ABSTRACT
Objectives: The relevance of detecting antibodies against anticardiolipin, β2-glycoprotein I (β2gpI) or lupus anticoagulant (LA), collectively called antiphospholipid autoantibodies (APA), in subjects with immune thrombocytopenia (ITP) is still a debated issue. In particular, whether APA profile may affect the clinical course of ITP is unknown.
Methods: In this study, we report our experience in a cohort of ITP patients with APA with specific interest to the relevance of different antiphospholipid antibody profiles in clinical outcome and response to treatment.
Results: Thirty-seven out of 159 patients (23.2%) fulfilling ITP criteria had a platelet count ≤50 × 109/L and tested positive at APA at ITP onset. Twenty-three (62.1%) patients received at least one line of treatment for ITP. Fourteen subjects (37.8%) showing triple positivity for APA showed a significantly lower median platelet count compared to other APA patients (p = .006). Among these ITP subjects with triple positivity, 85.7% needed a treatment because of low platelet count compared to 47.8% ITP patients with non-triple-positive APA (p = .0094). ITP/APA subjects who received immunosuppressors had a higher rate of thrombosis (p = .024) as well as thrombosis developed in subjects who were on steroid therapy at a significantly higher dosage than subjects who did not develop thrombotic episodes (p < .001). When considering treatment, CR and SR rate were significantly higher in ITP/triple-positive patients compared to non-triple-positive subjects (p = .021 and p = .005).
Conclusions: The profile of APA may affect the outcome of patients with ITP.
Introduction
Immune thrombocytopenia (ITP) comprises a heterogeneous group of disorders characterized by autoimmune-mediated platelet destruction and impairment of platelet production leading to bleeding events [Citation1,Citation2]. Antiphospholipid syndrome (APS) is characterized by arterial and venous thrombosis, recurrent foetal loss, and thrombocytopenia in the presence of antiphospholipid antibodies (APAs). Outside the clinical syndrome, APAs can coexist in subjects with ITP and typically, platelet count is lower than in isolated APAs. The percentage of ITP patients positive for APA varies from 25 to 75% [Citation3–5].
It has been previously reported that APA positivity is a common finding in patients with ITP. The most addressed aspect of this association has been the increased risk of thrombosis and this implication seems the only reason for testing ITP subjects for APA. Beside thrombotic risk, previous studies failed to identify clinical features which may differentiate ITP from ITP with APA.
This is also true for response to treatment although very few data are available on more recent therapeutic approaches as immunosuppressants, rituximab and thrombopoietin agonist (TPOa) [Citation6]. Published experience in a limited number of patients supports a role of rituximab in the modulation of the pathogenetic effect of APA. In this study, we report our experience in a cohort of ITP patients with APA with specific interest to the relevance of different antiphospholipid antibody profiles in clinical outcome and response to treatment.
Methods
Study population
We retrospectively evaluated 244 medical records of outpatients with thrombocytopenia (platelet count <100 × 109/L) assessed at our Institution from January 2009 to December 2016. ITP diagnosis was based on the American Society of Haematology guidelines for ITP [Citation2]. Only patients over 18 years old at diagnosis with platelet counts <50 × 109/L were included.
Pregnant patients or those with concomitant human immunodeficiency (HIV) or hepatitis C virus infection were excluded from the study. Primary as well as secondary ITP were considered for the study.
Laboratory methods
Lupus anticoagulant, aCL and anti-β2GP1 were tested at the time of ITP diagnosis. All subjects considered for the study were retested at 12 weeks apart. Results, expressed as IgG antiphospholipid or IgM antiphospholipid units, were considered negative (<15 μg/dl), weakly positive (15–39 μg/dl) or strongly positive (≥40 μg/dl). A solid-phase immunoassay on human β2GP1-coated plates (BMD) was used to detect anti-β2GP1. Lupus anticoagulant was sought using standard coagulation assays, according to the guidelines of the International Society of Thrombosis and Haemostasis [Citation7].
Briefly, LA were considered present when activated thromboplastin time and/or kaolin-clotting time were prolonged and the prolongation was not corrected by 1/1 mixture of patient plasma/normal pooled plasma. LA presence was then confirmed by shortening or correcting the prolonged coagulation time by the addition of excess phospholipids.
Outcome
Response to treatments had been defined as recommended [Citation2]. The response was considered to be related to a specific treatment only if a temporal correlation was indisputable evidence. The sustained response has been considered as partial remission (PR) or complete remission (CR) lasting >6 months. Loss of response or relapse were defined as thrombocytopenia <30 × 109/L in two assessments.
Thrombotic events were considered if confirmed by Doppler ultrasonography, ventilation–perfusion lung scintigraphy and/or computed tomography, angiography or other tests considered diagnostic for a specific thrombotic episode.
Results were presented as a mean standard deviation for continuous data and as percentages for categorical data. Comparisons of the numerical data between groups were performed using Student’s t-test or the Mann–Whitney U-test. Qualitative differences between groups were analysed by the chi-square test or Fisher’s exact test. Cox regression survival analysis was used to detect independent predictors of thromboembolism among clinical and serological factors. Two-sided P values of ≤.05 were considered statistically significant.
Results
Following patient selection according to inclusion criteria, 233 subjects were considered for the study. Among 159 patients (68.2%) fulfilling ITP criteria, 37 (23.2%) had a platelet count ≤50 × 109/L and were found to be positive for APA at the time of diagnosis.
Fourteen subjects did not receive any therapy at the time of ITP diagnosis and during the follow up because of platelet count >30 × 109/L and no bleeding symptoms. Thirteen subjects (35.1%) had an underlying disorder and were diagnosed as secondary ITP ().
Table 1. Patients’ characteristics.
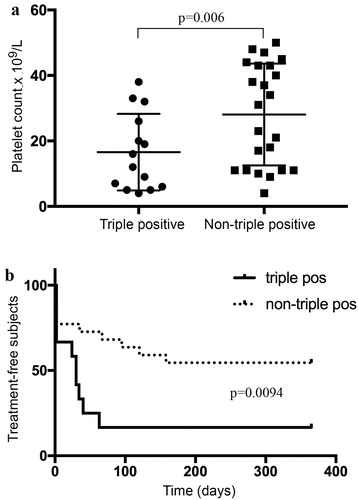
Twenty-three (62.1%) patients received treatment over the course of ITP disease. Clinical characteristics are summarized in . Fourteen subjects (37.8%) had LAC, anti-β2GP1 and anticardiolipin antibodies and were defined as triple-positive. Triple-positive subjects showed a significantly lower median platelet count compared to other APA patients (15 ± 2.5 vs 28.1 ± 3.1 × 109/L, p = .006; ). Therefore, a significantly higher fraction of triple-positive ITP subjects (12/14, 85.7%) were treated as a result of low platelet count compared to non-triple-positive APA over the course of the disease (11/23, 47.8%; p = .0094, Mantel–Cox test; (b)).
Figure 1. Platelet count in APL triple-positive and non-triple-positive ITP subjects. Solid horizontal lines represent mean values (a). Kaplan–Meier curves showing treatment-free outcome according to triple-positive or non-triple-positive APL pattern (p = .0094; log-rank test) (b).
The rate of thrombotic events was higher in the treated group compared to untreated subjects although it did not reach statistical significance (17.4% vs 7.1%, p = ns). When considering therapeutic agents, only the use of immunosuppressors (azathioprine, ciclosporine, cyclophosphamide, mycophenolate mofetil, vincristine) was associated with a higher rate of thrombosis (80% vs 28.1%, p = .024). Furthermore, although steroid use did not affect rate of thrombosis, all subjects who experienced thrombosis were on steroid therapy at a significantly higher dosage than subjects who did not develop thrombotic episodes (mean dosage 33 ± 5.9 mg vs 8.3 ± 0.8 mg die; p < .001). All subjects who experience thrombosis had platelet count >50 × 109/L at the time of the thrombotic event. One subject who was on eltrombopag therapy developed two sequential episodes of upper arm superficial thrombophlebitis when platelet count raised >100 × 109/L.
Twenty-two out of 23 subjects (95.6%) who received treatment for ITP showed a response to treatment. Among these patients, the CR rate was significantly higher in ITP/triple-positive patients (10/12, 83%) compared to non-triple-positive subjects (4/11, 36.3%; p = .021). Sustained response was observed in 11/12 (91.6) subjects with triple-positive APA compared to 4/11 (36.4%) non-triple-positive subjects (p = .005).
Sixteen out of 23 treated subjects received rituximab during the course of the disease. Overall response to rituximab was 68.7% (11/16), with 6 subjects reaching CR. No significative difference was observed in term of overall response rate to rituximab between ITP/triple- vs non-triple-positive subjects. A total of 10 subjects (27%) experienced at least a relapse. Eight patients relapsed between 6 and 15 months after rituximab treatment. Five of these patients had received a second course of rituximab which led to sustained remission (follow-up 55 months) in 4 cases and transitory remission (8 months) in one case. In all cases who achieved remission, this was long lasting compared to the time elapsed following the first course. All cases reaching the second remission were APA triple-positive subjects. Five patients who relapsed after rituximab or other treatment were treated with steroid, all reaching partial or complete remission.
The only factor independently related to relapse was the presence of anti-β2GP1 antibodies (p = .024). No relationship between APA titre and outcome was found.
Discussion
The relevance of APA in patients with ITP is open to debate. Although it seems advisable to test for APA any ITP patient developing thrombosis, it is still unclear if patients with primary ITP and APA should be treated differently.
ITP patients with normal and elevated levels of APAs have similar clinical profiles. Previous authors have reported a 30% prevalence of APA in ITP patients at the time of diagnosis, yet many researchers have questioned the clinical significance of this observation
The most addressed issue in ITP patients with APA is the thrombotic risk. Conflicting results have been published. Stasi and Ruggeri did not observe an increased incidence of thrombotic episodes in ITP subjects with APA whereas Diz-Kucukkaya et al provided data supporting that persistent APA positivity may be a risk factor for thrombosis [Citation3,Citation6,Citation8]. The overall rate of thrombotic events in our cohort was 13.5% and, although not statistically significant, subjects who received treatment for ITP experienced more thrombotic episodes compared to untreated subjects. Therefore, a higher rate of thrombotic events was found in our ITP patients with APA, compared to the overall rate of thrombosis reported in the literature for ITP. Although we could not confirm previous observation with regard to an association between steroid therapy and a higher rate of thrombosis, we found a direct correlation between the development of thrombosis and steroid dosage [Citation8]. In agreement with previous studies, immunosuppression (use of azathioprine, cyclophosphamide, cyclosporine, mycophenolate mofetil and vincristine) was significantly associated with an increased rate of thrombosis.
Our experience further strengthens the importance of balancing the benefit-risk ratio in term of bleeding and thrombosis when considering the treatment of a patient with ITP. In particular, not all subjects may need normalization of platelet count and some of them may benefit from prophylaxis for thrombosis. In this regard, there is a general consensus that prophylaxis should be done in ITP patients with high-risk situations like immobilization and surgery (including splenectomy) if platelet count is at least greater than 30 x109/L [Citation9]. On the other hand, although the presence of APA per se does not alter management of a patient with ITP, in selected cases increasing platelet count would allow to manage anticoagulation necessary to prevent thrombosis [Citation10].
The presence of triple-positive APA was associated with a lower platelet count in our cohort of ITP subjects. Previous studies failed to report any association between platelet count and the presence of APA in ITP subjects but none of them compared triple-positive vs single or double APA positivity. Severe thrombocytopenia in triple-positive APA may be explained by the presence of different antiplatelet antibodies with different specificity in ITP subjects with antiphospholipid antibodies, in some cases leading to a more relevant platelet destruction [Citation11,Citation12].
Our observations are in agreement with previous observations that thrombocytopenia could protect patients against thrombotic events as we observed thrombosis only in treated subjects with platelet count above 50 × 109/L [Citation6,Citation13]. It should be kept in mind that thromboses can occur during ITP and, as our data suggest, a close follow up is essential in ITP/APA subjects who are started on ITP-specific therapy.
Although there are no clinical trials on the role of rituximab in APS, some reports suggest a beneficial role in this setting, in particular with regard to the response of thrombocytopenia of APS subjects [Citation14]. Our data confirm a rate of response close to 60%, which is consistent with the expected rate in ITP subjects. Interestingly, triple-positive subjects showed longer remission compared to non-triple-positive patients. Although only from a speculative point of view, this observation may reflect a specific beneficial effect of rituximab in presence of anti-β2GP1 antibodies which were present on triple-positive subjects. In fact, there is evidence that anti-β2GPI antibodies may contribute to thrombocytopenia as a result of binding to platelet β2GPI able to induce platelet activation and aggregation [Citation15]. Interestingly, triple-positive patients who relapsed following rituximab reached second long-lasting remission, then identifying a subset of ITP/APA subjects who may benefit from rituximab retreatment.
In conclusion, different antiphospholipid antibody profile in ITP patients may affect the outcome and particularly indication and response to ITP-specific treatment.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable standards.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Fabrizio Vianello http://orcid.org/0000-0002-7174-4651
References
- Cines DB, Liebman HA. The immune thrombocytopenia syndrome: a disorder of diverse pathogenesis and clinical presentation. Hematol Oncol Clin North Am. 2009;23:1155–1161. doi: 10.1016/j.hoc.2009.09.003
- Neunert C, Lim W, Crowther M, et al. The American society of hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984
- Diz-Küçükkaya R, Hacihanefioğlu A, Yenerel M, et al. Antiphospholipid antibodies and antiphospholipid syndrome in patients presenting with immune thrombocytopenic purpura: a prospective cohort study. Blood. 2001;98:1760–1764. doi: 10.1182/blood.V98.6.1760
- Pierrot-Deseilligny Despujol C, Michel M, Khellaf M, et al. Antiphospholipid antibodies in adults with immune thrombocytopenic purpura. Br J Haematol. 2008;142:638–643. doi: 10.1111/j.1365-2141.2008.07228.x
- Uthman I, Godeau B, Taher A, et al. The hematologic manifestations of the antiphospholipid syndrome. Blood Rev. 2008;22:187–194. doi: 10.1016/j.blre.2008.03.005
- Stasi R, Stipa E, Masi M, et al. Prevalence and clinical significance of elevated antiphospholipid antibodies in patients with idiopathic thrombocytopenic purpura. Blood. 1994;84:4203–4208.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardisation committee of the international society on thrombosis and haemostasis. J Thromb Haemost. 2009;7:1737–1740. doi: 10.1111/j.1538-7836.2009.03555.x
- Ruggeri M, Tosetto A, Palandri F, et al. Thrombotic risk in patients with primary immune thrombocytopenia is only mildly increased and explained by personal and treatment-related risk factors. J Thromb Haemost. 2014;12:1266–1273. doi: 10.1111/jth.12636
- Rodeghiero F. Is ITP a thrombophilic disorder? Am J Hematol. 2016;91:39–45. doi: 10.1002/ajh.24234
- Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155
- Fabris F, Steffan A, Cordiano I, et al. Specific antiplatelet autoantibodies in patients with antiphospholipid antibodies and thrombocytopenia. Eur J Haematol. 1994;53:232–236. doi: 10.1111/j.1600-0609.1994.tb00195.x
- Macchi L, Rispal P, Clofent-Sanchez G, et al. Anti-platelet antibodies in patients with systemic lupus erythematosus and the primary antiphospholipid antibody syndrome: their relationship with the observed thrombocytopenia. Br J Haematol. 1997;98:336–341. doi: 10.1046/j.1365-2141.1997.2243038.x
- Galli M, Finazzi G, Barbui T. Thrombocytopenia in the antiphospholipid syndrome. Br J Haematol. 1996;93:1–5. doi: 10.1046/j.1365-2141.1996.390969.x
- Kumar D, Roubey RA. Use of rituximab in the antiphospholipid syndrome. Curr Rheumatol Rep. 2010;12:40–44. doi: 10.1007/s11926-009-0074-5
- Shi T, Giannakopoulos B, Yan X, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54:2558–2567. doi: 10.1002/art.21968
