Abstract
Stress as a modern civilization factor significantly affects our lives. While acute stress might have a positive effect on the organism, chronic stress is usually detrimental and might lead to serious health complications. It is known that stress induced by the physical environment (temperature-induced cold stress) can significantly impair the efficacy of cytotoxic chemotherapies and the anti-tumor immune response. On the other hand, epidemiological evidence has shown that patients taking drugs known as β-adrenergic antagonists (“β-blockers”), which are commonly prescribed to treat arrhythmia, hypertension, and anxiety, have significantly lower rates of several cancers. In this review, we summarize the current knowledge about catecholamines as important stress hormones in tumorigenesis and discuss the use of β-blockers as the potential therapeutic agents.
Stress as a risk factor in development and progression of various diseases
Stress is a modern civilization factor that affects our life and health. Selye (Citation1985) defined stress as a nonspecific response of the body to any demand imposed upon it. However, according to a relatively new concept, stress responses have a primitive kind of specificity, with differential responses of the sympathetic nervous and adrenomedullary hormonal systems, depending on the type and intensity of the stressor as sensed by the organism and interpreted in a light of experience (Pacak & Palkovits, Citation2001). Stress occurs when the organism perceives a disruption or a threat of disruption of homeostasis. In modern civilizations, humans can be exposed to a variety of stressors – social stress, emotional stress, pain, hypoxia, glucose deprivation, etc. Common stress-related symptoms may include a wide range of negative emotions or altered mood and behavior (i.e. anxiety, irritability, anger, abrupt startle response, hostility, depression), which exert adverse effects on various body organs (e.g. cardiovascular and nervous systems) (Hering et al., Citation2015).
Stress responses differ depending on their duration and intensity. Acute, short-term stress is generally considered to be beneficial for the organism, while prolonged, chronic stress may result in detrimental consequences. Chronic stress is associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, leading to an increase in the production of cortisol with simultaneous elevations in catecholamines, i.e. epinephrine and norepinephrine. Together with the length of stress duration, intensity of a stressor is an important factor. In order to study mechanisms of the acute and chronic stress response, experimental models of stress were employed. These models include strong, severe stressors, e.g. immobilization (Kvetnansky & Mikulaj, Citation1970), which activates both the adrenomedullary and sympathoneural system, or mild stressors, e.g. cold, which activates the sympathoneural pathway (Fukuhara et al., Citation1996).
Catecholamines are among the first signaling molecules that respond to stress. When an adequately sensitive assay for plasma levels of epinephrine and norepinephrine became available, evidence for different noradrenergic versus adrenergic responses in different situations accumulated rapidly. A new concept began to emerge, in which the sympathoneural system plays key roles in appropriate redistribution of blood flow in situations such as orthostasis, cold exposure, mild blood loss, locomotion, exercise, altered salt intake and water immersion. Adrenomedullary hormonal system (AHS) responds to global or metabolic threats, such as hypoglycemia, hemorrhagic hypotension, exercise beyond an anaerobic threshold, emotional distress and shock. Evidence also accumulated for an association of sympathoneural activation with active escape, avoidance, or attack, and an association of AHS activation with passive, immobile fear (Goldstein & Kopin, Citation2008).
Nowadays, stress is considered to be a risk factor for the development of several diseases or is thought to significantly worsen their progress and prognosis. The cardiovascular system is among the most affected tissues by the stress responses, primarily through the action of catecholamines, through both α- and β-adrenergic receptors. β-Adrenergic receptor (β-AR) blockade is widely used to treat heart failure, since adverse effects of chronic β-AR stimulation are central to the pathogenesis of this disease state (Yan et al., Citation2014). Nevertheless, cardiotoxicity/cardioprotection depends on the type of β-ARs. Prevention of cardiac events caused by surgical stress in aged rats was studied by Sun et al. (Citation2014). These authors achieved the best results with simultaneously activating β2-ARs and inhibiting β1-ARs. Different function of β1-, β2-ARs was described also in oxidative stress, where β1-ARs play a cardiotoxic and β2-ARs play a cardioprotective role (Fajardo et al., Citation2006). β2-ARs activate pro-survival kinases and attenuate mitochondrial dysfunction during oxidative stress; absence of β2-ARs enhances cardiotoxicity via negative regulation of survival kinases and enhancement of intracellular Ca2+, thus predisposing the mitochondria to opening of the MPT (Fajardo et al., Citation2011). Also, upregulation of the β3-AR could represent an adaptation mechanism that might be related to altered physiological function of the left ventricle and atrium during prolonged emotional stress. Upregulation of β3-AR may also be cardioprotective function during catecholamine overload (Laukova et al., Citation2014). Besides the catecholaminergic pathway, stress also affects other metabolic pathways, e.g. calcium transport (Hudecova et al., Citation2010; Krizanova et al., Citation2008), cortisol levels, pro- and anti-inflammatory cytokines (Furtado & Katzman, Citation2015), glucocorticoids (Papadopoulou et al., Citation2015), etc. These physiological changes are frequently implicated in stress-related disorders.
Chronic stress may be involved in the growth of cancer cells, along with accelerated angiogenesis (Strange et al., Citation2000). Epidemiologic studies found the most consistent relationships between stressful conditions and progression of already existing tumors, and relatively little data suggests that stress affects the initial incidence of cancer (Antoni et al., Citation2006; Chida et al., Citation2008). For example, chronic stress can accelerate the progression of human acute lymphoblastic leukemia via β-adrenergic signaling (Lamkin et al., Citation2012). Psychological stress promotes the progression of pancreatic cancer xenografts via neurotransmitter-induced activation of multiple pathways and increases systemic and tumor levels of norepinephrine, epinephrine, cortisol, vascular endothelial growth factor (VEGF), and cAMP (Schuller et al., Citation2012). Social stress also stimulates non-small cell lung carcinoma growth by increasing nicotinic acetylcholine receptor-mediated stress neurotransmitter signaling (Al-Wadei et al., Citation2012). These findings are consistent with sympathetic effects on the cell growth in cancer. Importantly, stress may also affect therapeutic efficacy in cancer. Psychiatric and psychosocial disorders among cancer patients have been reported as a major consequence of the disease and treatment.
Several mechanisms are implicated in the action of catecholamines in tumorigenesis. The first includes changes in cell-mediated immunity. Due to the fact that changes in expression of ARs are observed in many types of tumors, scientific interest has focused on the role of ARs in cancer. Epinephrine itself is able to stimulate secretion of VEGF, a key mediator of angiogenesis in cancer, by human prostate cancer cells (Bavadekar et al., Citation2013). Stimulation of cell proliferation via β-AR-dependent transactivation of the ERK/COX-2 pathway together with eliciting a differential response on the expression of cell cycle regulators has been shown in esophageal squamous-cell carcinoma cell line HKESC-1 (Liu et al., Citation2008). Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD (Bcl-2-associated death promoter) phosphorylation (Sastry et al., Citation2007). Norepinephrine increases aggressiveness of ovarian cancer cells via inducing Slug, C2H2 type of zinc-finger transcription factor. In addition, norepinephrine enhances expression of human telomerase, reverse transcriptase (hTERT), and metastasis of ovarian cancer cells to the lung (Choi et al., Citation2015). Similar results have been shown for isoprenaline (induction of epithelial-mesenchymal transition in gastric cancer cells) (Lu et al., Citation2015). Probably the most complex mechanism of involvement of adrenergic signaling in cancer progression has been proposed for breast cancer, and includes: (1) increasing tumor cell survival after exposure to chemotherapeutic agents; (2) increasing breast cancer cell proliferation; and (3) altering the tumor microenvironment via changes in angiogenesis and the inflammatory response (Obeid & Conzen, Citation2013). Prostate cancer is another type of cancer where the role of adrenergic signaling is intensively studied. Treatment with norepinephrine (β1-AR agonist) in mice xenografted with human DU145 prostate cancer cells increase the metastatic potential of these cells (Barbieri et al., Citation2015).
Pathways in cancer development and progression that might be affected by stress
Several studies have attempted to address key mechanisms of organism reactions to stress. It is well known that stress triggers a neuroendocrine chain reaction under the control of specific sites in the central nervous system, which mainly include secretion of catecholamines and glucocorticoids from the adrenal gland and the sympathetic nervous system.
Release of epinephrine and norepinephrine from the adrenal medulla constitutes the most rapid stress response. Both epinephrine and norepinephrine are critical in converting the perceived threat into action by activating the heart and muscles to prepare for the “fight or flight” response. Some acute stressors have been shown to elicit increases in the secretion of epinephrine and norepinephrine from the sympathoadrenal axis (Gerra et al., Citation2001; Schoder et al., Citation2000). Subsequently, enzymes of the catecholaminergic pathway are up-regulated to ensure further production of catecholamines. Although originally the role of catecholamines (mainly epinephrine and norepinephrine) was attributed to cardiac function, recently these stress hormones were likewise shown to play an important role in cancer.
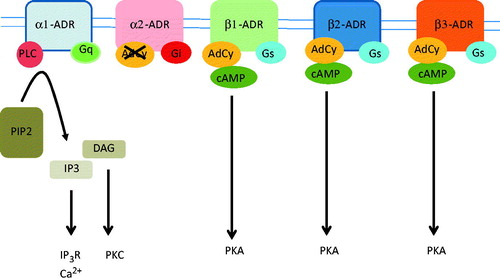
ARs are members of the G-protein coupled receptor family and they mediate physiological responses to norepinephrine and epinephrine. ARs are subdivided into three major families (α1, α2 and β) based on their structure, pharmacology and signaling mechanisms (Hieble et al., Citation1995). The α1-ARs bind to Gq protein and activate phospholipase C, which results in the production of diacylglycerol and IP3. The α2-ARs inhibit the activity of adenylate cyclase, whereas β-ARs of all types bind to Gs and activate adenylate cyclase (). Blockade of α2-ARs also increases noradrenaline release, since increased noradrenaline might mediate the ‘pro-tumor’ effect of α2 blockade.
Figure 1. Adrenergic receptors bind epinephrine and norepinephrine with a different affinity and they activate different metabolic pathways. The α1-ADRs are bound to Gq protein and they activate phospholipase C (PLC), which cleaves phosphatidylinositol 1,4-bisphosphate (PIP2) tp IP3 and diacylglycerol (DAG). IP3 activates IP3-receptors (IP3R), which are calcium-releasing channels that release calcium from the endoplasmic reticulum. DAG activates protein kinase C (PKC). The α2-ADRs bind Gi protein, which result in the inhibition af adenylate cyclase (AdCy) and inhibition of cAMP production. The β1, β2 and β3-ADRs are bound to the Gs protein and they activate AdCy, resulting in an increase of cAMP and activation of protein kinase A (PKA).
Chronic stress interferes with immune function and is thought to play a role in the etiology of tumor growth (Thaker et al., Citation2006). Clinical and animal studies indicate that chronic stress can be a factor in the growth and angiogenesis of carcinoma (Lutgendorf et al., Citation2002; Strange et al., Citation2000). Chronic stress results in greater tumor size, higher levels of plasma catecholamines, and more invasive growth of oral carcinoma cells in a mouse model (Xie et al., Citation2015).
The influence of the β-adrenergic system on energy metabolism and the immune system is thought to regulate cancer metastasis (Li et al., Citation2013). Epidemiological evidence has shown that patients taking drugs known as β-adrenergic antagonists (“β-blockers”), commonly prescribed to treat hypertension and anxiety, have significantly lower rates of several cancers (Fitzgerald, Citation2012; Jansen et al., Citation2014). On the other hand, stress induced by the physical environment, i.e. temperature-induced cold stress, can significantly impair the efficacy of cytotoxic chemotherapies and the anti-tumor immune response (Eng et al., Citation2014).
The three subtypes of β-adrenergic receptor are present in many sites of tumor growth and metastasis, including the brain, lung, liver, kidney, adrenal gland, breast, ovary, prostate, lymphoid tissues, bone marrow, and vasculature. β-Adrenergic signaling regulates the biological activity of several cancer-relevant cell types including epithelial cells, vascular myocytes and pericytes, adipocytes, fibroblasts, neural/glial cells, and most lymphoid and myeloid immune cells (; Baker et al., Citation2011; Daly & McGrath, Citation2011). Previous studies have shown that various human solid tumors, such as breast, colon, prostatic, ovary, nasopharyngeal and oral cancer express β2-AR, raising the possibility that such receptors may affect the invasion and dissemination processes (Palm et al., Citation2006; Shang et al., Citation2009; Yang et al., Citation2006). Moreover, some stress neurotransmitters, such as norepinephrine and epinephrine, have been demonstrated to contribute to the regulation of tumor cell invasion, at least in part through Ö-AR activation.
Table 1. Modulation of the β-ARs in various tumors.
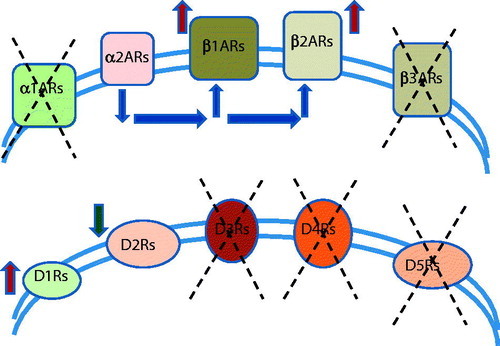
Recently, it has been documented that β-ARs are more prevalent in cancer pathogenesis than α-ARs (). Experimental studies in preclinical mouse models of breast cancer have shown that chronic restraint stress enhances disease progression by increasing catecholamine levels via β-AR signaling. Moreover, increased α-adrenergic signaling has also been shown to promote breast cancer in vitro and in vivo (Lamkin et al., Citation2015). The α-adrenergic antagonist, phentolamine, inhibits the detrimental effect of chronic restraint stress on both the primary tumor growth and metastatic dissemination in a preclinical orthotopic model of human breast cancer.
Figure 2. From all types of adrenergic receptors, only type1 (β1ARs) and type 2 (β2ARs) adrenergic receptors were described to be up-regulated in solid tumors. The α2-adrenergic signaling can act through an autoreceptor mechanism to inhibit sympathetic catecholamine release and, thus, modulate established effects of β-adrenergic signaling on tumor progression-relevant biology. Involvement of other types of adrenergic receptors – α1ARs and β3ARs was not described yet. From all 5 types of dopamine receptors, only type 1 (D1Rs) were downregulated in breast tumors. Also, dopamine, through its specific dopamine receptor 2 (D2Rs), inhibits tumor growth by suppressing the actions of vascular permeability factor/vascular endothelial growth factor A on both tumor endothelial cells and bone marrow–derived endothelial progenitor cells. Involvement of other types of dopaminergic receptors – D3Rs and D4Rs and D5Rs was not described yet.
Increased α-adrenergic signaling boosts breast cancer progression in vivo under non-stress conditions that involve subcutaneous injection into the flank and an orthotopic model that exhibits primary mammary tumor and distant metastasis. Thus, under non-stress conditions, phentolamine increased primary tumor size and distant metastasis at a rate that was commensurate with the effect of chronic restraint. Selective α1-adrenergic blockade by prazosin did not have any effect on tumor size or metastasis. Results obtained by Lamkin et al. (Citation2015) are consistent with the hypothesis that α2-adrenergic signaling can act through an autoreceptor mechanism to inhibit sympathetic catecholamine release and thus modulate established effects of β-adrenergic signaling on tumor progression-relevant biology. With regard to β-adrenergic signaling pathway in cancer, binding of ligand to β-adrenergic receptor activates G-protein-mediated activation of adenylate cyclase and subsequent conversion of ATP into cAMP, which activates phosphorylation of target proteins including transcription factors of CREB/ATF and GATA families. These factors have diverse functions in controlling cell proliferation, survival, and apoptosis (Persengiev & Green, Citation2003). The α2-ARs bind to Gαi and thus inhibit adenylate cyclase and decrease cAMP levels (; Ziolkowski & Grover, Citation2010). This mechanism may contribute to the explanation of “pro-tumor” effect of selective α2-adrenergic blockade. On the other hand, specific agonists of α2-AR (such as clonidine) stimulate incorporation of [3H]-thymidine in cancerous IBH-6, IBH-7, and MCF-7 cell lines as well as α2B- and α2C-adrenoceptor-subtype expressing non-tumor HBL-100 cells (Vazquez et al., Citation2006). Clarification of this controversy may be explained by the fact that different cells express different subtypes of α2-AR. Also, clonidine is a nonselective α2-AR agonist. This research group has also shown that malignant, hormone-insensitive HS-578T and MDA-MB-231 tumor cell lines express only a single subtype, α2A, whereas “normal” and hormone-sensitive tumor cells express both α2B and α2C subtypes (Vazquez et al., Citation2006). The presence of two different subtypes of α2-AR is probably necessary to regulate adrenergic signal transduction (Philipp et al., Citation2002). Moreover, Chiesa et al. (Citation2008), who investigated the involvement of α2-AR and estrogen receptors in a proliferation enhancement in human breast cancer MCF-7 cell line treated with catecholestrogenes, hydroxylation products of 17β-estradiol and estrone. The authors demonstrate that α2-AR may participate, at least in part, to the mitogenic effect of 2-hydroxy-estradiol, 2-hydroxy-estrone, and 4-hydroxy-estrone (Chiesa et al., Citation2008). In conclusion, the role of α-adrenergic signaling in cancer should be investigated, and further studies are necessary. On the other hand, specific agonists/antagonists of α2-AR subtypes are still missing, which make the research more difficult.
Up until now, three types of β-ARs have been identified and characterized. β1 and β2-ARs bind to Gs proteins (although β2-AR also couples to Gi) (Chen-Izu et al., Citation2000), which in turn are linked to adenylate cyclase activation (). The mechanism by which the activated β3-ARs transmit the signals across the plasma membrane also involves the stimulation of Gs (Guan et al., Citation1995). Catecholamine binding thus causes a rise in the intracellular concentration of the second messenger cAMP. Downstream effectors of cAMP include cAMP-dependent protein kinase (PKA), which mediates some of the intracellular events following hormone binding. These receptors differ in the sensitivity to norepinephrine/epinephrine. In β1-ARs, the binding affinities of epinephrine and norepinephrine are about the same. The β2-ARs are more sensitive to epinephrine, while the β3-ARs are more sensitive to norepinephrine. In many common cancers, production of cAMP boosts cancer proliferation, survival, and aggressiveness, reflecting the fact that, through mechanisms that require further clarification, cAMP can promote tyrosine phosphorylation, notably transactivation of the epidermal growth factor receptor (EGFR) (McCarty, Citation2014).
Interestingly, recent evidence suggests that dopamine (DA) is also involved in cancer development (). DA, an antiangiogenic neurotransmitter, promotes maturation of ovarian cancer vasculature by enhancing pericyte recruitment to tumor endothelial cells during chronic stress (Moreno-Smith et al., Citation2013). Dopamine acts through the five types of dopamine receptors. Borcherding et al. (Citation2016) discovered expression of DA type-1 receptors (D1Rs) in breast cancer, thereby identifying these receptors as novel therapeutic targets in these diseases. Thus, D1R overexpression is associated with advanced breast cancer and poor prognosis. In vivo and in vitro studies have shown that DA, through its specific dopamine receptor 2 (DR2), inhibits tumor growth by suppressing the actions of vascular permeability factor/vascular endothelial growth factor A on both tumor endothelial cells and bone marrow-derived endothelial progenitor cells (Chakroborty et al., Citation2009). The antiangiogenic and pro-vessel maturation effects of DA make this catecholamine an attractive choice to improve the efficacy of therapies for cancer and other diseases with abnormal vasculature (Moreno-Smith et al., Citation2013).
Catecholamine-producing tumors, stress and cancer
Pheochromocytoma and paraganglioma are neural crest tumors derived from chromaffin cells in either the adrenal gland or extra-adrenally (so called, paraganglioma). The most important characteristic of these tumors is that they produce catecholamines and their excess can have catastrophic consequences including lethal arrhythmia, stroke, hypertensive crisis, and other organ-related consequences as well as anxiety and nervousness (Lenders et al., Citation2005). In some patients, profound catecholamine release may result in multiorgan failure. For example, any direct tumor stimulation may lead to abrupt and significant catecholamine release that exceeds normal plasma values 1000 times or more with devastating consequences, often presenting as an emergency (Pacak, Citation2011). Although catecholamine release from these tumors is usually unpredictable, there are some situations under which these tumors are almost always stimulated, resulting in catecholamine release. From these situations, various stressful conditions are the most important ones, including psychological and physical stress. Thus, it is not uncommon to detect severe hypertension and tachyarrhythmia episodes in such patients who face some critical or life-threatening situations (divorce, job loss, chronic pain, accident, chronic anxiety, etc.). Alpha- and beta-adrenergic receptor blockers are the best therapies to be used for these tumors before they are surgically removed (Lenders et al., Citation2014). Thus, these tumors mimic stress-related situations, in which catecholamines are almost always released. According to the Selye theory of stress, these tumors have some similarities and differences related to the general adaptation syndrome where stress responses occur in three stages: alarm, resistance, and exhaustion. Similarly, in pheochromocytoma/paraganglioma patients, the alarm situation is reflected by the occasional increase in heart rate or blood pressure, the resistance situation is reflected by constantly elevated heart rate and blood pressure, and the exhaustion reflects multiorgan failure by enormously elevated catecholamine levels (e.g. if a large tumor or metastatic disease is present). In contrast to other stress-related conditions and the general adaptation syndrome, in patients with these tumors, there is no activation of other stress-related systems (especially the HPA axis), central stress-related components are less common (e.g. anxiety, depression) and as described above, there is not often any trigger for catecholamine release.
Although data related to acute or chronic stress and pheochromocytoma/paraganglioma progression and metastasis is missing, (pseudo)hypoxia is most likely an exemption. This is mainly related to pheochromocytomas/paragangliomas, which are part of a cluster 1 that has a pseudohypoxic signature and are gasping for air since the utilization of oxygen is impaired (Jochmanová et al., Citation2015). These pheochromocytomas/paragangliomas mainly belong to mutations in succinate dehydrogenase, hypoxia-inducible factor 2α, and von Hippel-Lindau (VHL) genes (Martucci & Pacak, Citation2014). All of these tumors are characterized by the disruptions of the hypoxia signaling pathway, due to either abnormal hypoxia-inducible factor α hydroxylation or binding to the von Hippel–Lindau protein. Hypoxia-inducible factor α is stabilized and effects transcription of many downstream genes, most of them involved in tumorigenesis, particularly proliferation, apoptosis, migration, invasiveness, and metastasis (Jochmanová et al., Citation2013). This data is well supported by the initial findings that high altitude is associated with carotid body paragangliomas (Arias-Stella & Valcarcel, Citation1973). These findings call for new therapeutic options of this hypoxia stress-related tumors. Nowadays, several agents affecting HIF-1α signaling have been introduced, with varying results depending on a cancer’s HIF-α phenotype and they mainly include antiangiogenic agents, such as inhibitors of vascular endothelial growth factor (VEGF), mTOR, heat shock protein 90 inhibitors, or agents that restore or activate PHD enzyme activity (Jochmanová et al., Citation2015). Currently, drugs selectively targeting HIF-2α signaling are under development. For example, two membrane diterpenes were found to selectively inhibit HIF-2α and modulate its downstream effectors (Grkovic et al., Citation2011; Scheuermann et al., Citation2013). In terms of inhibiting the HIF signaling pathway, agents targeting both HIF-1α and HIF-2α subunits, such as PI3K inhibitors, dual PI3K/mTOR inhibitor, or JNK inhibitor are of great interest since they can activate different genes and, in many cancers, both are expressed in a certain balanced ratio (for review see Jochmanová et al., Citation2015).
Other types of tumors, stress and cancer
Adrenergic activation plays an important role in colon cancer cell proliferation, most probably through β-AR. The β-blockers were able to reverse the proliferation induced by epinephrine and isoprenaline, and some of these blockers significantly decreased the proliferation of HT-29 cells. The elucidation of the intracellular pathways involved in CA-induced proliferation of colon cancer cells, and in the reversion of this effect by β-blockers, may contribute to identifying promising strategies in cancer treatment (Coelho et al., Citation2015). Repression of β2-AR but not β1-AR signaling selectively suppressed cell viability induced G1-phase cell cycle arrest, causing both intrinsic and extrinsic pathways-mediated apoptosis of specific CRC cells and inhibiting CRC-xenograft growth in vivo. However, the expression of β2-AR did not correlate with the progression of CRC in vitro or in clinical samples. These data are evidence that the expression profiles, signaling, and blockage of β2-AR have a unique pattern in CRC comparing to other cancers (Chin et al., Citation2016).
Chronic stress also promotes the growth of ovarian carcinoma in nude mice through increasing serum levels of norepinephrine (Gao et al., Citation2013). Lutgendorf et al. (Citation2012) showed that both epinephrine and norepinephrine are elevated in a sustained fashion in ovarian and other peritoneal tissues in preclinical models of chronic stress. These hormonal increases are associated with greater tumor burden, mediated by increased tumor angiogenesis. Increased adrenergic stimulation through the β2-ARs resulted in increased metastasis in ovarian cancer via elevated prostaglandin E2 synthesis (Nagaraja et al., Citation2016). On the other hand, dopamine levels are decreased in ovarian carcinomas from stressed mice, and dopamine replacement counteracts the stimulatory effects of epinephrine and norepinephrine on tumor growth by inhibiting tumor angiogenesis (Moreno-Smith et al., Citation2011). Dopamine treatment blocked stress-mediated increases in tumor growth and increased pericyte coverage of tumor endothelial cells in the ovary (Moreno-Smith et al., Citation2013).
Melanoma represents the most aggressive type of skin cancer, with an increasing incidence found especially in young adults. Expression of genetically determined stress-related phenomena may be a key factor in the epidemiology of melanoma (Ragan et al., Citation2013). The observation that β-ARs are upregulated in malignant melanoma tissues supports the hypothesis that circulating catecholamines norepinephrine and epinephrine, by activating their receptors, could favor melanoma progression in vivo. Indeed, norepinephrine and epinephrine produced in vitro via β-ARs activation a number of biological responses that exert a pro-tumorigenic effect in melanoma cell lines (Moretti et al., Citation2013).
The finding that melanoma outcomes for men are poorer when compared to women, and that cancer outcomes for the vast majority of cancer types are poorer in men, is an observation that holds true across different countries and over time (Micheli et al., Citation2009; Nosrati & Wei, Citation2014). The sex difference is a strong indication that in addition to cancer specific factors, there are likely modifying factors exogenous to the cancers, found in the host, that contribute to mediating outcomes. Stress affects males and females differently. Although sex-related differences exist in stress susceptibility and response, the mechanism responsible for such differential response is not clearly defined. Altemus (Citation2006) showed that after chronic restraint stress, female rats do not exhibit the impairment of spatial memory that was observed in males. The question of how sex affects stress-induced change cancer progression in males and females remains to be addressed.
Stress pathways as other anticancer targets
Antagonists of the β-adrenergic receptors (“β-blockers”) are commonly used in cardiovascular medicine in a number of cardiovascular conditions, which reduce both the morbidity as well as the mortality. In addition, they have found application in psychiatry. However, the observation that β-blockers produced an anticancer effect and improved survival of cancer patients opened a new front of investigations for the role of β-blockers in various cancers (for review, see Akbar & Alsharidah, Citation2014). Studies of β-adrenergic influence on tumor biology were motivated by epidemiologic observations associating stressful life circumstances with accelerated progression of incident cancers (Antoni et al., Citation2006; Chida et al., Citation2008) and studies linking the use of β-adrenergic antagonists (“β-blockers”) to attenuate disease progression. The studies revealed that the mechanism of action of (mainly nonselective) β-blockers is complex, and may be related to their ability to induce apoptosis in various tumor cell lines (Fuchs et al., Citation2015). A recent study of Chang et al. (Citation2015a) revealed that β-blockers may also act preventively as chemoprotective agents. These authors have determined that a dose of carvedilol (a nonselective β-/α1-blocker) dependently inhibited EGF-induced malignant transformation of JB6 P + cells, indicating chemopreventive activity against skin cancer. Propranolol as a nonselective β-blocker appears to reduce the risk of head and neck, esophagus, stomach, colon, and prostate cancers (Chang et al., Citation2015b), as well as the risk of hepatocellular carcinoma in patients with compensated viral C cirrhosis (Nkontchou et al., Citation2012). Propranolol may prevent the development of pancreatic ductal adenocarcinoma by blocking cAMP-dependent intracellular signaling, cAMP-dependent release of epidermal growth factor, and PKA-dependent release of vascular endothelial growth factor, while also downregulating the alpha 7 nicotinic acetylcholine receptors by inhibiting cAMP-mediated subunit assembly (Al-Wadei et al., Citation2009). A reduced risk of prostate cancer associated with sotalol (a nonselective β-blocker) has been shown in the study of Kaapu and coworkers (Kaapu et al., Citation2015). Similar observations have also been made for carvedilol. Data obtained from the Taiwan National Health Insurance Research Database shows that long-term treatment with carvedilol is associated with a reduced risk of upper gastrointestinal tract and lung cancers (Lin et al., Citation2015). Due to the fact that all above-mentioned β-blockers represent diverse generations with different selectivity to ARs and properties (propranolol and sotalolol – the first generation of β-blockers, nonselective with no vasodilatation activity; carvedilol – the third generation, nonselective with vasodilatation), the role of the selectivity and properties in their “anticancer” activity should be further investigated.
Altogether, these data suggest that β-AR plays an important role in prostate cancer metastasis formation. The treatment with antagonist propanolol with moderate affinity to both β1- and β2-ARs could represent an interesting tool to control this process in the cells overexpressing β2-AR. The above-mentioned data have significant clinical overlap: the study of Deng et al. (Citation2014) has demonstrated that norepinephrine attenuates the efficacy of sunitinib. Nevertheless, these authors suggest that a combination of sunitinib and propranolol might be suggested as a new strategy for treatment of solid tumors (Deng et al., Citation2014). These studies suggest indicates possible application of β-blockers in the field of cancer biology.
Propranolol, a nonselective blocker of β-ARs that is used to treat high blood pressure or a number of heart arrhythmias, is of considerable interest due to its possible application in treatment of some types of cancer. This compound is used as the first-line of treatment for infantile hemangiomas (Chiu et al., Citation2012; Ji et al., Citation2015; Porcel Chacon et al. Citation2015). In vitro studies indicate propranolol reduces the expression of HIF-1α in hemangioma cells in dose- and time-dependent manners, mainly by acting on β2-AR. In addition, propranolol inhibited the signal transducer and activator of transcription 3 (STAT3), a critical oncogenic signaling molecule, and the anti-apoptotic protein Bcl-2 (Li et al., Citation2015). Similar results (including apoptosis) were observed in cells from patients suffering from von Hippel–Lindau (VHL) disease, which is characterized by the growth of different types of tumors including hemangioblastomas in the central nervous system (CNS) and retina, renal carcinoma, pheochromocytomas, pancreatic serous cystadenoma, and endolymphatic sac tumors. The study confirmed that this β-blocker could reduce the growth of HIF-dependent tumors and may thus be a promising treatment to delay surgery in VHL patients (Albinana et al., Citation2015).
Antiangiogenic properties of propranolol have also been confirmed in the in vitro study of Lamy et al. (Citation2010) and in the study of Pan et al. (Citation2015). These authors conclude that regression of the cells occurs via the inhibition of cell cycle progression, invasion, and tube formation. In addition, propranolol decreases NO and VEGF levels through the down-regulation of the PI3K/Akt/eNOS/VEGF pathway (Pan et al., Citation2015), and is beneficial in treatment of solid Ehrlich Carcinoma (SEC) in xenograft models (however, only its high dose exhibited significant impact on survival rate in this study) (Abdin et al., Citation2014) in nude mice bearing BE(2) C neuroblastoma xenografts. In nude mice, the antiangiogenic effect of propranolol was mediated via inhibitory effect on the tumor growth and angiogenic factors expression in neuroblastoma (NB) xenografts (Xu et al., Citation2013). Together with the study of Wolter et al. (Citation2014), this report suggests that propranolol may negatively impact NB and may be considered in combination treatments for patients with relapsed and refractory NB. The ability of propranolol to induce apoptosis in endothelial cells through activation of the intrinsic and extrinsic apoptotic pathways contributes to its therapeutic effects against infantile hemangiomas (Tu et al., Citation2013). However, the rate of the apoptosis induced by propranolol should be modified by overexpression of some proteins, e.g. CD147, and downregulation of its expression can contribute to pro-apoptic effect of propranolol (Xie et al., Citation2013). Propranolol has antiproliferative and apoptotic effects on multiple myeloma cells, which has been confirmed in a study by Kozanoglu et al. (Citation2013). The authors of the study conclude that propranolol may be a good and economical way to treat multiple myeloma patients.
Synergistic effects of β-blockers and conventional anticancer therapy may be beneficial. For example, propranolol in combination with radiation therapy may be effective for reduction of clonogenic survivability, as has been shown on human gastric adenocarcinoma cell lines SGC-7901 and BGC-823 (Liao et al., Citation2010b). In general, combination of β-blockers with conventional chemotherapy may also be beneficial. A combination of propranolol with cisplatin may be useful in treatment of head and neck squamous cell carcinoma (Wolter et al., Citation2012). Carvedilol, a drug with well-known protective effect on mitochondria, induced severe mitochondria damage, and imatinib mesylate induced autophagy in C6 rat glioma chemoresistant experimental brain tumor cells. Imatinib mesylate is a tyrosine kinase inhibitor used in the therapy of some types of cancer, including Philadelphia chromosome-positive chronic myelogenous leukemia. These results suggest that carvedilol showed antitumor activity against rat C6 glioma cells and a combination of carvedilol with imatinib mesylate resulted in enhanced in vitro antitumor (Erguven et al., Citation2010).
A study by Coelho et al. (Citation2015) described a complex design that including application of both agonists and antagonists of β-AR. The study has shown that colon cancer cells express β-AR, and their activation is involved in carcinogenesis and tumor progression. The authors incubated HT-29 cells, which were derived from a human colon adenocarcinoma in the absence (control) or presence of the AR agonists, epinephrine, norepinephrine, and isoprenaline and observed pro-proliferative effect of these compounds. β-blockers propranolol, carvedilol, atenolol, and ICI-118,551 (selective β2-AR antagonist) were able to block the pro-proliferative effect of AR agonists and decrease the proliferation potential of the cells (Coelho et al., Citation2015).
The anti-tumor effect of propranolol has been shown on different models including both cell (e.g. human pancreatic carcinoma cells PC-2 together with all above-mentioned cell lines) and animal models (Zhang et al., Citation2009). Animal cell models are necessary to understand the mechanism of antitumor effects of β-blockers. β-AR are reported to be associated with the biologic behavior of breast cancer (see above-mentioned text) and may influence glucose metabolism. Reduced hexokinase (HK-2) expression in propranonol-treated 4T1 breast tumor cells and in cells of tumors of propranolol-treated mice point to the ability of propranolol to block glucose metabolism in tumor cells (Kang et al., Citation2014). The ability of propranolol to induce apoptosis in tumor cells has been described (Liao et al., Citation2010a). Its ability to modulate immune response, which may contribute to anti-tumor effect, should be further investigated (Khalili et al., Citation2013). The ability of propranolol to change cytokine production by tumor-infiltrating lymphocytes and to determine β2-AR expression in these cells probably contributes significantly to modulation of immune response (Seyedi et al., Citation2012). Immunomodulatory effect of esmolol, a cardioselective β1-AR blocker with rapid onset, has been shown in patients undergoing laparoscopic gastrectomy due to gastric cancer (Kim et al., Citation2013). All of the above-mentioned facts point at the novel promising strategy in cancer treatment. This strategy also includes synthesis of novel compounds, for example, isoflavene-propranolol hybrids, as anti-tumor agents (Yee et al., Citation2013).
Summary
There is a clear relationship between stressful conditions and progression of already incident tumors. Catecholamines as the stress hormones are involved in the progression of some tumors, preferentially through the β-ARs. Thus, β-blockers could have significant implications in cancer therapy by blocking β-ARs in tumor tissue. The observation that β-blockers produced an anticancer effect and improved the survival of cancer patients opens a new front of investigations for the role of β-blockers in various cancers.
Funding information
Work of the authors is supported by the following grants – APVV-14-0351 and VEGA 2/0082/16 (OK) and KAMOTEPRE – MUNI/A/1365/2015 with the support of the Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2016 (PB).
Disclosure statement
The authors declare no conflicts of interest. They alone are responsible for the whole content of this manuscript.
References
- Abdin AA, Soliman NA, Saied EM. (2014). Effect of propranolol on IL-10, visfatin, Hsp70, iNOS, TLR2, and survivin in amelioration of tumor progression and survival in solid ehrlich carcinoma-bearing mice. Pharmacol Rep 66:1114–21.
- Akbar S, Alsharidah MS. (2014). Are beta blockers new potential anticancer agents? Asian Pac J Cancer Prev 15:9567–74.
- Albinana V, Villar Gomez de Las Heras K, Serrano-Heras G, Segura T, Perona-Moratalla AB, Mota-Perez M, de Campos JM, Botrella LM. (2015). Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J Rare Dis 10:118.
- Altemus M. (2006). Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav 50:534–8.
- Al-Wadei HA, Al-Wadei MH, Schuller HM. (2009). Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs 20:477–82.
- Al-Wadei HA, Al-Wadei MH, Ullah MF, Schuller HM. (2012). Gamma-amino butyric acid inhibits the nicotine-imposed stimulatory challenge in xenograft models of non-small cell lung carcinoma. Curr Cancer Drug Targets 12:97–106.
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. (2006). The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6:240–8.
- Arias-Stella J, Valcarcel J. (1973). The human carotid body at high altitudes. Pathol Microbiol (Basel) 39:292–7.
- Baker JG, Hill SJ, Summers RJ. (2011). Evolution of β-blockers: from anti-anginal drugs to ligand-directed signalling. Trends Pharmacol Sci 32:227–34.
- Barbieri A, Bimonte S, Palma G, Luciano A, Rea D, Giudice A, Scognamiglio G, et al. (2015). The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int J Oncol 47:527–34.
- Bavadekar S, Budajaja F, Patel K, Vansal S. (2013). Epinephrine stimulates secretion of VEGF by human prostate cancer cells, LNCaP, through a beta2-adrenergic receptor-mediated pathway. FASEB J 27:1105–11.
- Borcherding DC, Tong W, Hugo ER, Barnard DF, Fox S, LaSance K, Shaughnessy E, Ben-Jonathan N. (2016). Expression and therapeutic targeting of dopamine receptor-1 (D1R) in breast cancer. Oncogene 35:3103–13.
- Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. (2009). Catecholamines regulate tumor angiogenesis. Cancer Res 69:3727–30.
- Chang A, Yeung S, Thakkar A, Huang KM, Liu MM, Kanassatega RS, Parsa C, et al. (2015a). Prevention of skin carcinogenesis by the beta-blocker carvedilol. Cancer Prev Res (Phila) 8:27–36.
- Chang PY, Huang WY, Lin CL, Huang TC, Wu YY, Chen JH, Kao CH. (2015b). Propranolol reduces cancer risk a population-based cohort study. Medicine (Baltimore) 94:e1097.
- Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG. (2000). G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels. Biophys J 79:2547–56.
- Chida Y, Hamer M, Wardle J, Steptoe A. (2008). Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 5:466–75.
- Chiesa IJ, Castillo LF, Luthy IA. (2008). Contribution of alpha2-adrenoceptors to the mitogenic effect of catecholestrogen in human breast cancer MCF-7 cells. J Steroid Biochem Mol Biol 110:170–5.
- Chin CC, Li JM, Lee KF, Huang YC, Wang KC, Lai HC, Cheng CC, et al. (2016). Selective β2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J Cell Physiol 231:459–72.
- Chiu YE, Drolet BA, Blei F, Carcao M, Fangusaro J, Kelly ME, Krol A, et al. (2012). Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer 59:934–8.
- Choi MJ, Cho KH, Lee S, Bae YJ, Jeong KJ, Rha SY, Choi EJ, et al. (2015). hTERT mediates norepinephrine-induced Slug expression and ovarian cancer aggressiveness. Oncogene 34:3402–12.
- Coelho M, Moz M, Correia G, Teixeira A, Medeiros R, Ribeiro L. (2015). Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol Rep 33:2513–20.
- Daly CJ, McGrath JC. (2011). Previously unsuspected widespread cellular and tissue distribution of β-adrenoceptors and its relevance to drug action. Trends Pharmacol Sci 32:219–26.
- Deng GH, Liu J, Zhang J, Wang Y, Peng XC, Wei YQ, Jiang Y. (2014). Exogenous norepinephrine attenuates the efficacy of sunitinib in a mouse cancer model. J Exp Clin Cancer Res 33:21.
- Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. (2014). A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother 63:1115–28.
- Erguven M, Yazihan N, Aktas E, Sabanci A, Li CJ, Oktem G, Bilir A. (2010). Carvedilol in glioma treatment alone and with imatinib in vitro. Int J Oncol 36:857–66.
- Fajardo G, Zhao M, Powers J, Bernstein D. (2006). Differential cardiotoxic/cardioprotective effects of beta-adrenergic receptor subtypes in myocytes and fibroblasts in doxorubicin cardiomyopathy. J Mol Cell Cardiol 40:375–83.
- Fajardo G, Zhao M, Berry G, Wong LJ, Mochly-Rosen D, Bernstein D. (2011). β2-adrenergic receptors mediate cardioprotection through crosstalk with mitochondrial cell death pathways. J Mol Cell Cardiol 51:781–9.
- Fitzgerald PJ. (2012). Beta blockers, norepinephrine, and cancer: an epidemiological viewpoint. Clin Epidemiol 4:151–6.
- Fuchs R, Schwach G, Stracke A, Meier-Allard N, Absenger M, Ingolic E, Haas HS, et al. (2015). The anti-hypertensive drug prazosin induces apoptosis in the medullary thyroid carcinoma cell line TT. Anticancer Res 35:31–8.
- Fukuhara K, Kvetnansky R, Weise VK, Ohara H, Yoneda R, Goldstein DS, Kopin IJ. (1996). Effects of continuous and intermittent cold (SART) stress on sympathoadrenal system activity in rats. J Neuroendocrinol 8:65–72.
- Furtado M, Katzman MA. (2015). Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res 229:37–48.
- Gao G, Sun J, Gao J, Xiong L, Yu L, Gao Y. (2013). Chronic stress promoted the growth of ovarian carcinoma via increasing serum levels of norepinephrine and interleukin-10 and altering nm23 and NDRG1 expression in tumor tissues in nude mice. Biosci Trends 7:56–63.
- Gerra G, Zaimovic A, Mascetti GG, Gardini S, Zambelli U, Timpano M, Raggi MA, Brambilla F. (2001). Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology 26:91–107.
- Goldstein DS, Kopin IJ. (2008). Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul 42:111–19.
- Grkovic T, Whitson EL, Rabe DC, Gardella RS, Bottaro DP, Linehan WM, McMahon JB, et al. (2011). Identification and evaluation of soft coral diterpenes as inhibitors of HIF-2α induced gene expression. Bioorg Med Chem Lett 21:2113–15.
- Guan XM, Amend A, Strader CD. (1995). Determination of structural domains for G protein coupling and ligand binding in beta 3-adrenergic receptor. Mol Pharmacol 48:492–8.
- Hering D, Lachowska K, Schlaich M. (2015). Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Curr Hypertens Rep 17:80.
- Hieble JP, Bondinell WE, Ruffolo RR Jr. (1995). Alpha- and beta-adrenoceptors: from the gene to the clinic. 1. Molecular biology and adrenoceptor subclassification. J Med Chem 38:3415–44.
- Hudecova S, Sedlakova B, Kvetnansky R, Ondrias K, Krizanova O. (2010). Modulation of the sodium-calcium exchanger in the rat kidney by different sequential stressors. Stress 13:15–21.
- Jansen L, Hoffmeister M, Arndt V, Chang-Claude J, Brenner H. (2014). Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer 120:1178–86.
- Ji Y, Chen S, Xu C, Li L, Xiang B. (2015). The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. Br J Dermatol 172:24–32.
- Jochmanová I, Yang C, Zhuang Z, Pacak K. (2013). Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst 105:1270–83.
- Jochmanová I, Zhuang Z, Pacak K. (2015). Pheochromocytoma: gasping for air. Horm Cancer 6:191–205.
- Kaapu KJ, Ahti J, Tammela TL, Auvinen A, Murtola TJ. (2015). Sotalol, but not digoxin is associated with decreased prostate cancer risk: a population-based case-control study. Int J Cancer 137:1187–95.
- Kang F, Ma W, Ma X, Shao Y, Yang W, Chen X, Li L, Wang J. (2014). Propranolol inhibits glucose metabolism and F-18-FDG uptake of breast cancer through posttranscriptional downregulation of hexokinase-2. J Nucl Med 55:439–45.
- Khalili A, Hassan ZM, Shahabi S, Pourfathollah AA, Ostad SN, Noori S, Mahdavi M, et al. (2013). Long acting propranolol and HSP-70 rich tumor lysate reduce tumor growth and enhance immune response against fibrosarcoma in Balb/c mice. Iran J Immunol 10:70–82.
- Kim YS, Kang SH, Song KY, Cho ML, Her YM, Huh JW, Lee J. (2013). The immunomodulatory role of esmolol in patients undergoing laparoscopic gastrectomy due to gastric cancer. Anaesthesia 68:924–30.
- Kozanoglu I, Yandim MK, Cincin ZB, Ozdogu H, Cakmakoglu B, Baran Y. (2013). New indication for therapeutic potential of an old well-known drug (propranolol) for multiple myeloma. J Cancer Res Clin Oncol 139:327–35.
- Krizanova O, Holotnakova T, Jurkovicova D, Polakova E, Zahradnikova A, Lacinova L, Kvetnansky R, et al. (2008). Type 1 and 2 IP3 receptors respond differently to catecholamines and stress. Ann N Y Acad Sci 1148:331–7.
- Kvetnansky R, Mikulaj L. (1970). Adrenal and urinary catecholamines in rats during adaptation to repeated immobilization stress. Endocrinology 87:738–43.
- Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, Arevalo JM, et al. (2012). Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun 26:635–41.
- Lamkin DM, Sung HY, Yang GS, David JM, Ma JC, Cole SW, Sloan EK. (2015). α2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology 51:262–70.
- Lamy S, Lachambre MP, Lord-Dufour S, Béliveau R. (2010). Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vasc Pharmacol 53:200–8.
- Laukova M, Tillinger A, Novakova M, Krizanova O, Kvetnansky R, Myslivecek J. (2014). Repeated immobilization stress increases expression of β3 -adrenoceptor in the left ventricle and atrium of the rat heart. Stress Health 30:301–9.
- Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Endocrine Society, et al. (2014). Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99:1915–42.
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. (2005). Phaeochromocytoma. Lancet 366:665–75.
- Li P, Guo Z, Gao Y, Pan W. (2015). Propranolol represses infantile hemangioma cell growth through the beta 2-adrenergic receptor in a HIF-1 alpha-dependent manner. Oncol Rep 33:3099–107.
- Li S, Sun Y, Gao D. (2013). Role of the nervous system in cancer metastasis. Oncol Lett 5:1101–11.
- Liao X, Che X, Zhao W, Zhang D, Bi T, Wang G. (2010a). The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep 24:1669–76.
- Liao X, Che X, Zhao W, Zhang D, Long H, Chaudhary P, Li H. (2010b). Effects of propranolol in combination with radiation on apoptosis and survival of gastric cancer cells in vitro. Radiat Oncol 5:98.
- Lin CS, Lin WS, Lin CL, Kao CH. (2015). Carvedilol use is associated with reduced cancer risk: a nationwide population-based cohort study. Int J Cardiol 184:9–13.
- Liu X, Wu WK, Yu L, Sung JJ, Srivastava G, Zhang ST, Cho CH. (2008). Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J Cell Biochem 105:53–60.
- Lu YJ, Geng ZJ, Sun XY, Li YH, Fu XB, Zhao XY, Wei B. (2015). Isoprenaline induces epithelial-mesenchymal transition in gastric cancer cells. Mol Cell Biochem 408:1–13.
- Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Zimmerman MB, et al. (2012). Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol 30:2885–90.
- Lutgendorf SK, Johnsen EL, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. (2002). Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer 95:808–15.
- Martucci VL, Pacak K. (2014). Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment. Curr Probl Cancer 38:7–41.
- McCarty MF. (2014). A role for cAMP-driven transactivation of EGFR in cancer aggressiveness – therapeutic implications. Med Hypotheses 83:142–7.
- Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, Zambon P, et al. (2009). The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer 45:1017–27.
- Moreno-Smith M, Lee SJ, Lu C, Nagaraja AS, He G, Rupaimoole R, Han HD, et al. (2013). Biologic effects of dopamine on tumor vasculature in ovarian carcinoma. Neoplasia 15:502–10.
- Moreno-Smith M, Lu C, Shahzad MM, Pena GN, Allen JK, Stone RL, Mangala LS, et al. (2011). Dopamine blocks stress-mediated ovarian carcinoma growth. Clin Cancer Res 17:3649–59.
- Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, Cecchi R, Chiarugi P. (2013). β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest 93:279–90.
- Nagaraja AS, Dorniak PL, Sadaoui NC, Kang Y, Lin T, Armaiz-Pena G, Wu SY, et al. (2016). Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene. 35:2390–7.
- Nkontchou G, Aout M, Mahmoudi A, Roulot D, Bourcier V, Grando-Lemaire V, Ganne-Carrie N, et al. (2012). Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev Res (Phila) 5:1007–14.
- Noda H, Miyaji Y, Nakanishi A, Konishi F, Miki Y. (2007). Frequent reduced expression of alpha-1B-adrenergic receptor caused by aberrant promoter methylation in gastric cancers. Br J Cancer 96:383–90.
- Nosrati A, Wei ML. (2014). Sex disparities in melanoma outcomes: the role of biology. Arch Biochem Biophys 563:42–50.
- Obeid EI, Conzen SD. (2013). The role of adrenergic signaling in breast cancer biology. Cancer Biomark 13:161–9.
- Pacak K, Palkovits M. (2001). Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–48.
- Pacak K. (2011). Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul 45:65–90.
- Palm D, Lang K, Niggemann B, Drell TL 4th, Masur K, Zaenker KS, Entschladen F. (2006). The norepinephrine driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer 118:2744–9.
- Pan W, Li P, Guo ZT, Huang Q, Gao Y. (2015). Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr Blood Cancer 62:1414–20.
- Papadopoulou A, Siamatras T, Delgado-Morales R, Amin ND, Shukla V, Zheng YL, Pant HC, et al. (2015). Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl Psychiatry 5:e578.
- Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, Gayoso-Diz P, Otero-Rey EM, Gándra-Rey JM, García-García A. (2012). Activity of β2-adrenergic receptor in oral squamous cell carcinoma is mediated by overexpression of the ADRBK2 gene: a pilot study. Biotech Histochem 87:179–86.
- Persengiev SP, Green MR. (2003). The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis 8:225–8.
- Philipp M, Brede M, Hein L. (2002). Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol – Regul Integr Comp Physiol 283:287–95.
- Porcel Chacon R, del Boz Gonzalez J, Navarro Morón J. (2015). Delayed-onset of multiple cutaneous infantile hemangiomas due to propranolol: a case report. Pediatrics 135:1064–6.
- Powe DG, Voss MJ, Habashy HO, Zänker KS, Green AR, Ellis IO, Entschladen F. (2011). Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat 130:457–63.
- Ragan AR, Lesniak A, Bochynska-Czyz M, Kosson A, Szymanska H, Pysniak K, Gajewska M, et al. (2013). Chronic mild stress facilitates melanoma tumor growth in mouse lines selected for high and low stress-induced analgesia. Stress 16:571–80.
- Sardi I, Giunti L, Bresci C, Buccoliero AM, Degl'innocenti D, Cardellicchio S, Baroni G, et al. (2013). Expression of β-adrenergic receptors in pediatric malignant brain tumors. Oncol Lett 5:221–5.
- Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A, Carson JP, et al. (2007). Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem 282:14094–100.
- Scheuermann TH, Li Q, Ma HW, Key J, Zhang L, Chen R, Garcia JA, et al. (2013). Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat Chem Biol 9:271–6.
- Schoder H, Silverman DH, Campisi R, Sayre JW, Phelps ME, Schelbert HR, Czernin J. (2000). Regulation of myocardial blood flow response to mental stress in healthy individuals. Am J Physiol – Heart Circ Physiol 278:360–6.
- Schuller HM, Plummer HK 3rd, Bochsler PN, Dudric P, Bell JL, Harris RE. (2001). Co-expression of beta-adrenergic receptors and cyclooxygenase-2 in pulmonary adenocarcinoma. Int J Oncol 19:445–9.
- Schuller HM, Al-Wadei HA, Ullah MF, Plummer HK 3rd. (2012). Regulation of pancreatic cancer by neuropsychological stress responses: a novel target for intervention. Carcinogenesis 33:191–6.
- Selye H. (1985). The nature of stress. Basal Facts 7:3–11.
- Seyedi S, Andalib A, Rezaei A, Hosseini SM, Mohebbi SR, Zali MR, Vafai M, et al. (2012). The effects of isoproterenol and propranolol on cytokine profile secretion by cultured tumor-infiltrating lymphocytes derived from colorectal cancer patients. Cell J 13:281–93.
- Shang ZJ, Liu K, Liang de F. (2009). Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med 38:371–6.
- Strange KS, Kerr LR, Andrews HN, Emerman JT, Weinberg J. (2000). Psychosocial stressors and mammary tumor growth: an animal model. Neurotoxicol Teratol 22:89–102.
- Sun Y, Wang Y, Zhang L, Xu C, Liu Y, Kang S, Yan C, et al. (2014). Prevention of cardiac events caused by surgical stress in aged rats: simultaneously activating β2-adrenoceptor and inhibiting β1-adrenoceptor. Stress 17:373–81.
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, et al. (2006). Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12:939–44.
- Tu JB, Ma RZ, Dong Q, Jiang F, Hu XY, Li QY, Pattar P, Zhang H. (2013). Induction of apoptosis in infantile hemangioma endothelial cells by propranolol. Exp Ther Med 6:574–8.
- Vazquez SM, Mladovan AG, Perez C, Bruzzone A, Baldi A, Luthy IA. (2006). Human breast cell lines exhibit functional alpha2-adrenoceptors. Cancer Chemother Pharmacol 58:50–61.
- Wolter JK, Wolter NE, Blanch A, Partridge T, Cheng L, Morgenstern DA, Podkowa M, et al. (2014). Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 5:161–72.
- Wolter NE, Wolter JK, Enepekides DJ, Irwin MS. (2012). Propranolol as a novel adjunctive treatment for head and neck squamous cell carcinoma. J Otolaryngol Head Neck Surg 41:334–44.
- Xie H, Li C, He Y, Griffin R, Ye Q, Li L. (2015). Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol 51:991–7.
- Xie W, Xie H, Liu F, Li W, Dan J, Mei Y, Dan L, et al. (2013). Propranolol induces apoptosis of human umbilical vein endothelial cells through downregulation of CD147. Br J Dermatol 168:739–48.
- Xu T, Xiao X, Zheng S, Zheng J, Zhu H, Ji Y, Yang S. (2013). Antiangiogenic effect of propranolol on the growth of the neuroblastoma xenografts in nude mice. J Pediatr Surg 48:2460–5.
- Yan L, Vatner SF, Vatner DE. (2014). Disruption of type 5 adenylyl cyclase prevents β-adrenergic receptor cardiomyopathy: a novel approach to β-adrenergic receptor blockade. Am J Physiol Heart Circ Physiol 307:1521–8.
- Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, et al. (2006). Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 66:10357–64.
- Yee EM, Pasquier E, Iskander G, Wood K, Black DS, Kuman N. (2013). Synthesis of novel isoflavene-propranolol hybrids as anti-tumor agents. Bioorg Med Chem 21:1652–60.
- Zhang D, Ma Q, Shen S, Hu H. (2009). Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist's anticancer effect in pancreatic cancer cell. Pancreas 38:94–100.
- Ziolkowski N, Grover AK. (2010). Functional linkage as a direction for studies in oxidative stress: alpha-adrenergic receptors. Can J Physiol Pharmacol 88:220–32.
