTS13 in plasma was measured using the ACTIFLUOR ADAMTS13 activity assay kit purchased from SEKISUI Diagnostics, LLC (Stamford, CT). The standard curve was constructed using normal plasma of known concentrations of ADAMTS13 in the kit. The activity of ADAMTS13 in plasma was determined by interpolating the fluorescence change from the standard curve, and the activity was expressed as ng/ml according to the curve. According to our method, the local reference range of ADAMTS-13 (above the 2.5th percentile and below the 97.5th percentile) is determined to be 481–785 ng/ml, while ADAMTS-13 below 481 ng/ml (equivalent to about 60% activity) is considered to be low level.
Statistics
All analyses were carried out with GraphPad Prime 5.5 software. All data are expressed as mean ± SD. Student's t test and χ2 statistics (Fisher exact test) were used to evaluate differences between groups. The Kaplan-Meier method was used to analyze survival, and the groups were compared by use of the Mantel–Cox and Breslow tests. Receiver operating characteristics (ROC) curves were used to evaluate the predictive value of ADAMTS13 after BMT. Area under curves (AUC) was calculated and optimal cut-off values were analyzed according to the maximum value of Youden index. All the statistical tests were two tailed. A value of P < 0.05 was considered statistically significant.
Results
AML patients with lower ADAMTS13 activity show extended APTT, PT and elevated CRP and D-Dimer levels after BMT
46 AML patients admitted to the hospital were included in our research. All were performed BMT afterwards and their ADAMTS13 activity was measured before pretreatment of BMT. According the local reference range, we divided the patients into two groups: Low ADAMTS13 Group (ADAMTS13 activity lower than 481 ng/ml) and Normal Group (with ADAMTS13 activity 481–785 ng/ml). The detailed sub-types of AML and demographic information of the patients were listed in and there was no significant difference about the constitution between two groups. Followed-up medical records of the patients were collected. We evaluated APTT, PT, CRP, WBC and hemoglobin values one month after BMT to reflect the physical condition of the patients. As shown in , we found APTT and PT in Low ADAMTS13 Group were 42.9 ± 15.1s and 20.2 ± 15.2s, which were extended compared with Normal Group (33.6 ± 7.7s, 11.9 ± 2.2s). As a marker of inflammation, CRP of the Low ADAMTS13 Group is 86.2 ± 95.3 mg/L, significantly higher than that of Normal Group (24.8 ± 32.0 mg/L). We also evaluated the level of D-Dimer, and Low ADAMTS-13 Group was also higher than Normal Group, 3157 ± 3672 ng/ml to 587 ± 1021 ng/ml.
Table 1. Demographic and clinical characteristics of the AML patients before BMT.
Table 2. Summary of the clinical characteristics of the AML patients one month after BMT.
AML patients with low ADAMTS13 activity have more BMT-related complications after BMT
All the patients were followed up for one year after BMT. For the BMT-related complications, each patient was recorded, including infection, bleeding, GVHD, organ failure and thrombotic microangiopathies (TMA), etc. The numbers of the occurrence in two groups were listed in . We found that Low ADAMTS13 Group suffered higher percentage of each complication than Normal Group, but only for pulmonary infection, cytomegalovirus infection, TMA and respiratory failure, the differences were significant (P < .05). We had also analyzed the relapse rates within one year after transplantation in the two groups. The low ADAMTS-13 group was (8/25), the normal group ADAMTS-13 group was (5/21), and the low ADAMTS-13 group was slightly higher than normal. Group, but the P value checked by Chi-square did not reach a significant level (P > .05).
AML patients with low ADAMTS13 activity suffer higher mortality
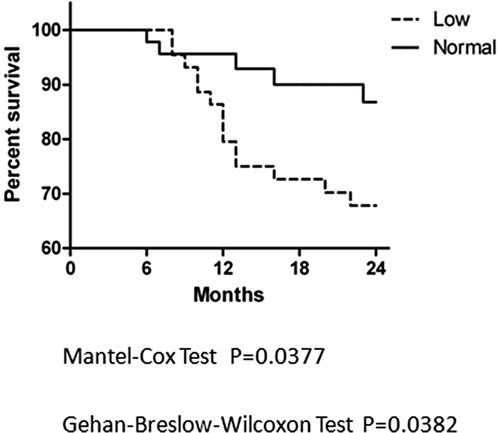
All the AML patients were followed for one year since BMT and the situations of mortality were recorded. The cause of death includes recurrence, infection and GVHD, or mixed causes. The Kaplan-Meier method was used to analyze survival, and the two groups were compared by use of the Mantel–Cox and Breslow tests. The survival curves is shown in , for Mantel–Cox test between curves, P = 0.0163, for Breslow test between curves, P = 0.0155, meaning that there was significant difference between the survival curves of two groups. We also analyze the survival curve started from the onset day of AML. Situation of each patient was recorded for two years (24 months) since the day the leukemia was onset, which could exclude the influence of the time course from onset to BMT. The curve is shown in , for Mantel–Cox test between curves, P = 0.0377 and for Breslow test between curves, P = 0.0382, which suggest that there are also significant difference between survival curves of two groups.
Figure 1. Kaplan-Meier curve analysis since BMT of two groups of AML patients. AML patients were followed for one year after BMT and the Kaplan-Meier curves were used to show percentage of survival of Low ADAMTS13 Group (dashed) and Normal Group (solid). Two groups were compared by use of the Mantel-Cox and Breslow tests and the P values were listed below.
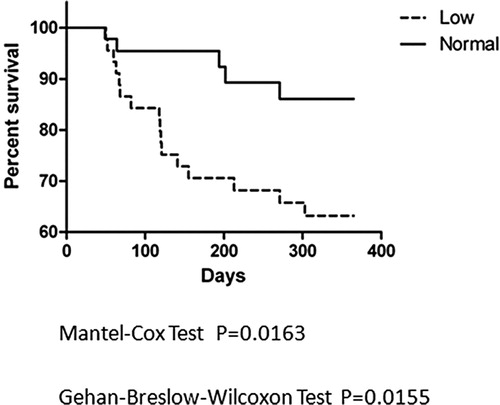
Figure 2. Kaplan-Meier curve analysis since onset of two groups of AML patients. The survival curve started from the day of onset, followed for 24 months since the day the leukemia was onset. The Kaplan-Meier curves were used to show percentage of survival of Low ADAMTS13 Group (dashed) and Normal Group (solid) of AML patients. P values of the Mantel-Cox and Breslow tests were listed below.
ROC analysis of the predictive value of ADAMTS13 for infection complications and survival
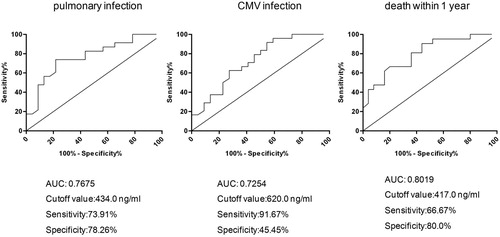
Based on the patient's complications and observations about death during the first year after transplantation, we performed ROC curve analysis in combination with continuous variables of ADAMTS-13. The potential effect of the activity level of ADAMTS13 on predicting prognosis was verified by the curve. The results are shown in . Based on the results, we found that the activity of ADAMTS13 has AUC of more than 0.7 for lung infection, CMV infection and death within one year after BMT, suggesting that ADAMTS13 has predictive value for prognosis of AML patients after BMT. According to the Yoden index, the cutoff values for each index were 434.0, 620.0 and 417.0 ng/ml, respectively. The cutoff values of lung infection and death within one year were close to 481 ng/ml, the cut-off value we used for AML patients grouping. For CMV infection, the second highest Yoden index was with the cutoff value of 430.5 ng/ml (sensitivity is 62.5% and specificity is 72.7%), which is also close to 481 ng/ml, suggesting that our grouping criteria are more reasonable.
Figure 3. ROC analysis of ADAMTS-13 for predicting infections and death of AML patients. The ROC curves of ADAMTS-13 for predicting pulmonary infection, CMV infection and death within 1 year after BMT were analyzed and AUC were calculated. Data were obtained from statistical analysis of 46 AML patients. Optimal cut-off values were analyzed according to the maximum value of Youden index. The sensitivity and specificity at the cut-off value were also listed.
Discussion
Concerning the significance of ADAMTS-13 in AML, our group had reported that ADAMTS-13 level is relatively reduced during AML and is related to inflammation factors and infections of AML patients [Citation15]. BMT represents a completely different treatment from conventional treatments. For patients who had already been treated with BMT, the role of ADAMTS-13 is still unclear and is worth studying. In this study we demonstrated that for AML, level of ADAMTS13 activity could reflect the prognosis of the patients after bone marrow transplantation. For patients with low ADAMTS13 activity, the prognosis was worse and the probability of serious complications and mortality was significantly higher than AML patients with normal ADAMTS13 activity, which suggest predictive role of ADAMTS13 activity for the prognosis of AML after BMT.
Why the low activity of ADAMTS13 predicts a worse prognosis after BMT? We believe this is also related to changes in coagulation function in patients. Although ADAMTS13 levels are not as low as in TTP patients, lower than normal ADAMTS13 activity may still play a role in disease progression. After bone marrow transplantation, severe changes happened in the body's coagulation system, and low ADAMTS13 activity can lead to increased vWF levels, so the risk of excessive platelet aggregation is higher. From our results, we also found that the likelihood of TMA in patients with AML is higher than normal. Blood coagulation worsens, further leading to organ failure or even death. According to our results, ADAMTS-13 may be associated with relapse rate, but their relationship was relatively week. The detailed mechanism for low ADAMTS13 to produce more complications and mortality need to be studied in further studies.
Our research still has limitations. The conditions of patients with acute leukemia vary, and individual differences should be considered. For the classification of AML, they are better to be studied separately, though we have shown that the classification of low ADAMTS13 groups does not differ from the normal group. There are also some factors that may affect the results, such as the efficiency of BMT, the time course between onset and BMT, and so on. However, we tried our best to eliminate the interference factors and make the two groups comparable. As to what causes some patients to have low ADAMTS13 activity, this still cannot be answered by our present study. It has been reported that the activity of ADAMTS13 in patients with acute systemic inflammation is reduced [Citation11]. From our own perspective, inflammation may play its part in the decrease of ADAMTS13 activity.
In this study we inquired into the role of low ADAMTS13 activity in the prognosis of AML after BMT, which will be beneficial to comprehensively understand the clinical significance of ADAMTS13, especially its predictive role in the prognosis of acute leukemia.
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Description of the authors
Dr Chen Liu designed the research and wrote the paper; Ms Man Han and Ms Ying Song were in charge of making the figures and tables and correcting the manuscript; Dr Lei Zhao measured the clinical parameters and ADAMTS-13 activity levels; Ms Mengjie Zhu and Qinzhu Xu collected the data and helped to analyze the data; Prof. Hui Wang helped to write the paper. All the authors are members of department of Clinical Laboratory, Peking University People's Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395
- Moake JL, Turner NA, Stathopoulos NA, et al. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986;78:1456. doi: 10.1172/JCI112736
- Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200
- Vesely SK, George JN, Lämmle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura–hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–68. doi: 10.1182/blood-2003-01-0193
- Bianchi V, Robles R, Alberio L, et al. Von Willebrand factor–cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100:710–713. doi: 10.1182/blood-2002-02-0344
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11–18. doi: 10.1182/blood-2008-02-078170
- Oleksowicz L, Bhagwati N, DeLeon-Fernandez M. Deficient activity of von Willebrand’s factor-cleaving protease in patients with disseminated malignancies. Cancer Res. 1999;59:2244–2250.
- Pereboom ITA, Adelmeijer J, Van Leeuwen Y, et al. Development of a severe von Willebrand factor/ADAMTS13 dysbalance during orthotopic liver transplantation. Am J Transplant. 2009;9:1189–1196. doi: 10.1111/j.1600-6143.2009.02621.x
- Banno F, Kokame K, Okuda T, et al. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood. 2006;107:3161–3166. doi: 10.1182/blood-2005-07-2765
- Chauhan AK, Motto DG, Lamb CB, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203:767–776. doi: 10.1084/jem.20051732
- Reiter RA, Varadi K, Turecek PL, et al. Changes in ADAMTS13 (von-Willebrand-factor-cleaving protease) activity after induced release of von Willebrand factor during acute systemic inflammation. Thromb Haemost. 2005;93:554–558.
- Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562.
- Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. New Engl J Med. 1995;332:217–223. doi: 10.1056/NEJM199501263320403
- Liu C, Zhao L, Zhao J, et al. Reduced ADAMTS-13 level negatively correlates with inflammation factors in plasma of acute myeloid leukemia patients. Leukemia Res. 2017;53:57–64. doi: 10.1016/j.leukres.2016.12.004
