Abstract
In rodents, chronic social defeat stress promotes deficits in social interest and social interaction. We further explored these antisocial effects by comparing the consequences of two different defeat stress protocols (episodic vs. continuous stress) in a social investigation test. We expected that continuous, but not episodic, stress would induce social deficits in this model. Furthermore, we tested whether a potentially anxiolytic dose of ethanol reverses social deficits induced by defeat stress. Male Swiss mice were exposed to a 10-day social defeat protocol, using daily confrontations with an aggressive resident mouse. Episodic stress consisted of brief defeat episodes, after which the defeated mouse was returned to its home cage, until the next defeat 24 h later (n = 7–11/group). For continuous stress, similar defeat episodes were followed by cohabitation with the aggressive resident for 24 h, separated by a perforated divider, until the following defeat (n = 8–14/group). Eight days after stress termination, defeated and control mice were assessed in a social investigation test, after treatment with ethanol (1.0 g/kg, i.p.) or 0.9% saline. Considering the time spent investigating a social target, mice exposed to episodic or continuous social stress showed less social investigation than controls (p < .05). Deficits in social interest were not reversed by acute ethanol treatment. However, ethanol reduced time spent in social interaction in one control group (p < .05). Locomotor activity was not affected by social stress or ethanol. Thus, a history of social defeat stress, whether episodic or continuous, promotes deficits in social investigation that were not reversed by acute treatment with ethanol.
Introduction
Chronic exposure to stressful events enhances vulnerability to neuropsychiatric disorders, including anxiety and mood disorders, as well as increased risk for drug abuse (Sinha, Citation2001, Citation2008). Currently, the main source of stressful stimuli in humans stems from social adversities (Martinez et al., Citation1988; Ruis et al., Citation1999; Sinha, Citation2008). In rodents, repeated exposure to social defeat stress promotes behavioral and neurobiological consequences, such as increased anxiety-like behavior, anhedonia, deficits in social interaction and altered responses to drugs of abuse (Avgustinovich et al., Citation1997; Citation2005; Berton et al., Citation2006; Hammels et al., Citation2015; Miczek et al., Citation2008; Norman et al., Citation2015; Rygula et al., Citation2005; Vasconcelos et al., Citation2015; Venzala et al., Citation2012; Yu et al., Citation2011).
However, two different protocols of social defeat stress can induce opposite behavioral consequences, which are accompanied by different neurobiological alterations (Miczek et al., Citation2011). Hence, rats exposed to continuous social stress present anxiety-like behavior, an anhedonic response toward a sucrose solution, and reduced cocaine intake (Miczek et al., Citation2011). In contrast, brief, intermittent defeat episodes produce no anhedonia, no change in exploratory behavior, and increased cocaine consumption (Miczek et al., Citation2011). The main difference between the protocols (continuous vs. episodic social stress) concerns the period of sensory contact with the aggressive resident, after each defeat episode (24 h in continuous stress; 5 min in episodic stress). (Golden et al., Citation2011; Yap et al., Citation2005).
Social inhibition, or social avoidance, is one of the most important and impairing consequences of chronic exposure to social stress, as described by Kudryavtseva et al. (Citation1991) and much explored more recently (Bagot et al., Citation2015; Berton et al., Citation2006; Golden et al., Citation2011). Interestingly, cohabitation with the aggressive resident seems to be required for social deficits to arise (Challis et al., Citation2013). Challis et al. (Citation2013) reported reduced social interest in C57BL6 mice exposed to continuous social stress, but not in defeated mice not exposed to further sensory contact with the aggressive resident. Social avoidance has been shown to persist for at least 4 weeks, after 10 days of continuous social stress in mice (Berton et al., Citation2006).
Social inhibition and deficits in social interest are considered to be symptoms of mood and anxiety disorders, and may be reversed by antidepressants (Berton et al, Citation2006) and anxiolytic drugs, including ethanol (File, Citation1980; File & Hyde, Citation1978). In low to moderate doses, ethanol reduces anxiety-like behavior in different behavioral tests (File, Citation1980; File & Hyde, Citation1976; Kameda et al., Citation2007; Sakharkar et al., Citation2014; Stewart et al., Citation1993). However, bidirectional effects of ethanol administration on social behavior may be observed depending on age of the animals, previous manipulations (e.g. stress), and testing conditions (Hilakivi & Lister, Citation1989; Varlinskaya & Spear, Citation2002, Citation2012). The aim of this study was to compare in male mice the consequences of two protocols of social defeat (episodic vs. continuous) on a social investigation test, and to reverse defeat-induced social deficits with acute ethanol treatment. We hypothesized that continuous, but not episodic, stress would induce long-term deficits in social interest in this model.
Methods
Animals
Male Swiss albino mice were obtained from CEDEME (Universidade Federal de São Paulo, Brazil), arriving at 8–10 weeks old (approximate body weight of 35 g at arrival). Mice (n = 190 in total, including experimental mice, residents, and stimulus mice) were individually housed, except when noted in the protocols below, and kept in a temperature controlled room (21 ± 2 °C) and a 12 h/12 h light–dark cycle (lights on at 07:00 h and off at 19:00 h). Water and food were available ad libitum at all times. All manipulations and experiments were conducted during the light phase (between 11:00 h and 16:00 h). All experiments were previously approved by the Ethics Committee on Animal Use at the Universidade Federal de São Paulo (CEUA #8964170714).
Behavioral procedures
Social defeat stress
Male resident mice (n = 30) were housed in pairs with a female (1 resident male and 1 female per cage) and acclimated for 3 weeks before being trained for aggressive behavior. During training, younger, smaller, and nonaggressive male naïve mice (n = 30) were introduced into the home cage of the residents, after removal of the female, and residents could threaten, pursue, and attack the male intruders, as previously described (Yap et al., Citation2005). Training for aggressive behavior was carried out for 5–9 sessions, every other day, until aggressive residents expressed stable levels of aggression toward stimulus mice (variation in attack bites <15% over three consecutive sessions).
Episodic defeat stress
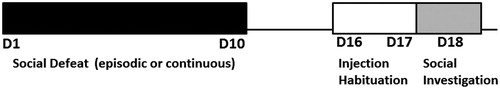
Experimental male mice from both stress and control groups were individually housed for approximately 10 days prior to the experiment. Mice in the episodic stress group were submitted to defeat episodes with a maximum duration of 15 min. Defeat consisted of a 5-min period of instigation and threats in the home cage of the aggressive resident, where the experimental mouse was physically separated from the resident by a perforated divider. Next, the phase of physical confrontation occurred, in which the resident typically pursues, attacks, and bites the intruder, until the intruder assumes a submissive posture (for four consecutive seconds) or until 5-min has elapsed. The confrontation is then interrupted by returning the perforated divider into the cage, once again separating the resident and the defeated mouse, allowing for another 5 min of psychological threats (Tornatzky & Miczek, Citation1993; Yap et al., Citation2005). Episodically defeated mice were then returned to their home cages, until the next defeat session, 24 h later. For each confrontation, the experimental mouse faced a different aggressive resident, for a total of 10 defeats during 10 days. Episodic control mice were individually housed during the entire protocol, being handled daily for weighing. shows the experimental timeline.
Continuous defeat stress
In this protocol, the intruder was defeated daily, each day by a different resident, but remained in the resident’s home cage for 24 h until the following defeat, for a total of 10 days. Control mice for the continuous defeat stress protocol were housed in pairs with another male control, also separated by perforated dividers (similar conditions to the residents’ cages), during the 10-day procedure. The pairs of continuous control mice were switched and rotated every 24 h, as described by Golden et al. (Citation2011). At the end of the defeat protocol, experimental and control continuous mice were returned to individual housing until termination of the experiment.
Social investigation test
Eight days after termination of the social defeat protocol (episodic or continuous defeat stress), mice were submitted to a social investigation test, as shown in . Each mouse was placed in an open-field apparatus (42 × 42 cm), and behavior was monitored with a video tracking system (EthoVision XT, Noldus, the Netherlands). The open-field arena contained a cylindrical wire mesh cage (10 × 6.5 cm) in which a non-familiar naïve male mouse was placed (as social target). Mice used as social targets were young adults (∼8 weeks old), group-housed, with no previous aggressive experience. Initially, the experimental mouse was placed in the arena for exploration for 2.5 min, with the empty interaction cage (no social target). Then, experimental mice were removed from the apparatus and received an i.p. injection of 6.7 ml/kg of saline (NaCl 0.9% w/v), or ethanol (15% w/v in 0.9% saline, 1.0 g/kg; Ethanol, Synth®, Brazil) and were kept in their home cages for 5 min, to allow for alcohol effects to start to emerge, as previously reported (Quadros et al., Citation2002). Each mouse was then placed again in the open field, with the presence of a social target within the interaction wire cage, for another 2.5 min (similar to procedures described by Berton et al., Citation2006; Golden et al., Citation2011). An imaginary ring of 5 cm around the interaction cage was considered as the “interaction zone”. We were interested in long-term consequences of chronic social stress, and for this reason, we opted to test mice 8 days after termination of defeat stress. Furthermore, we have observed short-term locomotor impairment in stressed mice in our laboratory (unpublished data), which could potentially confound the results if mice were tested within 24 h post-stress.
Choice of ethanol dose for the experiments
Pilot tests were carried out to test the effects of different doses of ethanol on the social investigation test, using group-housed naïve mice. Mice were tested for a range of ethanol doses (from 1.0 g/kg up to 2.0 g/kg, i.p.) and behaviors were recorded as reported above. The dose of 1.0 g/kg was chosen because it did not induce nonspecific motor effects (such as locomotor stimulation), and seemed to modestly enhance social investigation (number of mice tested with each ethanol dose averaged 4–5 mice/dose, which was not sufficient for reliable statistical analysis; data not shown). Furthermore, previous studies in our laboratory showed that this dose of ethanol reduced anxiety-like behavior in an elevated plus maze test (unpublished data).
Statistical analyses
Behavioral parameters measured during the social investigation test (including time spent in the interaction zone, distance moved (cm), number of entries into the interaction zone, and average duration of visits to the interaction zone) were analyzed with three-way ANOVAs with repeated measures. Factors were group (stress, control), treatment (saline vehicle, ethanol), and test as the repeated measure (no target test; social target test). Data were checked for normal distribution using the Kolmogorov–Smirnov test (p > .05). When significant effects were detected by the ANOVA for any of the factors analyzed, post hoc Newman Keuls tests were run. Due to the complexity of the analysis design, we decided to run unprotected post hoc tests for the interaction between the three factors (group × treatment × test), whenever at least two effects (or interactions) were significant. Additionally, Cohen’s d test for effect size was run to estimate the strength of the effects indicated by the unprotected post hoc tests (“d” values above 0.8 are considered large effects; values between 0.5 and 0.8 are considered moderate; and below 0.5, small). The level of statistical significance was established at 5%.
Results
Experiment 1: consequences of repeated episodes of defeat stress on the social investigation test: effects of ethanol
In the current social investigation test, time spent in the interaction zone in the presence of the social target was used as an index of social approach and social interest (Berton et al., Citation2006; Golden et al., Citation2011). For this parameter, a three-way ANOVA detected the following effects: group (stress < controls; F[1,33] = 5.28, p = .028), treatment (ethanol < saline; F[1,33] = 6.21, p = .018), as well as a test × treatment interaction (F[1,33] = 5.24, p = .028); there were 7–11 mice per treatment condition. The post hoc analysis for the three-factor interaction revealed that only control mice treated with saline presented increased time in the interaction zone in the presence of a social target relative to the “no target” condition (p = .006, d = 1.79, ), while all other groups showed no differences. During the social target test, groups exposed to defeat stress and ethanol-treated controls showed reduced time in the interaction zone relative to saline-treated controls (p < .01, d values ranged from 1.48 to 1.84 in each comparison). No differences were observed in the number of visits to the interaction zone ().
Figure 2. Behavioral assessment during the social investigation test after episodic defeat stress. Mice were exposed to episodic defeat stress for 10 days, and treated with 1.0 g/kg ethanol or saline (i.p.) prior to the social investigation test (n = 7–11/group), 8 days post-stress. Behaviors were recorded in the absence (“no target”) or presence (“target”) of a social target inside a wire interaction cage. Mice received ethanol/saline administration immediately after the “no target” test and 5 min prior to the “target” test, thus mice were not under ethanol effects during the “no target” test. The duration of the test was 150 s for each condition. Behavioral parameters were analyzed by three-way ANOVAs with repeated measures (factors “group”, “treatment”, and “test” as the repeated measure). (A) Time spent (s) in the 5-cm interaction zone around the interaction cage; (B) Number of entries into the interaction zone; (C) Average duration of visiting bouts to the interaction zone (s/entry); (D) Distance moved (cm). Results shown as means ± SEM. *p < .05, difference between tests (target vs. no target); #p < .05, relative to episodic control saline group, during the target test.
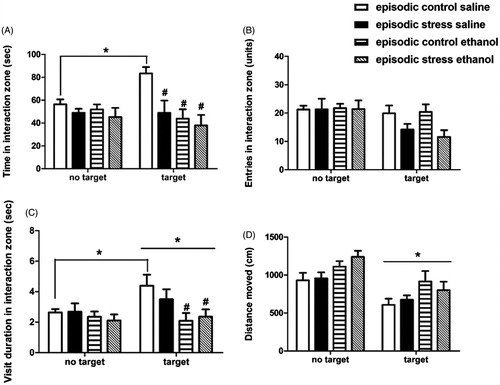
Next, we calculated the average duration of visits to the interaction zone (total time in interaction zone/number of entries; ). A treatment effect (ethanol < saline; F[1,33] = 6.67, p = .013), a test effect (target > no target; F[1,33] = 4.96, p = .033) and a test × treatment interaction (F[1,33] = 5.06, p = .031) were detected. The post hoc analysis for the three-factor interaction revealed that only saline-treated controls showed increased visiting bouts in the presence of a social target, relative to the no target trial (p = .022, d = 1.08). Further, in the target test, both ethanol-treated groups (control-ethanol and stress-ethanol) showed shorter visiting duration to the interaction zone, relative to saline-treated control mice (p < .05, d values ranged from 1.05 to 1.19).
The analysis of locomotor activity during the tests, detected a test effect (no target > target; F[1,33] = 36.28, p = .000001) and a treatment effect (ethanol > saline; F[1,33] = 7.07, p = .012). A general reduction in distance moved was observed when mice were tested in the presence of the social target (p = .000001). The ethanol treatment effect observed was not confirmed in the post hoc comparisons considering all factors (). There were no significant differences in body weight gain between stress (−0.20 g ± 0.73; n = 18) and control (0.85 g ± 0.38; n = 19) mice during the course of the social defeat protocol (calculated as weight on day 10 of stress minus pre-stress baseline weight; one-way ANOVA, n.s.).
Experiment 2: consequences of repeated exposure to continuous defeat stress on the social investigation test: effects of ethanol
For mice exposed to the continuous defeat stress procedure, there was a significant test effect (target > no target; F[1,39] = 14.3937, p = .0005) and a group × test interaction (F[1,39] = 9.4345, p = .004) for time spent in the interaction zone during the social investigation test. Post hoc analyses for the three-factors interaction showed that both continuous control groups (treated with saline or ethanol) presented increased time spent in the interaction zone in the presence of a social target, relative to the “no target” condition (p < .01, d values ranged from 1.0 to 1.07, ). The analysis also showed that ethanol-treated stress group presented decreased time spent in the interaction zone relative to the saline-treated control group, in the presence of a social target (p = .020, d = 1.32). Number of entries into the interaction zone was not affected by either group, treatment, or test factors (); there were 8–14 mice per treatment condition.
Figure 3. Behavioral assessment during the social investigation test after continuous defeat stress. Mice were exposed to continuous defeat stress for 10 days, and treated with 1.0 g/kg ethanol or saline (i.p.) prior to the social investigation test (n = 8–14/group), 8 days post-stress. Behaviors were recorded in the absence (“no target”) or presence (“target”) of a social target inside a wire interaction cage. Mice received ethanol/saline administration right after the “no target” test and 5 min prior to the “target” test, thus mice were not under ethanol effects during the “no target” test”. The duration of the test was 150 s for each condition. Behavioral parameters were analyzed with three-way ANOVAs with repeated measures (factors “group”, “treatment”, and “test” as the repeated measure). (A) Time spent (s) in the 5-cm interaction zone around the interaction cage; (B) Number of entries into the interaction zone; (C) Average duration of visiting bouts to the interaction zone (s/entry); (D) Distance moved (cm). Results shown as means ± SEM. *p < .05, difference between tests (target vs. no target); #p < .05, relative to continuous control saline group, during the target test.
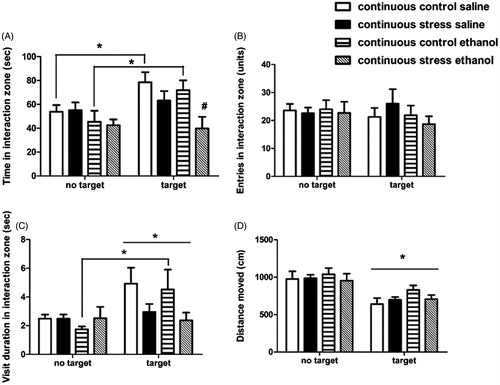
There was a significant test effect concerning the duration of visits to the interaction zone, with an overall increase in visiting duration in the presence of a social target (test effect: F[1,39] = 10.13; p = .003). A group × test interaction was also observed (F[1,39] = 7.9301; p = .007), showing that controls displayed increased visiting bout duration during the target test, relative to stressed mice (p = .005). The post hoc analysis for three-factors interaction showed that ethanol-treated controls presented increased visiting duration to the social target relative to the no target condition (p = .041, d = .99; with a trend for saline-treated controls, p = .07, d = .87). Differences in social interest were not due to locomotor effects, as only a test effect was found for distance moved (F[1,39] = 64.05 p = .00001), with a general reduction in locomotion during the social target test (). There were no significant differences in body weight gain between stress (−0.12 g ± 0.46, n = 23) and control (0.44 g ± 0.29, n = 20) mice during the course of social defeat stress protocol (calculated as weight on day 10 of defeat minus pre-stress baseline weight; one-way ANOVA, n.s.
Discussion
The current study replicates and expands the finding that mice exposed to chronic social defeat stress present social inhibition when allowed to explore a non-familiar non-aggressive conspecific (Berton et al., Citation2006; Kudryavtseva et al., Citation1991; Yu et al., Citation2011). Contrary to our hypothesis, no different outcomes emerged whether mice were exposed to repeated, brief episodes of social defeats by an aggressive mouse, or to defeats followed by continuous cohabitation with the aggressor. Both groups submitted to episodic or continuous defeat stress failed to show increased exploration of a stimulus mouse in a social investigation test. Unexpectedly, treatment with ethanol failed to increase social investigation in stressed mice. Instead, ethanol may have aggravated some stress-induced deficits, and also induced social inhibition in one of the control groups (episodic control group).
Our initial hypothesis was that episodic and continuous defeat stress would promote different outcomes in social investigation, similar to the study by Challis et al. (Citation2013). In that study, social aversion was only promoted when mice were submitted to a 20-min or 24-h cohabitation (sensory contact) with the aggressive resident after each defeat. When defeated mice were immediately returned to the home cage after defeats (absence of sensory contact post-defeat), no social deficits were observed (Challis et al., Citation2013). In our study, however, both social defeat protocols induced relevant social deficits in the social investigation test. Nonetheless, our protocol for episodic defeats included 5 min of sensory contact with the resident immediately after defeat, prior to returning to the home cage. Thus, it is possible that this 5-min sensory contact may be sufficient to promote long-term social deficits, at least in Swiss mice. In the study by Challis et al. (Citation2013), social investigation was assessed 24 h post-stress. Thus, requirements for a minimum period of sensory contact to promote social deficits may vary due to strain differences or to the interval between stress and testing.
In rats, another study also reported contrasting outcomes in behavioral and neurochemical effects by chronic exposure to episodic vs. continuous defeat stress (Miczek et al., Citation2011). In that case, however, stress protocols had discrepant durations (35 days for continuous stress vs. 10 days for episodic stress), which could account for some of the differential outcomes observed between the two protocols (Miczek et al. Citation2011). Our findings suggest that social behavior may be particularly sensitive to chronic defeat stress, since long-term impairment in social interest was observed after both episodic and continuous social stress protocols.
Most of the previous studies showing social avoidance after social defeat stress used C57BL6 mice as intruders, and Swiss-derived mice as resident aggressors and as social targets during the social investigation test (Avgustinovich et al., Citation1997; Golden et al., Citation2011; Hammels et al., Citation2015; Huhman et al., Citation2003; Venzala et al., Citation2012; Yu et al., Citation2011). In the present experiments, Swiss mice were used as both intruders and residents, and only naïve males were used as social targets during the social investigation test. Thus, during the social investigation test, there were no particular sensorial cues indicating that the social target was an aggressor, which could facilitate social avoidance by the defeated mouse. Our findings further confirm a more generalized form of social inhibition in defeated mice, which does not depend on the visual and/or olfactory recognition of a potential aggressor. Rather, defeated mice showed reduced social interest even toward naïve mice, with no prior aggressive experience. This is an important consideration, since the behavior of the social target can influence the behavioral response of the experimental mouse (Hammels et al., Citation2015; Krishnan et al., Citation2007).
In the present study, ethanol administration prior to the social investigation test failed to reverse social inhibition in mice with a history of episodic or continuous defeat stress. Defeated mice treated with ethanol showed no increases in exploration of the interaction cage in the presence of a social target. Thus, pro-social effects of ethanol were not observed in our tests. Indeed, in some behavioral parameters, ethanol treatment may have further aggravated the effects of social stress. Furthermore, in the continuous stress experiment, both saline- and ethanol-treated stressed mice failed to increase investigation of the cage in the presence of the social stimulus. However, only ethanol-treated stressed mice spent significantly less time in the interaction zone in the presence of the social stimulus, relative to saline-controls. In the episodic stress experiment, acute ethanol treatment per se produced social deficits in control mice, perhaps due to an interaction with prolonged social isolation (see below). Additionally, only ethanol-treated episodically stressed mice showed shorter visiting bouts to the social target relative to saline-controls. Thus, it seems that in some parameters, ethanol potentiated the negative effects of social stress. Since ethanol treatment can be considered a stressful stimulus, it is possible that such effects may be due to stress sensitization, in which previously defeated (or isolated) mice become more sensitive to effects of ethanol.
In several studies, ethanol has been shown to increase social investigation in rodents, but in a dose-, age-, and protocol-dependent manner (File & Hyde, Citation1978; File, Citation1980; Varlinskaya et al., Citation2001; Varlinskaya & Spear, Citation2002, Citation2012; Vetter-O’Hagen et al., Citation2009; Willey & Spear, Citation2014). In one study, social deficits induced by chronic restraint stress were reversed by 0.5 g/kg ethanol, but not other doses, in both adult and adolescent rats (Varlinskaya et al., Citation2010). In another case, pro-social effects of ethanol were only observed in adolescent rats, while a higher ethanol dose produced social aversion in adult, stressed rats (Varlinskaya & Spear, Citation2012). While the present study tested a single ethanol dose on social investigation, this dose was carefully chosen based on pilot studies and on the finding that doses of 1.6 g/kg and above already promote increases in locomotor activity in this line of mice (Macedo et al., unpublished). Nonetheless, testing of different ethanol doses in the current testing conditions would be desirable, and the use of a single ethanol dose is a limitation of the present study. However, it is possible that anxiolytic drugs such as ethanol are not effective in reversing social deficits promoted by social defeat stress. Chlordiazepoxide, a benzodiazepinic anxiolytic drug, also failed to improve social interaction after chronic defeat in mice (Berton et al., Citation2006).
Interestingly, the control groups for the different protocols were differentially affected by ethanol. Social investigation was reduced in control mice for episodic stress, while not affecting social interest in controls for the continuous defeat stress. This suggests that the different control conditions in the two protocols importantly affect sensitivity and response to ethanol treatment. This is likely due to different housing conditions during the 10-day stress procedure. Controls for episodic stress were maintained in social isolation during the stress protocol (as in Yap et al., Citation2005), whereas controls for continuous defeat stress were maintained in cohabitation with another control, separated by a perforated divider, and with changing cage mates every 24 h during the 10-day manipulation (Golden et al., Citation2011). Even though both control groups (as well as stressed groups) were individually housed prior to, and after the 10-day manipulation, the differences in housing during these 10 days seem to be relevant for effects of ethanol on social behavior.
Social isolation is recognized as a stressful condition that can promote social avoidance (Hermes et al., Citation2011; Hol et al., Citation1999) and increase anxiety-like behavior in other nonsocial tests, particularly in younger animals (Hermes et al., Citation2011; Weiss et al., Citation2004). Importantly, social isolation can change behavioral responses to drugs of abuse, including ethanol (Bardo et al., Citation2013). For example, in mice submitted to 10 days of social isolation, a dose of 0.8 g/kg of ethanol was shown to reduce social interaction (Hilakivi & Lister, Citation1989). Thus, antisocial effects of ethanol may have been triggered in non-defeated mice that were kept in social isolation for a more prolonged period of time. It is also possible that the lack of pro-social effects of ethanol in defeat-stressed mice may be associated with the individual housing conditions that mice experienced during several days in both protocols.
Conclusions
In summary, the present study shows that both episodic and continuous defeat stress promote deficits in social investigation, which are not attenuated by ethanol administration. In our conditions, administration of 1.0 g/kg of ethanol seemed to aggravate some stress-induced deficits in social behavior, and further promoted social avoidance in control episodic mice that were kept in isolated housing conditions for a longer period of time.
Acknowledgements
The authors would like to thank Liz Paola Domingues for scientific support and contributions.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Avgustinovich DF, Gorbach OV, Kudryavtseva NN. (1997). Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol Behav 61:37–43.
- Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. (2005). A model of anxious depression: persistence of behavioral pathology. Neurosci Behav Physiol 35:917–24.
- Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, Deisseroth K. (2015). Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun 6:7062.
- Bardo MT, Neisewander JL, Kelly TH. (2013). Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev 65:255–90.
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, et al. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–8.
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O. (2013). Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci 33:13978–88.
- File SE. (1980). The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods 2:219–38.
- File SE, Hyde JR. (1978). Can social interaction be used to measure anxiety?. Br J Pharmacol 62:19–24.
- File SE, Hyde J, Pool M. (1976). Effects of ethanol and chlordiazepoxide on social interaction in rats [proceedings]. Br J Pharmacol 58:465P.
- Golden SA, Covington HEIII, Berton O, Russo SJ. (2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6:1183–91.
- Hammels C, Pishva E, De Vry J, van der Hove DLA, Prickaerts J, van Winkel R, Selten JP, et al. (2015). Defeat stress in rodents: from behavior to molecules. Neurosci Biobehav Rev 59:111–40.
- Hermes G, Li N, Duman C, Duman R. (2011). Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav 104:354–9.
- Hilakivi LA, Lister RG. (1989). Effect of ethanol on the social behavior of group-housed and isolated mice. Alcohol Clin Exp Res 13:622–5.
- Hol T, Van den Berg CL, Van Ree JM, Spruijt BM. (1999). Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res 100:91–7.
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. (2003). Conditioned defeat in male and female Syrian hamsters. Horm Behav 44:293–9.
- Kameda SR, Frussa-Filho R, Carvalho RC, Takatsu-Coleman AL, Ricardo VP, Patti CL, Ribeiro RDA. (2007). Dissociation of the effects of ethanol on memory, anxiety, and motor behavior in mice tested in the plus-maze discriminative avoidance task. Psychopharmacology (Berl) 192:39–48.
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404.
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. (1991). Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav 38:315–20.
- Macedo GC, Suchecki D, Quadros IMH. Repeated social defeat and repeated ethanol: consequences on ethanol drinking and ethanol induced psychomotor effects in mice. Unpublished.
- Martinez M, Calvo-Torrent A, Pico-Alfonso MA. (1988). social defeat and subordination as models of social stress in laboratory rodents: a review. Aggr Behav 24:241–56.
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE III. (2011). Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci 31:9848–57.
- Miczek KA, Yap JJ, Covington HE III. (2008). Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120:102–28.
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA. (2015). Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology (Berl) 232:991–1001.
- Quadros IM, Hipólide DC, Frussa-Filho R, De Lucca EM, Nobrega JN, Souza-Formigoni MLO. (2002). Resistance to ethanol sensitization is associated with increased NMDA receptor binding in specific brain areas. Eur J Pharmacol 442:55–61.
- Ruis MAW, te Brake JHA, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. (1999). Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 24:285–300.
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. (2005). Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162:127–34.
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC. (2014). Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol 17:2057–67.
- Sinha R. (2001). How does stress increase risk of drug abuse and relapse?. Psychopharmacology (Berl) 158:343–59.
- Sinha R. (2008). Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–30.
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. (1993). Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol 10:1–10.
- Tornatzky W, Miczek KA. (1993). Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav 53:983–93.
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. (2010). Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav 96:228–35.
- Varlinskaya EI, Spear LP. (2002). Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res 26:1502–11.
- Varlinskaya EI, Spear LP. (2012). Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav 100:440–50.
- Varlinskaya EI, Spear LP, Spear NE. (2001). Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res 25:377–85.
- Vasconcelos M, Stein DJ. Almeida RMMD (2015). Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: a systematic review of the last decade. Trends Psychiatry Psychother 37:51–66.
- Venzala E, García-García AL, Elizalde N, Tordera RM. (2012). Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur Neuropsychopharmacol 23:697–708.
- Vetter-O’Hagen C, Varlinskaya E, Spear L. (2009). Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol 44:547–54.
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. (2004). Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res 152:279–95.
- Willey AR, Spear LP. (2014). Effects of ethanol on social approach and 50 kHz ultrasonic vocalization production in adolescent male Sprague‐Dawley rats. Dev Psychobiol 56:857–63.
- Yap JJ, Covington HE III, Gale MC, Datta R, Miczek KA. (2005). Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology (Berl) 179:230–9.
- Yu T, Guo M, Garza J, Rendon S, Sun XL, Zhang W, Lu XY, Cognitive, et al. (2011). Cognitive and neural correlates of depression-like behaviour in socially defeated mice: an animal model of depression with cognitive dysfunction. Int J Neuropsychopharmacol 14:303–17.