Abstract
Post-traumatic stress disorder (PTSD), a disorder that develops following exposure to traumatic experience(s), is frequently associated with agitation, aggressive behavior and psychotic symptoms. Monoamine oxidase (MAO) degrades different biogenic amines and regulates mood, emotions and behavior, and has a role in the pathophysiology of various neuropsychiatric disorders. The aim of the study was to investigate the association between different symptoms occurring in PTSD [PTSD symptom severity assessed by the Clinician Administered PTSD Scale (CAPS), agitation and selected psychotic symptoms assessed by the Positive and Negative Syndrome Scale (PANSS)] and platelet MAO-B activity and/or genetic variants of MAOB rs1799836 and MAOA-uVNTR polymorphisms in 249 Croatian male veterans with PTSD. Our study revealed slightly higher platelet MAO-B activity in veterans with PTSD with more severe PTSD symptoms and in veterans with agitation, and significantly higher platelet MAO-B activity in veterans with more pronounced psychotic symptoms compared to veterans with less pronounced psychotic symptoms. Platelet MAO-B activity was associated with smoking but not with age. Genetic variants of MAOB rs1799836 and MAOA-uVNTR were not associated with agitation and selected psychotic symptoms in veterans with PTSD. A marginally significant association was found between MAOB rs1799836 polymorphism and severity of PTSD symptoms, but it was not confirmed since carriers of G or A allele of MAOB rs1799836 did not differ in their total CAPS scores. These findings suggest an association of platelet MAO-B activity, but a lack of association of MAOB rs1799836 and MAOA-uVNTR, with selected psychotic symptoms in ethnically homogenous veterans with PTSD.
Introduction
Post-traumatic stress disorder
Post-traumatic stress disorder (PTSD) is a trauma and stressor related disorder that develops in some, but not all persons after exposure to extreme traumatic event(s) (APA, Citation1994, Citation2013). The etiology of PTSD is still poorly understood and involves interaction of different environmental, biological and genetic factors (Domschke, Citation2012; Schmidt et al., Citation2011). Besides major symptoms such as re-experiencing, avoidance, numbing and hyper-arousal (including hypervigilance, sleep problems and irritability), PTSD is frequently associated with social withdrawal, anger, aggression and suicidal behavior, which interfere with personal functioning and quality of life (Begic & Jokic-Begic, Citation2001; Hoge et al., Citation2007; Kehle et al., Citation2011; Kessler et al., Citation1999; Mann, Citation2003). PTSD also commonly co-occurs with other psychiatric disorders such as depression, substance use and anxiety disorders, that substantially complicate the treatment of PTSD (Axelrod et al., Citation2005; Scherrer et al., Citation2008; Wolf et al., Citation2010).
Various studies have demonstrated that PTSD symptoms may trigger violent behavior caused by anger and hostility (Elbogen et al., Citation2010; Jakupcak et al., Citation2007; Novaco & Chemtob, Citation2015). The strong and positive association between hyperarousal PTSD symptom cluster and aggressive behavior was found in male combat veterans (Taft et al., Citation2007a). In addition, PTSD symptoms have contributed to aggressive behavior in elderly, medically ill, and cognitively impaired male patients in a long-term care unit for veterans (Carlson et al., Citation2008). Agitation, which includes increased responsiveness to external or internal stimuli, irritability, hostility and inappropriate psychomotor and/or verbal activity, may also be associated with verbal or physical aggression (Mohr et al., Citation2005; Montoya et al., Citation2011; Volavka, Citation2014).
Intrusive thoughts and recollections, images and “flashback” experiences, which are the hallmark symptoms of PTSD (Ehlers & Steil, Citation1995), can take the form of hallucinations (Hamner, Citation1996; Mueser & Butler, Citation1987; Wilcox et al., Citation1991), delusional thought processes (Pinto & Gregory, Citation1995) or both (Butler et al., Citation1996), and are often accompanied by paranoia, therefore showing similarities with psychosis (Butler et al., Citation1996; Hamner & Gold, Citation1998; Heins et al., Citation1990; Ivezic et al., Citation2000; Romme & Escher, Citation1989; Sansonnet-Hayden et al., Citation1987; Shaner & Eth, Citation1989). As the psychotic symptoms are found in 30–40% of the PTSD cases (Hamner, Citation1997), PTSD with secondary psychotic features (PTSD-SP) is emerging as a syndrome (Braakman et al., Citation2008; Kozaric-Kovacic et al., Citation2005; Kozaric-Kovacic & Pivac, Citation2007; Pivac et al., Citation2004). Veterans with PTSD and a comorbid psychotic disorder display more positive symptoms of psychosis, general psychopathology, paranoia and violent thoughts, feelings and behaviors, than patients who suffer primarily from PTSD or psychotic disorder, suggesting higher levels of cognitive, emotional and behavioral disturbance (Sautter et al., Citation1999).
Monoamine oxidase
Monoamine oxidase (MAO) is an enzyme involved in degradation of different biogenic amines. MAO-A and MAO-B isoforms share 70% homology in amino acid sequence (Chen & Shih, Citation1998), but differ in their substrate specificities, immunological properties and tissue distribution (Bortolato & Shih, Citation2011). Due to its important role in the regulation of mood, emotions and behavior (Edmondson et al., Citation2009), MAO has been suggested to play a role in the pathophysiology of various mental and neurodegenerative disorders. A point mutation in the MAOA gene, resulting in MAO-A deficiency (Brunner et al., Citation1993) has been associated with impulsive aggression, irritability, assaultive behavior, property destruction or other antisocial behaviors. In addition, the changes in the MAO-B activity and expression have been associated with depression, alcoholism, psychotic disorders, impulsivity and neurodegenerative diseases (Adolfsson et al., Citation1980; Sandler et al., Citation1993).
Platelet MAO-B activity has been proposed as peripheral biomarker of different disinhibited behaviors such as sensation- and novelty-seeking, extraversion, low harm avoidance, impulsive and risky behavior, psychopathy- and aggression-related personality traits (Harro et al., Citation2004; Oreland, Citation2004; Oreland & Hallman, Citation1995; Ruchkin et al., Citation2005; Shih et al., Citation1999; Stalenheim, Citation2004). Both increased and decreased platelet MAO-B activity have been associated with vulnerability to different behaviors and traits, suggesting no linear relationship (Paaver et al., Citation2006; Schalling et al., Citation1987). Namely, in contrast to the study showing significantly lower platelet MAO-B activity in the subjects with PTSD when compared to healthy control group (Davidson et al., Citation1985), our previous results demonstrated that platelet MAO-B activity did not differ significantly between war veterans with or without PTSD, and prisoners of war with PTSD (Pivac et al., Citation2002). However, higher platelet MAO-B activity, but a lack of differences in MAOB rs1799836 allele frequencies, was observed in veterans with PTSD, especially those with psychotic compared to non-psychotic features (Pivac et al., Citation2007).
The mutations in genes coding for both MAO-A and MAO-B have been associated with diverse clinical phenotypes (Bortolato & Shih, Citation2011). The G/A substitution (rs1799836) in the MAOB gene has been proposed to affect the stability and/or translation of MAOB mRNA (Balciuniene et al., Citation2002) and therefore increase MAO-B protein expression and enzymatic activity (Jakubauskiene et al., Citation2012). For MAOB rs1799836, an association between G allele and increased platelet MAO-B activity has been reported in a small sample (Garpenstrand et al., Citation2000), but not confirmed in our previous studies including healthy subjects (Pivac et al., Citation2006), veterans with PTSD (Pivac et al., Citation2007) or alcohol-dependent patients (Nedic Erjavec et al., Citation2014).
A functional variable number tandem repeat (uVNTR) within MAOA gene promotor is a 30-bp repeat sequence with 2, 3, 3.5, 4 and 5 copies (Huang et al., Citation2004) that influences transcriptional activity and expression of the MAOA gene (Sabol et al., Citation1998). Among the most frequent alleles in the population, the four repeat variant has been associated with higher, and the three repeat variants with lower MAO-A activity (Bortolato & Shih, Citation2011; Deckert et al., Citation1999; Sabol et al., Citation1998). The association of increased aggression and impulsivity with both low (Antypa et al., Citation2013; Bortolato & Shih, Citation2011; Stetler et al., Citation2014) and high-activity MAOA variants (Manuck et al., Citation2002), as well as lack of such association (Kiive et al., Citation2014; Sjoberg et al., Citation2008; Tikkanen et al., Citation2011) have been reported. Moreover, although interaction of environmental factors, such as childhood trauma or maltreatment, with MAOA-uVNTR polymorphism may increase the risk for over-aggressive behavior (Aslund et al., Citation2011; Caspi et al., Citation2002; Kim-Cohen et al., Citation2006; Widom & Brzustowicz, Citation2006; Williams et al., Citation2009), some studies failed to observe such findings (Haberstick et al., Citation2014).
The focus of our research was on aggression (i.e. severe agitation) and PTSD symptom severity, as both platelet MAO-B activity and MAOA-uVNTR polymorphism were reported to be associated with aggression (Antypa et al., Citation2013; Bortolato & Shih, Citation2011; Stetler et al., Citation2014) that was related to severity of PTSD symptoms (Taft et al., Citation2007a), and the association of MAOB rs1799836 polymorphism and platelet MAO-B activity has been reported as well (Balciuniene et al., Citation2002; Garpenstrand et al., Citation2000). However, the data from the literature have been inconclusive, probably due to many confounding factors such as gender, smoking, age, ethnicity and medication, which have been shown to influence MAO activity (Kiive et al., Citation2002; Muck-Seler et al., Citation2008, Citation2009; Nedic Erjavec et al., Citation2014; Oreland, Citation2004; Pivac et al., Citation2003, Citation2006, Citation2007; Roth et al., Citation1976).
Hence, in order to further clarify these relationships, the aim of our study was to evaluate the association of different symptoms occurring in PTSD (PTSD symptom severity, agitation and selected psychotic symptoms) with platelet MAO-B activity and with the genetic variants of the MAOB rs1799836 and MAOA-uVNTR polymorphisms, in ethnically homogenous, relatively large group of medication naïve Caucasian male veterans with PTSD. Our hypothesis was that platelet MAO-B activity, as well the distribution of genetic variants of MAOB rs1799836 and MAOA-uVNTR polymorphisms, will differ between male Croatian veterans with more pronounced PTSD symptoms, agitation and psychotic symptoms compared to veterans with PTSD with less pronounced symptoms, after controlling for the effect of smoking and age.
Methods
Veterans with PTSD
The study included 249 male participants, veterans with real combat experience, who developed chronic and current combat related PTSD. Veterans were evaluated and recruited at the National Center for Psychotrauma, Referral Center for the Stress-related Disorders of the Department of Psychiatry in University Hospital Dubrava, Zagreb, in the period from 2001 to 2004. Veterans were drug naïve and did not receive any kind of psychological treatment (e.g. CBT). They were all Caucasians of Croatian origin, all served in the Croatian armed forces between 1991 and 1995 in the Homeland war. They were soldiers with active military duty, comparable traumatic combat experience (3.0 ± 1.0 years of duration) and comparable duration of time passed after the traumatic experience (6.1 ± 2.7 years). The exact number of traumatic experiences (i.e. direct combat experience in a war, involvement in a life-threatening accident, witnessing of someone being badly injured or killed and being threatened with various weapons) could not be assessed, since the war lasted for 5 years, but all veterans were exposed during the active military duty to similar combat events since they participated in the same military operations. They were 43.8 ± 7.2 years old (mean ± SD). As smoking significantly affects MAO activity, veterans with PTSD were subdivided according the present smoking status into smokers (≥10 cigarettes per day) and non-smokers.
Post-traumatic stress disorder (PTSD) diagnosis was done using the Structured Clinical Interview (SCID) for DSM-IV based on DSM-IV criteria (APA, Citation1994). In addition, all participants completed a questionnaire which included: basic demographic characteristics, smoking, alcohol consumption (not quantified), physical activity, the presence of acute or chronic physical illness, history of diabetes, hypertension, cardiovascular diseases and medication use. Exclusion criteria were: a positive family history of psychiatric disorder, earlier history of acute psychosis, dementia, cognitive dysfunction, mental retardation, schizophrenia, mood disorders, personality disorders, substance abuse, past or current alcohol or other substance abuse within 3 months, clinically significant abnormalities in electrocardiogram or laboratory findings and positive urine screen for illicit drugs and alcohol, symptoms of acute or chronic physical illness, history of cardiovascular or neurological disorder, hypertension, diabetes or other metabolic and/or endocrine disorder. After complete description of the study protocol to the participants, written informed consent was obtained. All the studies were approved by the Ethics committee of the University Hospital Dubrava and conducted in accordance with the Helsinki Declaration as revised 1989. Veterans were also evaluated for symptoms using the Clinician Administered PTSD Scale (CAPS) and the Positive and Negative Syndrome Scale (PANSS).
PTSD severity symptoms
For assessing the PTSD symptom severity, patients were evaluated according to CAPS (Weathers et al., Citation2001) scores, and 56 (22.5%) subjects had mild (range 46–65 scores) PTSD symptoms, 132 (53.0%) subjects had moderate (range 66–95 scores) PTSD symptoms and 61 (24.5%) subjects had severe (range 96–136 scores) PTSD symptoms.
Agitation
For assessing agitation, patients were rated according to the PANSS Excited Component (PANSS-EC). The PANSS-EC consists of five PANSS items: excitement (P4), hostility (P7), tension (G4), uncooperativeness or lack of cooperation (G8) and poor impulse control (G14). All PANSS items are rated from 1; (absent); 2 (minimal); 3 (mild); 4 (moderate); 5 (moderate/severe), 6 (severe) to 7 (extreme). The score range in these five items in the PANSS-EC ranges from 5 to 35 and agitation was defined as present when a person had a mean score of ≥14 (Lesem et al., Citation2011).
Psychotic symptoms
For assessing psychotic symptoms, veterans with PTSD were evaluated according to the particular items: delusions (P1), conceptual disorganization (P2); hallucinatory behavior (P3) and suspiciousness/persecution (P6) on the PANSS Positive subscale. Evidence of hallucinations or delusions was assessed during the mental status examination and the onset of PTSD in psychotic PTSD patients preceded the onset of psychosis. The score range in these four selected items in the PANSS-psychotic subscale was 4–28, and veterans with a score of at least 4 (moderate severity) on these particular selected items were defined as patients with psychotic symptoms. Therefore, they were rated psychotic if they had mean score of ≥16 on these particular selected items, and if psychotic symptoms emerged after the onset of PTSD (Braakman et al., Citation2008).
Sampling
Blood sampling was performed in the period from 2001 to 2004, around 8 am, following the overnight fasting. Blood samples (4 ml) were drawn in a plastic syringe containing 1 ml of anticoagulant (acid citrate dextrose). Series of centrifugations of whole blood and platelet-rich-plasma were performed in order to obtain platelets, which were then disrupted by sonication.
Measurement of MAO-B activity
Platelet MAO-B activity was determined immediately following collection of blood samples (in the period from 2001 to 2004) by modified method described by Krajl (Citation1965). Briefly, standard (4-hydroxyquinoline, 4-HOQ), blank (water) and platelet sonicates were incubated with substrate kynuramine at 37 °C. After 1 h, the reaction was stopped with 1 N NaOH. Varian Spectrophotofluorimeter Cary Eclipse was used for the measurement of 4-HOQ fluorescence, a product of kynuramine (exciting λ = 310 nm, emitted λ = 380 nm). Following protein level determination by the method of Lowry et al. (Citation1951), platelet MAO-B activity was expressed in nmol 4-OHQ/mg protein/h.
Genotyping of rs1799836 polymorphism in MAOB gene
Genomic DNA was isolated from all blood samples immediately following their collection (in the period from 2001 to 2004) using the salting out method (Miller et al., Citation1988) and the isolated DNA was stored at −20 °C. MAOB intron 13 A/G polymorphism (rs1799836) was genotyped in the DNA samples isolated from 238 veterans using ABI Prism 7300 Real time PCR System apparatus (Applied Biosystems, Foster City, CA), during the year 2007, according to the procedures described by Applied Biosystems. The probe-primer combinations were purchased from Applied Biosystems as TaqMan® SNP Genotyping Assay (C_8878790_10 for MAO-B). Briefly, 20 ng of genomic DNA was amplified in 96-well plates using a 10 μl reaction volume for 40 cycles at 95 °C for 15 s followed by 60 °C for 60 s. Around 20% of samples were genotyped again as a quality control for genotyping assays.
Genotyping of uVNTR polymorphism in MAOA gene
The MAOA promoter uVNTR polymorphism was genotyped during the year 2015 in the DNA samples isolated from 199 veterans, using PCR with primers MAOA-F: 5′-ACAGCCTCGCCGTGGAGAAG-3′ and MAOA-F: 5′-GAACGGACGCTCATTCGGA-3′. Final PCR reaction volume (15 μl) contained ∼100 ng of DNA, 1.7 mM MgCl2, 1 × Q solution, 20 pmol of each primer, 0.25 mM dNTP and 1 U of Taq polymerase (Qiagen, Hilden, Germany). The conditions of PCR performed on 2720 Thermal Cycler (Applied Biosystems, Foster City, CA) were: 5 min at 95 °C, 35 cycles at 94 °C for 30 s, 30 s at 57 °C and 1 min at 72 °C, with 7 min at 72 °C (final extension). After the separation of PCR products on 2.5% agarose gels and staining with ethidium bromide, the bands were visualized using Uvitec BXT-20-M transiluminator. All participants had alleles with 3 or 4 repeats, except two carriers of the 5-repeat variant. The 3-repeat and 5-repeat variants represent low activity variants, while variants with four repeats represent high-activity variants.
Data analysis
The results were expressed as numbers and percentages, means ± SEM and or median and 25th (Q1) and 75th (Q3) percentiles, and evaluated with SigmaStat 3.5 (Jandel Scientific Corp., San Jose, CA). The frequencies of the MAOB rs1799836 and MAOA-uVNTR variants were compared using χ2-tests with Yates correction for continuity. The deviation from the Hardy–Weinberg equilibrium in MAOB rs1799836 and MAOA-uVNTR could not be tested in male subjects since the genes encoding MAOA and MAOB are located on the short arm of the X chromosome. Normality of the distribution was assessed with the Kolmogorov–Smirnov test. Two groups were compared with Student’s t-test or when the normality of data failed, with a Mann–Whitney test. Three groups of data were analyzed with one-way ANOVA followed by the Tukey test. Multiple regression analysis was performed with platelet MAO-B activity as the dependent variable, and particular symptoms or scores and smoking as independent variables. G*Power 3 Software (Faul et al., Citation2009) was used for conducting power analyses, i.e. to determine a priori sample size and actual power. For multiple regression analysis (with α = 0.05; power (1 − β) = 0.800; a small effect size (ω = 0.30); number of predictors =2), total desired sample size was 36, and the actual sample size evaluating platelet MAO-B activity was 249. For genetic analyses with a χ2-test (with α = 0.05; power (1 − β) = 0.800 and a small effect size (ω = 0.30); df =2), total desired sample size was 108, and if df =1; total desired sample size was 88, while the actual total sample size was 238 for MAOB rs1799836 and 199 for MAOA-uVNTR polymorphism.
Results
Frequency of PTSD symptoms and smoking in veterans with PTSD
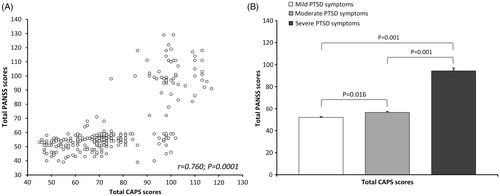
According to the CAPS scores, veterans with PTSD were subdivided into categories of veterans with mild, moderate and severe PTSD symptoms. Since more than 2/3 (77.5%) of veterans with PTSD had moderate to severe PTSD symptoms, compared to those with mild PTSD symptoms, the correlation between total CAPS and total PANSS scores was evaluated and showed a significant positive correlation (r = 0.760; p = 0.0001, Pearson’s coefficient of correlation, . As shown in , when total PANSS scores were subdivided according to CAPS severity categories, total PANSS scores differed significantly (F2,246 = 277.32; p = 0.001; one-way ANOVA) between veterans with mild, moderate and severe PTSD symptoms. The Tukey test revealed that veterans with severe PTSD symptoms had significantly higher total PANSS scores than veterans with mild (p = 0.001) or moderate (p = 0.001) PTSD symptoms, and veterans with moderate PTSD symptoms had significantly higher total PANSS scores than veterans with mild (p = 0.016) PTSD symptoms (.
Figure 1. Correlation between total CAPS and PANSS scores in veterans with PTSD (A); Total PANSS scores (means ± SEM) in veterans subdivided according to PTSD symptoms severity into mild, moderate and severe PTSD symptoms (B).
A significant positive correlation (r = 0.699; p = 0.0001, Pearson’s coefficient of correlation) was also detected between total CAPS and PANSS-EC scores (showing agitation). When veterans were subdivided according to CAPS severity categories, PANSS-EC scores (means ± SEM) differed significantly (F2,246 = 166.73; p = 0.001; one-way ANOVA) between veterans with mild (9.1 ± 0.87), moderate (10.0 ± 0.73) and severe (16.0 ± 1.74) PTSD symptoms. Multiple comparisons (Tukey’s test) showed that veterans with mild PTSD symptoms had significantly lower PANSS-EC scores than veterans with moderate or severe (p < 0.050) PTSD symptoms, and that veterans with moderate PTSD symptoms had significantly (p < 0.050) lower PANSS-EC scores than veterans with severe PTSD symptoms.
In addition, when veterans were subdivided into those with or without selected psychotic symptoms according to PANSS, a significant positive correlation (r = 0.758; p = 0.0001, Pearson’s coefficient of correlation) was also detected between total CAPS and PANSS psychotic scores. After subdivision of veterans according to CAPS severity categories, PANSS psychotic scores (means ± SEM) differed significantly (F2,246 = 275.88; p = 0.001; one-way ANOVA) between veterans with mild (4.1 ± 0.02), moderate (4.9 ± 0.21) and severe (14.1 ± 0.59) PTSD symptoms. Multiple comparisons (Tukey’s test) showed that veterans with mild PTSD symptoms had significantly lower PANSS-EC scores than veterans with moderate or severe (p < 0.050) PTSD symptoms, and that veterans with moderate PTSD symptoms had significantly (p < 0.050) lower PANSS-EC scores than veterans with severe PTSD symptoms.
Veterans with PTSD were more frequently smokers (N = 162, 65%) than non-smokers (N = 87, 35%; ). When subdivided according to the mild, moderate and severe PTSD symptoms and smoking status, a similar (χ2 = 4.975; df = 2; p = 0.083) distribution of veterans with PTSD, who were classified according to the smoking status and PTSD symptoms severity, was detected. In veterans with PTSD, subdivided into smokers and non-smokers, there was no significant differences in the frequency of the psychotic symptoms (χ2 = 0.178; df =1; p = 0.673) or agitation (χ2 = 1.389; df =1; p = 0.239) between those with mild and those with moderate/severe symptoms.
Table 1. Demographic and clinical data (age, total CAPS, total PANSS, PANSS–EC and PANSS psychotic scores) and platelet MAO-B activity of veterans with PTSD subdivided according to the smoking status.
To assess the possible association between smoking and CAPS or PANSS (total and subscale) scores, all veterans with PTSD were subdivided according to their smoking status into smokers and non-smokers, and their CAPS or PANSS scores were evaluated using Student’s t-test (). There were no significant differences between smokers and non-smokers in their total CAPS (p = 0.578), total PANSS (p = 0.681), PANSS-EC (p = 0.159) and PANSS psychotic (p = 0.603) scores. In addition, smokers and non-smokers did not differ significantly (p = 0.807) in their age ().
Platelet MAO-B activity and smoking
There was a significant difference in platelet MAO-B activity between smokers and non-smokers with PTSD, since non-smokers had significantly (p = 0.0007) higher platelet MAO-B activity compared to smokers ().
Platelet MAO-B activity and various symptoms in veterans with PTSD
Platelet MAO-B activity and PTSD symptoms severity
To evaluate the association of the severity of PTSD symptoms, determined using the CAPS score categories, with platelet MAO-B activity, veterans with PTSD were subdivided into categories of veterans with mild, moderate and severe PTSD symptoms. Multiple regression analysis, with platelet MAO-B activity as the dependent variable, and PTSD symptom severity and smoking as independent variables, was used. This analysis showed a significant main effect (F2,243 = 7.327, p = 0.001, Radj2 = 0.049), but no significant effect of PTSD symptom severity according to CAPS score categories (p = 0.102) on platelet MAO-B activity. The proposed model was significant due to a significant effect of smoking (p = 0.0004) on platelet MAO-B activity. These results suggested that smoking could explain up to 4.9% of variation in platelet MAO-B activity. Within both smokers (F2,157 = 0.474, p = 0.623) and non-smokers (F2,83 = 1.333, p = 0.269), there was a trend toward higher platelet MAO-B activity in veterans with severe PTSD symptoms compared to veterans with mild or moderate symptoms, which did not reach the level of statistical significance. Since there was a highly significant main effect, to further evaluate this relationship, the association between total CAPS scores (i.e. not the CAPS score categories) and platelet MAO-B activity was determined using multiple linear regression analysis. It showed a significant main effect (F2,243 = 8.216, p = 0.001, Radj2 = 0.056) that was due to a significant association of the total CAPS scores (p = 0.037), and a significant association of smoking (p = 0.0005) with platelet MAO-B activity, suggesting that both smoking and total CAPS scores might explain up to 5.6% of variation in platelet MAO-B activity.
Platelet MAO-B activity and agitation
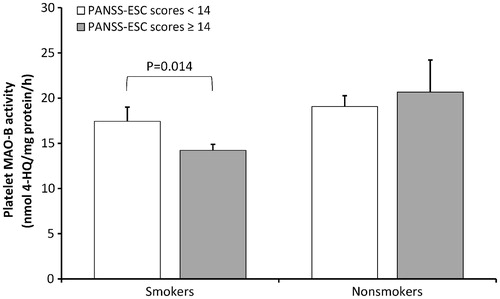
Veterans with PTSD are frequently agitated. For the evaluation of the association between agitation and platelet MAO-B activity, multiple regression analysis was used, with platelet MAO-B activity as the dependent variable, and categories of agitation (agitated versus non-agitated) and smoking as independent variables. It revealed a significant model (F2,243 = 7.979, p = 0.001, Radj2 = 0.054), due to a significant effect of agitation (p = 0.048), and a significant effect of smoking (p = 0.0004) on platelet MAO-B activity. These results suggested that both smoking and agitation could explain up to 5.4% of variation in platelet MAO-B activity. Within smokers, platelet MAO-B activity was significantly (t = 2.219; df =158; p = 0.014; Student’s t-test) increased in agitated compared to less agitated veterans with PTSD (). To confirm this significance, multiple regression analysis also evaluated the association between the total PANSS-EC scores (i.e. not the PANSS-EC categories) and platelet MAO-B activity, and showed a significant main effect (F2,243 = 6.439, p = 0.002, Radj2 = 0.042) that was due to the significant effect of smoking (p = 0.0005), but a lack of a significant association between agitation scores (p = 0.320) and platelet MAO-B activity.
Platelet MAO-B activity and psychotic symptoms
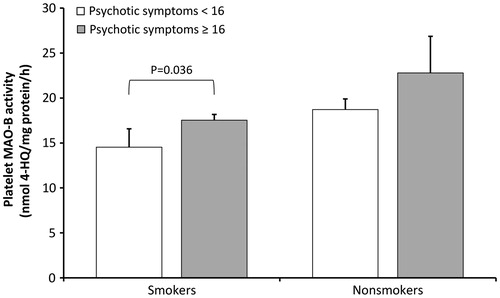
Veterans with PTSD frequently develop psychotic symptoms. To evaluate possible association of psychotic symptoms, determined using the particular items of the PANSS Positive subscale, with platelet MAO-B activity, veterans with PTSD were subdivided into veterans with and without psychotic symptoms. Multiple regression analysis, with platelet MAO-B activity as the dependent variable, and smoking and psychotic symptoms as independent variables, was used. This analysis revealed a significant model (F2,243 = 8.410, p = 0.001, Radj2 = 0.057), due to a significant effect of psychotic symptoms (p = 0.030) and significant effect of smoking (p = 0.0005) on platelet MAO-B activity. These results suggested that both smoking and psychotic symptoms could explain up to 5.7% of variation in platelet MAO-B activity. Within smokers, veterans with more severe psychotic symptoms had significantly higher platelet MAO-B activity (t = 3.002; df =158; p = 0.036; Student t-test) than veterans with less pronounced psychotic symptoms. These results are shown in . To confirm this relationship, the association between selected PANSS Positive scores and platelet MAO-B activity was also determined using multiple linear regression analysis. It showed a significant main effect (F2,243 = 8.189, p = 0.001, Radj2 = 0.055), significant effect of selected PANSS Positive scores (p = 0.038) and significant effect of smoking (p = 0.0005) on platelet MAO-B activity. These results suggest that both smoking and selected PANSS Positive scores could explain up to 5.5% of variation in platelet MAO-B activity.
The frequency of MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD subdivided according to various symptoms
The frequency of MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD subdivided according to the different symptom categories
In veterans with PTSD the frequency of the G and A alleles of MAOB rs1799836 was 46.2 and 53.8%, respectively, while the frequency of MAOA-uVNTR variants with five repeats was 1%, with three repeats was 34.7% and with four repeats was 64.3%. The distribution of MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD, subdivided according to the different categories: PTSD severity symptoms, symptoms of agitation and psychotic symptoms, is shown in . To elucidate the possible association between different symptoms categories and MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD, χ2-test was used. The frequency of MAOB rs1799836 A and G alleles differed significantly (p = 0.046) in veterans subdivided according to the mild, moderate and severe PTSD symptoms. On the other hand, a similar frequency of the MAOB rs1799836 A and G alleles was detected in veterans with PTSD who were classified according to the agitation (p = 0.271) and psychotic (p = 0.937) symptoms (). There was no significant difference in the distribution of the MAOA-uVNTR low activity and high activity variants in veterans with PTSD subdivided according to the PTSD severity symptoms (p = 0.365), symptoms of agitation (p = 0.195) and psychotic symptoms (p = 0.257; ).
Table 2. The distribution of the MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD subdivided according to the different categories: PTSD severity symptoms, symptoms of agitation and psychotic symptoms.
The scores for PTSD severity symptoms, symptoms of agitation and psychotic symptoms in veterans with PTSD subdivided according to the particular MAOB rs1799836 and MAOA-uVNTR variants
To assess the possible relationship between the scores for PTSD, agitation and psychotic symptoms and MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD, the differences in the particular scores in veterans subdivided according to the presence of the MAOB rs1799836 A and G alleles, or according to the presence of the MAOA-uVNTR low and high activity variants, were tested (). There were no significant (Mann–Whitney test) differences in the scores describing PTSD symptom severity (p = 0.336), agitation (p = 0.728) and psychotic symptoms (p = 0.643) in veterans, carriers of the A or G allele of MAOB rs1799836 (). In addition, no significant differences were detected in the scores measuring PTSD severity symptoms (p = 0.388), agitation (p = 0.366) and psychotic symptoms (p = 0.422) in veterans, carriers of the low-activity and high-activity variants of MAOA-uVNTR ().
Table 3. Association of the scores for PTSD severity symptoms, symptoms of agitation and psychotic symptoms with the MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD.
Platelet MAO-B activity, smoking, age and MAOB rs1799836 and MAOA-uVNTR variants in veterans with PTSD
To assess whether there is any association between age, smoking, MAOB rs1799836 and MAOA-uVNTR variants and platelet MAO-B activity, a multiple linear regression analysis was used, with platelet MAO-B activity as the dependent variable and age, smoking, MAOB rs1799836 and MAOA-uVNTR variants as independent variables. The model was significant (F4,185 = 2.440, p = 0.049, Radj2 = 0.030) due to the significant effect of smoking (p = 0.006) on platelet MAO-B activity. The results revealed no significant association of age (p = 0.358), MAOB rs1799836 (p = 0.468) and MAOA-uVNTR variants (p = 0.494) with platelet MAO-B activity, and showed that smoking was the only factor that could explain up to 3.0% of variation in platelet MAO-B activity.
Discussion
Our study evaluated the association between different symptoms occurring in PTSD (PTSD symptom severity assessed by the CAPS scores, agitation and selected psychotic symptoms assessed by the PANSS scores) and platelet MAO-B activity or the genetic variants of MAOB rs1799836 and MAOA-uVNTR polymorphisms in male medication-free veterans with PTSD. The main findings are slightly higher platelet MAO-B activity in veterans with PTSD with more severe PTSD symptoms and in veterans with agitation, and significantly higher platelet MAO-B activity in veterans with more pronounced psychotic symptoms compared to veterans with less pronounced psychotic symptoms, suggesting that indices of more severe symptoms in PTSD were related to higher platelet MAO-B activity. A significant positive correlation between total CAPS and total PANSS scores (which among others included both PANSS-EC scores and selected PANSS psychotic scores) was observed, suggesting that in veterans enrolled in our study, the severity of PTSD symptoms correlated with overall psychopathology (total PANSS scores). This finding was confirmed with a positive correlation between total CAPS and PANSS-EC scores (measuring agitation) as well as between total CAPS and PANSS psychotic scores in veterans with PTSD. The association of agitation with PTSD symptom severity is in line with the important indirect associations of PTSD symptoms to aggression (Taft et al., Citation2007a). Our results, showing that specific psychotic symptoms (e.g. delusions, conceptual disorganization, hallucinatory behavior and suspiciousness/persecution, assessed using the particular PANSS positive items) were considerably associated with PTSD severity, do not agree with the findings that psychotic features do not reflect severity of PTSD symptoms (Hamner, Citation1997), and are not related to severity of PTSD (David et al., Citation1999). However, our results are in line with the data showing a positive correlation between the severity of hyperarousal symptoms and occurrence of psychotic symptoms (Braakman et al., Citation2009; Kastelan et al., Citation2007), and with the meta-analysis data showing that the clinical profile of the group with high PTSD and high psychosis was inferior compared to other PTSD groups (Shevlin et al., Citation2011). Therefore, agitation and psychotic symptoms were associated with more severe PTSD symptoms in our veterans with combat related PTSD.
Both forms of MAO are involved in modulation of human behavior and their alterations are associated with vulnerability to various psychopathologies. PTSD is frequently associated with aggressive and violent behavior (Hecker et al., Citation2015; Kessler et al., Citation1999; Mann, Citation2003), and psychotic symptoms (Braakman et al., Citation2008; Hamner Citation1997). In combat veterans, PTSD symptoms were directly associated with higher levels of aggression, while combat exposure was related indirectly through PTSD symptoms with aggression (Taft et al., Citation2007b). Therefore, there is an unmet need to further examine the neurobiological underpinning of the aggressive behavior in PTSD. Platelet MAO-B activity is assumed to be a peripheral biomarker associated with different personality traits and pathological behaviors including antisocial, violent and aggressive behavior (Kiive et al., Citation2002; Oreland, Citation2004). Several factors including gender, smoking, age, ethnicity and neurodegenerative disorders have been shown to affect platelet MAO-B activity (Kiive et al., Citation2002; Muck-Seler et al., Citation2009; Nedic Erjavec et al., Citation2014; Oreland, Citation2004; Roth et al., Citation1976; Sobell et al., Citation1997). Moreover, medication such as MAO-inhibitors (Oreland, Citation2004), haloperidol (Meszaros et al., Citation1998), clozapine (Ertugrul et al., Citation2007), antidepressants (Pivac et al., Citation2003) or lamotrigine (Muck-Seler et al., Citation2008) all alter platelet MAO-B activity. However, our study included medication naïve veterans with PTSD, and therefore the influence of drugs on platelet MAO-B activity might be excluded. We have found slightly higher platelet MAO-B activity in veterans with PTSD with more severe PTSD symptoms and in veterans with agitation, and significantly higher platelet MAO-B activity in veterans with more pronounced psychotic symptoms compared to veterans with less pronounced symptoms. Our results revealed no considerable association of age and significant association of smoking status with platelet MAO-B activity. Lower platelet MAO-B activity in smokers compared to non-smokers is in line with many studies showing decrease in platelet MAO-B activity due to cigarette smoking (Berlin & Anthenelli, Citation2001; Kiive et al., Citation2005; Oreland, Citation2004; Oreland et al., Citation2002; Whitfield et al., Citation2000).
In smokers with PTSD, platelet MAO-B activity was increased in agitated compared to non-agitated, and in psychotic compared to non-psychotic veterans. The slight increase in platelet MAO-activity has been also observed in agitated and psychotic non-smokers with PTSD, but it did not reach the level of statistical significance. Nevertheless, in the case of PANSS psychotic scores, the increase in platelet MAO-B activity in psychotic smokers (20.65%) has been the same as the increase in psychotic non-smokers (21.69%), and multiple linear regression analysis confirmed significant effect of both selected PANSS psychotic scores and smoking on platelet MAO-B activity. These results agree with our previous data showing higher platelet MAO-B activity in veterans with PTSD complicated with psychotic compared to non-psychotic features, assessed using different particular items on the PANSS Positive, Negative and General psychopathology scales (Pivac et al., Citation2007), and in high-risk taking drivers (Paaver et al., Citation2006).
Multiple regression analysis with platelet MAO-B activity as the dependent variable, and categories of agitation (agitated versus non-agitated) and smoking as independent variables revealed a significant effect of agitation, and a significant effect of smoking on platelet MAO-B activity. However, in the case of PANSS-EC scores, that determined agitation, the increase in platelet MAO-activity in agitated veterans with PTSD was considerably smaller in non-smokers than in smokers. As multiple regression analysis confirmed only the significant effect of smoking, it is possible that the relationship between PANSS-EC scores and platelet MAO-B activity is not causative. However, this might be also due to the fact that the number of non-smokers included in the study is twice smaller than the number of smokers. These data are in line with the results showing similar platelet MAO-B activity between aggressive and non-aggressive alcohol dependent patients (Nedic Erjavec et al., Citation2014; Netter et al., Citation2015).
In this study, no association of MAOB rs1799836 and MAOA-uVNTR variants and platelet MAO-B activity was observed. The association of MAOA and MAOB genes and the corresponding enzyme activities has been reported (Balciuniene et al., Citation2002; Garpenstrand et al., Citation2000) and disputed (Girmen et al., Citation1992; Nedic Erjavec et al., Citation2014; Netter et al., Citation2015; Pivac et al., Citation2006, Citation2007). Lower MAO-B activity was associated with either A allele of MAOB rs1799836 in a small number (N = 55) of male subjects (Garpenstrand et al., Citation2000) or with the G allele of MAOB rs1799836 in postmortem human brain (Balciuniene et al., Citation2002). In agreement with our data, no individual SNPs in MAOA or MAOB genes were significant associated with platelet MAO-B activity (Jansson et al., Citation2005).
Regarding the most frequently evaluated polymorphisms in MAOA and MAOB genes, the only association was found between the MAOB rs1799836 and mild, moderate and severe PTSD symptoms. Since this was a marginal significance, to further evaluate this possible relationship, veterans with PTSD were subdivided into carriers of the G or A allele of MAOB rs1799836 and according to total CAPS scores. No significant differences in total CAPS scores between G or A carriers was found. In addition, carriers of the G or A allele of MAOB rs1799836 did not differ in the PANSS-EC or selected PANSS-Positive scores. Since there were no substantial differences in the scores measuring PTSD severity symptoms, agitation and psychotic symptoms in veterans, carriers of the low activity and high activity variants of MAOA-uVNTR, our results revealed that both MAOB rs1799836 A and G alleles, or MAOA-uVNTR low and high activity variants, were not associated with PTSD symptom severity, agitation and psychotic symptoms in veterans with PTSD.
Although not concurring with our hypothesis, our results revealed that genetic variants of MAOB rs1799836 and MAOA-uVNTR were not linked to agitation and selected psychotic symptoms in male veterans with PTSD. As far as we are aware, there are no data showing the association or a lack of association between genetic variants of MAOB rs1799836 and MAOA-uVNTR and agitation in combat exposed veterans with PTSD. On the other hand, in line with present data, in our previous study no significant differences in MAOB rs1799836 allele frequencies were found between veterans with non-psychotic and psychotic PTSD (Pivac et al., Citation2007). In agreement with our data, aggression in schizophrenia, determined by the Overt Aggression Scale, was not associated with the allele frequency of MAOB rs1799836 (Zammit et al., Citation2004), and symptom severity in schizophrenia, using the Scale of the Assessment of Negative Symptoms, was not associated with MAOB rs1799836 and MAOA-uVNTR variants (Camarena et al., Citation2012). In addition, no major effect of MAOA-uVNTR variants on male adolescent criminal activity was found when MAOA genotype was considered without its psychosocial context (Nilsson et al., Citation2006). In contrast to our results, more frequent finding of the MAOA-uVNTR low activity allele associated with various aggressive traits (Bortolato et al., Citation2013; Bortolato & Shih, Citation2011) might be explained by the substantial effects of the environmental factors, such as unfavorable psychosocial conditions (Nilsson et al., Citation2006), or early traumatic experience, history of abuse or neglect (Caspi et al., Citation2002; Huang et al., Citation2004), that contribute to gene x environment interaction. As we did not have the access to data regarding early life stress exposure and experience of violent victimization, we could not assess the impact of the environmental factors in our study. When comparing a direct association between various symptoms and genetic variants of the MAOB rs1799836 and MAOA-uVNTR, our results did not support the theory that these two selected polymorphisms within the MAOA and MAOB genes, as determinants of catecholamine activity, are risk factors for PTSD severity, agitation and psychosis in PTSD.
However, besides these two gene polymorphisms, many other genetic, epigenetic and environmental factors may influence MAO-A and MAO-B activity in the brain. In addition to structural genes for MAO, genes that post-translationally modify MAO, genes that control the microenvironment of MAO, and genes encoding regulatory factors that modulate MAO transcription, might be also involved in the regulation of MAO activity in vivo (Shih et al., Citation1999). As decreased brain MAO-A activity has been associated with human aggression (Alia-Klein et al., Citation2008) and we have not determined the activity of MAO-A activity in the brain of participants with PTSD, observed slightly or significantly higher platelet MAO-B activity in veterans with agitation and psychotic symptoms, respectively, might represent a compensatory mechanism for low MAO-A activity in the brain. Namely, it has been suggested that MAO-B might play an auxiliary role in the metabolism of catecholamines when the activity of MAO-A is impaired (Lenders et al., Citation1996). Although both MAO subtypes degrade dopamine, tryptamine and tyramine, MAO-A catabolizes primarily serotonin, noradrenaline, adrenaline; while MAO-B degrades β-phenylethylamine and benzylamine. However, in the absence of one subtype, the other isoenzyme substitutes its role (Shih & Chen, Citation2004) and MAO-A and MAO-B can oxidize each other’s preferred substrates when the substrate or enzyme concentration is changed (Shih et al., Citation1999).
The limitation of the study is that the sample included only male combat exposed veterans with PTSD, and therefore conclusions may not be generalized to civilian victims of trauma. Moreover, based on the calculations of sample size and statistical power, the study was overpowered, which may generate false positive results.
In conclusion, in our study, both MAOB rs1799836 A and G alleles, or MAOA-uVNTR low and high activity variants were not associated with PTSD symptom severity, agitation and psychotic symptoms in veterans with PTSD. However, our results demonstrated slightly higher platelet MAO-B activity in veterans with PTSD with more severe PTSD symptoms and in veterans with agitation, and significantly higher platelet MAO-B activity in veterans with more pronounced psychotic symptoms compared to veterans with less pronounced psychotic symptoms. These results are in line with a variety of data suggesting a great complexity of PTSD with pronounced variations in specific symptoms and comorbidities, such as agitation and psychosis, which further complicate the clinical picture as well as treatment response. Our findings obtained on the ethnically homogenous, relatively large group of Caucasian veterans of Croatian origin, matched for the combat experience, with chronic and current combat related PTSD, might lead to a better understanding of the molecular basis of aggression and psychosis in PTSD.
Acknowledgements
Authors thank the psychiatric staff of the University Hospital Dubrava, National Center for Psychotrauma, Department of Psychiatry, Referral Center for the Stress related Disorders of Ministry of Health of Croatia.
Disclosure statement
This work was partly supported by Croatian Science Foundation, project No. IP-2014-09-4289, but this organization had no role in the writing or the decision to submit the article for publication. The authors declare no conflict of interest.
References
- Adolfsson R, Gottfries CG, Oreland L, Wiberg A, Winblad B. (1980). Increased activity of brain and platelet monoamine oxidase in dementia of Alzheimer type. Life Sci 27:1029–34.
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, et al. (2008). Brain monoamine oxidase A activity predicts trait aggression. J Neurosci 28:5099–104.
- Antypa N, Giegling I, Calati R, Schneider B, Hartmann AM, Friedl M, Konte B, et al. (2013). MAOA and MAOB polymorphisms and anger-related traits in suicidal participants and controls. Eur Arch Psychiatry Clin Neurosci 263:393–403.
- APA. (1994). Diagnostic and statistical manual of mental disorders (DSM-IV). 4th ed. Washington, DC: American Psychiatric Association. 886 p.
- APA. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-V). 5th ed. Washington, DC: American Psychiatric Association. 947 p.
- Aslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW. (2011). Maltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behav Genet 41:262–72.
- Axelrod SR, Morgan CA III, Southwick SM. (2005). Symptoms of posttraumatic stress disorder and borderline personality disorder in veterans of Operation Desert Storm. Am J Psychiatry 162:270–5.
- Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin EE. (2002). Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet 110:1–7.
- Begic D, Jokic-Begic N. (2001). Aggressive behavior in combat veterans with post-traumatic stress disorder. Mil Med 166:671–6.
- Berlin I, Anthenelli RM. (2001). Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol 4:33–42.
- Bortolato M, Shih JC. (2011). Behavioral outcomes of monoamine oxidase deficiency: preclinical and clinical evidence. Int Rev Neurobiol 100:13–42.
- Bortolato M, Pivac N, Muck-Seler D, Nikolac Perkovic M, Pessia M, Giovanni GD. (2013). The role of the serotonergic system at the interface of aggression and suicide. Neuroscience 236:160–85.
- Braakman MH, Kortmann FA, van den Brink W, Verkes RJ. (2008). Posttraumatic stress disorder with secondary psychotic features: neurobiological findings. Prog Brain Res 167:299–302.
- Braakman MH, Kortmann FA, van den Brink W. (2009). Validity of 'post-traumatic stress disorder with secondary psychotic features': a review of the evidence. Acta Psychiatr Scand 119:15–24.
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. (1993). Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262:578–80.
- Butler RW, Mueser KT, Sprock J, Braff DL. (1996). Positive symptoms of psychosis in posttraumatic stress disorder. Biol Psychiatry 39:839–44.
- Camarena B, Fresan A, Aguilar A, Escamilla R, Saracco R, Palacios J, Tovilla A, Nicolini H. (2012). Monoamine oxidase A and B gene polymorphisms and negative and positive symptoms in schizophrenia. ISRN Psychiatry 2012:852949.
- Carlson EB, Lauderdale S, Hawkins J, Sheikh JI. (2008). Posttraumatic stress and aggression among veterans in long-term care. J Geriatr Psychiatry Neurol 21:61–71.
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. (2002). Role of genotype in the cycle of violence in maltreated children. Science 297:851–4.
- Chen K, Shih JC. (1998). Monoamine oxidase A and B: structure, function, and behavior. Adv Pharmacol 42:292–6.
- David D, Kutcher GS, Jackson EI, Mellman TA. (1999). Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry 60:29–32.
- Davidson J, Lipper S, Kilts CD, Mahorney S, Hammett E. (1985). Platelet MAO activity in posttraumatic stress disorder. Am J Psychiatry 142:1341–3.
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, et al. (1999). Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 8:621–4.
- Domschke K. (2012). Patho-genetics of posttraumatic stress disorder. Psychiatr Danub 24:267–73.
- Edmondson DE, Binda C, Wang J, Upadhyay AK, Mattevi A. (2009). Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry 48:4220–30.
- Ehlers A, Steil R. (1995). Maintenance of intrusive memories in posttraumatic stress disorder: a cognitive approach. Behav Cogn Psychother 23:217–49.
- Elbogen EB, Wagner HR, Fuller SR, Calhoun PS, Kinneer PM. (2010). Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup, Beckham JC. Correlates of anger and hostility in Iraq and Afghanistan war veterans. Am J Psychiatry 167:1051–8.
- Ertugrul A, Ucar G, Basar K, Demir B, Yabanoglu S, Ulug B. (2007). Influence of clozapine on platelet serotonin, monoamine oxidase and plasma serotonin levels. Psychiatry Res 14:49–57.
- Faul F, Erdfelder E, Buchner A, Lang AG. (2009). Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41:1149–60.
- Garpenstrand H, Ekblom J, Forslund K, Rylander G, Oreland L. (2000). Platelet monoamine oxidase activity is related to MAOB intron 13 genotype. J Neural Transm (Vienna) 107:523–30.
- Girmen AS, Baenziger J, Hotamisligil GS, Konradi C, Shalish C, Sullivan JL, Breakefield XO. (1992). Relationship between platelet monoamine oxidase B activity and alleles at the MAOB locus. J Neurochem 59:2063–6.
- Haberstick BC, Lessem JM, Hewitt JK, Smolen A, Hopfer CJ, Halpern CT, Killeya-Jones LA, et al. (2014). MAOA genotype, childhood maltreatment, and their interaction in the etiology of adult antisocial behaviors. Biol Psychiatry 75:25–30.
- Hamner MB. (1996). Clozapine treatment for a veteran with comorbid psychosis and PTSD. Am J Psychiatry 153:841.
- Hamner MB. (1997). Psychotic features and combat-associated PTSD. Depress Anxiety 5:34–8.
- Hamner MB, Gold PB. (1998). Plasma dopamine beta-hydroxylase activity in psychotic and non-psychotic post-traumatic stress disorder. Psychiatry Res 77:175–81.
- Harro J, Fischer K, Vansteelandt S, Harro M. (2004). Both low and high activities of platelet monoamine oxidase increase the probability of becoming a smoker. Eur Neuropsychopharmacol 14:65–9.
- Hecker T, Fetz S, Ainamani H, Elbert T. (2015). The cycle of violence: associations between exposure to violence, trauma-related symptoms and aggression-findings from congolese refugees in Uganda. J Trauma Stress 28:448–55.
- Heins T, Gray A, Tennant M. (1990). Persisting hallucinations following childhood sexual abuse. Aust N Z J Psychiatry 24:561–5.
- Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. (2007). Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am J Psychiatry 164:150–3.
- Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. (2004). An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology 29:1498–505.
- Ivezic S, Bagaric A, Oruc L, Mimica N, Ljubin T. (2000). Psychotic symptoms and comorbid psychiatric disorders in Croatian combat-related posttraumatic stress disorder patients. Croat Med J 41:179–83.
- Jakubauskiene E, Janaviciute V, Peciuliene I, Soderkvist P, Kanopka A. (2012). G/A polymorphism in intronic sequence affects the processing of MAO-B gene in patients with Parkinson disease. FEBS Lett 586:3698–704.
- Jakupcak M, Conybeare D, Phelps L, Hunt S, Holmes HA, Felker B, Klevens M, McFall ME. (2007). Anger, hostility, and aggression among Iraq and Afghanistan War veterans reporting PTSD and subthreshold PTSD. J Trauma Stress 20:945–54.
- Jansson M, McCarthy S, Sullivan PF, Dickman P, Andersson B, Oreland L, Schalling M, Pedersen NL. (2005). MAOA haplotypes associated with thrombocyte-MAO activity. BMC Genetics 6:46.
- Kastelan A, Franciskovic T, Moro L, Roncevic GI, Grkovic J, Jurcan V, Lesica T, et al. (2007). Psychotic symptoms in combat-related post-traumatic stress disorder. Mil Med 172:273–7.
- Kehle SM, Greer N, Rutks I, Wilt T. (2011). Interventions to improve veterans' access to care: a systematic review of the literature. J Gen Intern Med 26:689–96.
- Kessler RC, Borges G, Walters EE. (1999). Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry 56:617–26.
- Kiive E, Ensoo D, Harro M, Harro J. (2002). Platelet monoamino oxidase activity in association with aggressive and hyperactive behavior: the effect of smoking? Pers Ind Diff 33:355–65.
- Kiive E, Merenäkk L, Harro M, Harro J. (2005). Changes in platelet monoamine oxidase activity, cholesterol levels and hyperactive behaviour in adolescents over a period of three years. Neurosci Lett 384:310–15.
- Kiive E, Laas K, Akkermann K, Comasco E, Oreland L, Veidebaum T, Harro J. (2014). Mitigating aggressiveness through education? The monoamine oxidase A genotype and mental health in general population. Acta Neuropsychiatr 26:19–28.
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. (2006). MAOA, maltreatment, and geneenvironment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry 11:903–13.
- Kozaric-Kovacic D, Pivac N, Muck-Seler D, Rothbaum BO. (2005). Risperidone in psychotic combat related posttraumatic stress disorder: an open trial. J Clin Psychiatry 66:922–7.
- Kozaric-Kovacic D, Pivac N. (2007). Quetiapine treatment in an open trial in combat-related post-traumatic stress disorder with psychotic features. Int J Neuropsychopharmacol 10:253–61.
- Krajl M. (1965). A rapid microfluorimetric determination of monoamine oxidase. Biochem Pharmacol 14:1684–6.
- Lenders JW, Eisenhofer G, Abeling NG, Berger W, Murphy DL, Konings CH, Wagemakers LM, et al. (1996). Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J Clin Invest 97:1010–19.
- Lesem MD, Tran-Johnson TK, Riesenberg RA, Feifel D, Allen MH, Fishman R, Spyker DA, et al. (2011). Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry 198:51–8.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75.
- Mann JJ. (2003). Neurobiology of suicidal behaviour. Nat Rev Neurosci 4:819–28.
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. (2002). A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res 95:9–23.
- Meszaros Z, Borsiczky D, Mate M, Tarcali J, Szombathy T, Tekes K, Magyar K. (1998). Platelet MAO-B activity and serotonin content in patients with dementia: effect of age, medication, and disease. Neurochem Res 23:863–8.
- Miller SA, Dykes DD, Polesky HF. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215.
- Mohr P, Pečeňak J, Švestka J, Swingler D, Treuer T. (2005). Treatment of acute agitation in psychotic disorders. Neuroendocrinol Lett26:327–35.
- Montoya A, Valladares A, Lizán L, San L, Escobar R, Paz S. (2011). Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSSEC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes 9:18.
- Muck-Seler D, Sagud M, Mustapic M, Nedic G, Babic A, Mihaljevic Peles A, Jakovljevic M, Pivac N. (2008). The effect of lamotrigine on platelet monoamine oxidase type B activity in patients with bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 32:1195–8.
- Muck-Seler D, Presecki P, Mimica N, Mustapic M, Pivac N, Babic A, Nedic G, Folnegovic-Smalc V. (2009). Platelet serotonin concentration and monoamine oxidase type B activity in female patients in early, middle and late phase of Alzheimer’s disease. Progr Neuro-Psychopharmacol Biol Psychiatry 33:1226–31.
- Mueser KT, Butler RW. (1987). Auditory hallucinations in combat-related chronic posttraumatic stress disorder. Am J Psychiatry 144:299–302.
- Nedic Erjavec G, Nenadic Sviglin K, Nikolac Perkovic M, Muck-Seler D, Jovanovic T, Pivac N. (2014). Association of gene polymorphisms encoding dopaminergic system components and platelet MAO-B activity with alcohol dependence and alcohol dependence-related phenotypes. Prog Neuropsychopharmacol Biol Psychiatry 54:321–7.
- Netter P, Montag C, Reuter M, Baars M, Gallhofer B. (2015). Genetic variation of the MAO B Gene is related to shorter reaction times in alcohol dependent patients. J Addict Med Ther 3:1014.
- Nilsson KW, Sjoberg RL, Damberg M, Leppert J, Ohrvik J, Olof Alm P, Lindstrom L, Oreland L. (2006). Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry 2:121–7.
- Novaco RW, Chemtob CM. (2015). Violence associated with combat-related posttraumatic stress disorder: The importance of anger. Psychol Trauma 7:485–92.
- Oreland L, Hallman J. (1995). The correlation between platelet MAO activity and personality: Short review of findings and a discussion on possible mechanisms. Prog Brain Res 106:77–84.
- Oreland L, Damberg M, Hallman J, Garpenstrand H. (2002). Smoking only explains part of the associations between platelet monoamine oxidase activity and personality. J Neural Transm (Vienna) 109:963–75.
- Oreland L. (2004). Platelet monoamine oxidase, personality and alcoholism: the rise, fall and resurrection. Neurotoxicology 25:79–89.
- Paaver M, Eensoo D, Pulver A, Harro J. (2006). Adaptive and maladaptive impulsivity, platelet monoamine oxidase (MAO) activity and risk-admitting in different types of risky drivers. Psychopharmacology (Berl) 186:32–40.
- Pinto PA, Gregory RJ. (1995). Posttraumatic stress disorder with psychotic features. Am J Psychiatry 152:471–2.
- Pivac N, Knezevic J, Kozaric-Kovacic D, Dezeljin M, Mustapic M, Rak D, Matijevic T, et al. (2007). Monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity in combat-related posttraumatic stress disorder. J Affect Disord 103:131–8.
- Pivac N, Knezevic J, Mustapic M, Dezeljin M, Muck-Seler D, Kozaric-Kovacic D, Balija M, et al. (2006). The lack of association between monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity among men. Life Sci 79:45–9.
- Pivac N, Kozaric-Kovacic D, Muck-Seler D. (2004). Olanzapine versus fluphenazine in an open trial in patients with psychoptic combat related posttraumatic stress disorder. Psychopharmacology 175:451–6.
- Pivac N, Mück-Seler D, Sagud M, Jakovljević M. (2002). Platelet serotonergic markers in posttraumatic stress disorder. Prog Neuropsychophar-macol Biol Psychiatry 26:1193–8.
- Pivac N, Muck-Seler D, Sagud M, Jakovljevic M, Mustapic M, Mihaljevic-Peles A. (2003). Long-term sertraline treatment and peripheral biochemical markers in female depressed patients. Prog Neuropsycho-pharmacol Biol Psychiatry 27:759–65.
- Romme MA, Escher AD. (1989). Hearing voices. Schizophr Bull 15:209–16.
- Roth JA, Young JG, Cohen DJ. (1976). Platelet monoamine oxidase activity in children and adolescents. Life Sci 18:919–24.
- Ruchkin V, Koposov RA, Klinteberg B, Oreland L, Grigorenko EL. (2005). Platelet MAO-B, personality and psychopathology. J Abnorm Psychol 114:477–82.
- Sabol SZ, Hu S, Hamer D. (1998). A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 103:273–9.
- Sandler M, Glover V, Clow A, Jarman J. (1993). Monoamine oxidase-B, monoamine oxidase-B inhibitors, and Parkinson's disease. A role for superoxide dismutase? Adv Neurol 60:238–41.
- Sansonnet-Hayden H, Haley G, Marriage K, Fine S. (1987). Sexual abuse and psychopathology in hospitalized adolescents. J Am Acad Child Adolesc Psychiatry 26:753–7.
- Sautter FJ, Brailey K, Uddo MM, Hamilton MF, Beard MG, Borges AH. (1999). PTSD and comorbid psychotic disorder: comparison with veterans diagnosed with PTSD or psychotic disorder. J Trauma Stress 12:73–88.
- Schalling D, Asberg M, Edman G, Oreland L. (1987). Markers for vulnerability to psychopathology: temperament traits associated with platelet MAO activity. Acta Psychiatr Scand 76:172–82.
- Scherrer JF, Xian H, Lyons MJ, Goldberg J, Eisen SA, True WR, Tsuang M, et al. (2008). Posttraumatic stress disorder; combat exposure; and nicotine dependence, alcohol dependence, and major depression in male twins. Compr Psychiatry 49:297–304.
- Schmidt U, Holsboer F, Rein T. (2011). Epigenetic aspects of posttraumatic stress disorder. Dis Markers 30:77–87.
- Shaner A, Eth S. (1989). Can schizophrenia cause posttraumatic stress disorder? Am J Psychother 43:588–97.
- Shevlin M, Armour C, Murphy J, Houston JE, Adamson G. (2011). Evidence for a psychotic posttraumatic stress disorder subtype based on the National Comorbidity Survey. Soc Psychiatry Psychiatr Epidemiol 46:1069–78.
- Shih JC, Chen K, Ridd MJ. (1999). Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 22:197–217.
- Shih JC, Chen K. (2004). Regulation of MAO-A and MAO-B gene expression. Curr Med Chem 11:1995–2005.
- Sjoberg RL, Ducci F, Barr CS, Newman TK, Dell'osso L, Virkkunen M, Goldman D. (2008). A nonadditive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior. Neuropsychophar-macology 33:425e30.
- Sobell JL, Lind TJ, Hebrink DD, Heston LL, Sommer SS. (1997). Screening the monoamine oxidase B gene in 100 male patients with schizophrenia: a cluster of polymorphisms in African-Americans but lack of functionally significant sequence changes. Am J Med Genet 74:44–9.
- Stalenheim EG. (2004). Long-term validity of biological markers of psychopathy and criminal recidivism: follow-up 6–8 years after forensic psychiatric investigation. Psychiatry Res 121:281–91.
- Stetler DA, Davis C, Leavitt K, Schriger I, Benson K, Bhakta S, Wang LC, et al. (2014). Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. J Psychiatr Res 58:69–75.
- Taft CT, Kaloupek DG, Schumm JA, Marshall AD, Panuzio J, King DW, Keane TM. (2007a). Posttraumatic stress disorder symptoms, physiological reactivity, alcohol problems, and aggression among military veterans. J Abnorm Psychol 116:498–507.
- Taft CT, Vogt DS, Marshall AD, Panuzio J, Niles BL. (2007b). Aggression among combat veterans: relationships with combat exposure and symptoms of posttraumatic stress disorder, dysphoria, and anxiety. J Trauma Stress 20:135–45.
- Tikkanen R, Auvinen-Lintunen L, Ducci F, Sjöberg RL, Goldman D, Tiihonen J, Ojansuu I, Virkkunen M. (2011). Psychopathy, PCL-R, and MAOA genotype as predictors of violent reconvictions. Psychiatry Res 185:382–6.
- Volavka J. (2014). Aggression in psychoses. Adv Psychiatry 2014: 196281.
- Weathers FW, Keane TM, Davidson JR. (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 13:132–56.
- Whitfield JB, Pang D, Bucholz KK, Madden PA, Heath AC, Statham DJ, Martin NG. (2000). Monoamine oxidase: associations with alcohol dependence, smoking and other measures of psychopathology. Psychol Med 30:443–54.
- Widom CS, Brzustowicz LM. (2006). MAOA and the”cycle of violence”; childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry 60:684–9.
- Wilcox J, Briones D, Suess L. (1991). Auditory hallucinations, posttraumatic stress disorder, and ethnicity. Compr Psychiatry 32:320–3.
- Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, Palmer DM, Paul RH, Song L, et al. (2009). A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology 34:1797–809.
- Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, Koenen KC. (2010). Posttraumatic stress disorder and the genetic structure of comorbidity. J Abnorm Psychol 119:320–30.
- Zammit S, Jones G, Jones SJ, Norton N, Sanders RD, Milham C, McCarthy GM, et al. (2004). Polymorphisms in the MAOA, MAOB, and COMT genes and aggressive behavior in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 128:19–20.