Abstract
Caring for offspring diagnosed with eating disorders (EDs) puts caregivers under high levels of chronic stress, which have negative consequences for their health. Unfortunately, caregivers have received little attention from mental health professionals. Chronic stress experienced by informal caregivers has been associated with the alteration of body homeostasis, and therefore, the functioning of various physiological systems. This could be the basis of health problems in informal caregivers of people with EDs. The main objective of this study was to analyze physiological response, in terms of heart rate (HR) and heart rate variability (HRV), to an acute laboratory stressor in a sample of informal caregivers of individuals with anorexia nervosa (n = 24) compared to a sample of noncaregivers (n = 26). In addition, the relationship between depressive mood and the aforementioned cardiovascular response parameters was analyzed in the group of caregivers. Caregivers had higher high-frequency (HF) power HRV, and lower HR, low-frequency (LF) power HRV and LF/HF ratio values than noncaregivers, which suggests lower cardiovascular reactivity to the acute stressor than noncaregivers. Moreover, a blunted HR response to stress was associated with high depressive mood scores in caregivers. Hence, it seems that the worse the mood the lower the cardiovascular reactivity to stressful events in this population. Developing and implementing psychotherapeutic interventions focused on stress management would help caregivers to reduce their stress levels and cope more effectively with stressors.
Introduction
Caring for offspring diagnosed with eating disorders (EDs) puts caregivers under high levels of chronic stress, which have negative consequences for their health (Chadda, Citation2014). Unfortunately, family caregivers have been paid little attention by mental health professionals; this is despite the fact that several studies have reported that such caregivers tend to experience considerable stress and burden, and need help in coping with it.
EDs in Western countries affect up to 3% of the population, 95% of those affected being women between the ages of 12 and 25 years old (Su et al., Citation2016). These disorders, namely anorexia nervosa, bulimia nervosa and binge eating disorder, are characterized by abnormalities in food intake behaviors and/or the presence of behaviors related to weight control, generating a range of health problems together with an alteration of the individual’s psychosocial functioning (Krassas, Citation2003). A person with this kind of psychological illness often needs help and support, family members being the primary caregivers of people with EDs in most of the Western world (Treasure et al., Citation2001).
Family caregivers play multiple roles in the care of individuals with EDs, including providing day-to-day care, supervising medications, taking the patient to medical appointments and looking after their financial needs. The daily challenges associated with this care can seriously affect caregivers’ health and quality of life. Caregivers of people with EDs have reported poorer quality of life and mental health, lower levels of vitality and worse mood than noncaregivers (Alda, Espina & Ortego, Citation2006; Highet, Thompson, & Schmidt, 2008; Sepúlveda, López, Todd, Whitaker, & Treasure, Citation2008).
In particular, caregivers may experience a number of changes in various physiological systems that may lead to health impairments (Corrêa et al., Citation2015). These changes may be adaptive in the short term, in that they allow the individual to deal with stress adaptively (alostasis), or maladaptive when these changes are prolonged over time (allostatic load) (McEwen & Gianaros, Citation2011). Allostatic loading, as a consequence of chronic stress, has been associated with a significant deterioration in health status and quality of life (Seeman, McEwen, Rowe, & Singer, Citation2001). Allostatic load can be measured in physiological systems as chemical imbalances in the autonomic nervous system (ANS) (McEwen & Gianaros, Citation2011), and these can be studied with two reliable indicators, namely the heart rate (HR) and heart rate variability (HRV). The HR provides relevant information about ANS functioning and HRV, measured as variation in the beat-to-beat interval, tends to reflect cardiovascular flexibility and its ability to respond and adjust to environmental (internal and external) demands (Fatisson, Oswald, & Lalonde, Citation2016). Therefore, measurements of HR and HRV may be very helpful to improve our understanding of the biological mechanisms that underlie deterioration in the health of caregivers of people with EDs.
An alteration in the functioning of the ANS increases the probability of negative health outcomes, especially in relation to cardiovascular disease (Tobaldini et al., Citation2017). Several studies in caregivers have assessed HRV response to acute stress, but their results are not consistent. For example, some studies have found that caregivers of people with cancer had higher sympathetic predominance at rest than noncaregivers (Lucini et al., Citation2008; Teixeira & Pereira, Citation2014), and one study found that caregivers of people with dementia had higher HR during a speech stressor (Von Känel, Dimsdale, Patterson, & Grant, Citation2003). On the other hand, another study found that caregivers of people with autism spectrum disorder had higher vagal tone than noncaregivers, and measures of the sympathovagal balance indicated lower than normal sympathetic activity in caregivers, but there were no significant differences in HR between groups (Ruiz-Robledillo, Bellosta-Batalla, & Moya-Albiol, Citation2015). Moreover, a previous study by the same group analyzed another marker of functioning of sympathetic branch of the ANS, namely electrodermal activity, in caregivers of people with EDs. This study showed that caregivers presented lower electrodermal response to acute stress than noncaregivers (Ruiz-Robledillo, Romero-Martínez, & Moya-Albiol, Citation2016). It is important to note that the chronic conditions differed between the aforementioned studies and this may have affected the caregivers’ HRV status. On the other hand, as far as we know, no studies have analyzed cardiovascular reactivity to acute stress in laboratory settings in caregivers of people with EDs.
HRV not only reflects physiological regulation, but also cognitive and emotional regulation (Appelhans & Luecken, Citation2008; Hamilton & Alloy, Citation2016; Thayer, Fredrikson, Sollers, & Wager, 2012). Several studies have demonstrated that individuals with greater resting HRV have greater emotion regulation and responsiveness, individuals with lower resting HRV having poorer emotion regulation abilities (Appelhans & Luecken, Citation2008; Thayer & Brosschot, Citation2005). As depression may be considered a disorder of emotion (Rottenberg, Citation2005) and is also marked by deficits in emotion regulation (Gotlib & Joormann, Citation2010), HRV has attracted interest in the context of depression. In particular, it has recently been reported that lower resting HRV is associated with depression (Hamilton & Alloy, Citation2016). However, the role of HRV reactivity in response to stress in depression remains less clear. Although the prevalence of depression is higher in caregivers than in noncaregivers (Romero-Martínez, Ruiz-Robledillo, & Moya-Albiol, Citation2016), there is a gap in the scientific literature analyzing the relationship of HR and HRV with depressive symptoms in caregivers, including in caregivers of people with EDs.
Given all the aforementioned results, the main aim of this study was to analyze the cardiovascular response (HR and HRV) to acute stress in the laboratory in informal caregivers of people with EDs compared with noncaregivers (caregivers of typically developing offspring). On the basis of previous research on electrodermal activity in caregivers of people with EDs (Ruiz-Robledillo et al., Citation2015), we hypothesized that caregivers would have a lower cardiovascular response to acute stress, showing lower sympathetic activation than noncaregivers (Carroll et al., Citation2005; Ruiz-Robledillo et al., Citation2015, Citation2016). We also aimed to examine the role of depressive mood in cardiovascular response to stress. Based on previous research in this field (Jandackova, Britton, Malik, & Steptoe, Citation2016), we expected to find depressive symptoms to be negatively associated with HR and HRV, especially in the case of the informal caregivers. We considered that evaluating the cardiovascular reactivity to experimentally induced acute stress, as a representation of usual stress reactivity to real-life events, could provide useful information to help us to understand the biological mechanisms of health disturbances in this population.
Method
Participants
The final sample was comprised of 52 participants, 24 caregivers (fathers [n = 11] and mothers [n = 13]) of offspring with EDs and 26 noncaregivers (fathers [n = 12] and mothers [n = 14]) who only cared for typically developing offspring. Parents of individuals with EDs were recruited from a hospital day care center specialized in EDs (Valencia, Spain). Parents participated voluntarily in the study and gave written informed consent in accordance with ethical principles regarding human research (Declaration of Helsinki). The study was approved by the University of Valencia’s Ethics Committee (code: H1360051962905).
The age of the offspring with anorexia nervosa ranged from 12 to 32 years (22.50 ± 5.77), with a sex ratio of 1 male to 23 females. These individuals were clinically diagnosed with anorexia nervosa by clinical staff, following the DSM-5 criteria.
Inclusion criteria for participating in the study were as follows: being a first-degree relative of an individual with a clinically diagnosed ED, living in the same home as this individual, and being his/her main caregiver for at least the last 2 years before the study. An adequate control group was selected, comprising nonadolescent fathers and mothers of healthy offspring without any chronic illnesses or dependence due to disability. Additionally, parents in the control group had not been caregivers for anyone with a chronic illness in the previous 2 years.
Procedure
Participants were instructed to abstain from eating, drinking stimulants (such as tea, coffee or alcohol), brushing their teeth, or smoking during the 2-h period before arriving at the laboratory. The experimental procedure was performed between 4:00 and 7:00 pm, in order to minimize hormonal and cardiovascular variations attributable to the circadian rhythm. Each session lasted approximately 2.5 h. After participants arrived, data were gathered on anthropometric characteristics (age, weight, height), and adherence to the instructions was checked.
Electrodes were attached and participants were encouraged to make themselves comfortable and relax for 10 min. After this period of habituation, participants completed psychological questionnaires to assess their prestress psychological states (anxiety and mood) and then baseline electrocardiogram signals were recorded for 10 min. General information regarding the stress stimuli and the evaluation of their performance during the stressor was then provided to the participants, and after that, participants remained silent for 8 min (preparatory period). After this preparatory period, participants were exposed to a psychosocial stressor consisting of a 20-min session in front of a committee of two men and three women performing a set of cognitive tasks (Stroop test, mirror-drawing test and arithmetic tasks). During this stressor period, a video camera was switched on to heighten the evaluative threat by simulating a recording, as suggested in previous studies (Dickerson, Gruenewald, & Kemeny, 2004). Following completion of the stressor, physiological measurements continued to be recorded during a 10-min recovery period. During this period, participants completed questionnaires to assess their poststress psychological states (anxiety and mood), and at the end of the period, they returned to the first room.
Finally, in a second session, participants completed the severe depression subscale of the shorter 28-item version of the General Health Questionnaire (GHQ-28). In addition, in the case of caregivers, researchers conducted an interview regarding the characteristics of the care recipient (diagnosis, gender, age, global activity and dependence rating) and the status of the caregiver (years since the definitive diagnosis of the care recipient, time spent caregiving per week, burden, level of worry regarding the future and the disorder).
Appraisal scores
The task and the outcomes obtained were assessed with ad hoc questions rated on a 10-point scale. Participants were asked about the stress that the task caused (“On a scale from 0 (no stress) to 10 (extreme stress), how much stress did you experience during the task?”). They also answered a series of questions related to satisfaction with the outcome (“On a scale from 0 (not at all) to 10 (highly), how satisfied are you with the outcome obtained in the task?”) and to their attribution for the outcome (internal and external locus of control) (“On a scale from 0 (not at all) to 10 (highly), how dependent do you feel the outcome of the task was on you, your cognitive abilities and your intelligence?” and “On a scale of 0 (not at all) to 10 (highly), how dependent do you feel the outcome of the task was on external factors, the events that occurred during the session, and the type of task?”).
Psychological response to task
Depressive mood states were measured using a suitably validated of the profile of mood states (POMS) (Balaguer, Fuentes, Meliá, & García-Merita, 1995). This questionnaire is composed of 29 items, rated on a 5-point Likert-type scale, grouped into five subscales (tension, depression, anger, vigor and fatigue) with a Cronbach’s alpha higher than 0.80. In this study, only the depression subscale was employed.
Depressive symptoms
Depressive mood was assessed with the severe depression subscale of the GHQ-28 designed by Goldberg and Hillier (Goldberg & Hillier, Citation1979). Items were ranked on a 4-point Likert scale, from 0 (better than usual) to 3 (worse than usual). Cronbach’s alpha was higher than 0.92.
Care recipient characteristics
To evaluate the offspring’s level of independence, caregivers answered the Barthel Index (Mahoney & Barthel, Citation1965) referring to their child. This questionnaire covers 12 different basic activities, including eating, showering and bladder control. The Cronbach’s α in this study was 0.79. Higher scores in this questionnaire indicate that the care recipient is more independent, with a maximum score of 100 indicating full independence.
The global activity of the care recipient was evaluated using the Global Assessment Scale (Endicott, Spitzer, Fleiss, & Cohen, Citation1976) with a reliability coefficient of 0.91. The scale is composed of 10 sentences resulting in a single score from 1 (severity of symptoms and risk of suicide attempt) to 100 (lack of symptoms).
Electrophysiological recordings in caregivers
A physiological recording system (BIOPAC Systems, Santa Barbara, CA) was used to capture, process and analyze electrocardiograms. This system was connected to a signal preamplifier UIM150 (Universal Interface Module) and this in turn was connected to a computer equipped with data acquisition hardware (MP150) and data storage software (AcqKnowledge 4.2 for Windows). Electrocardiogram recordings were visually screened and R-waves of problematic recordings with artifacts were deleted from the data file. HR was then calculated from the resultant file in accordance with published guidelines (Task Force, 1996). The electrocardiogram signal was transformed into a tachogram using the Acknowledge software. Fast Fourier transformation was then applied to the tachogram and the resultant power spectrum of the HRV was characterized in the frequency domain, considering the low frequency (LF) and high-frequency (HF) components, as well as the LF/HF ratio. Following the recommendations of the Task Force (1996), we expressed LF and HF powers in both absolute and normalized units, the normalized units reflecting the relative value of each component. LF power in normalized units (LFnu) is considered to be a marker of sympathetic activation only, while HF power in normalized units (HFnu) represents vagal activation (Burr, Citation2007). The normalized values, which always add up to 100 (LF + HF), are dominated by changes in the HF component. Hence, the measures are not independent and should not therefore be interpreted independently. The other commonly considered parameter describing HRV, the LF/HF ratio, indicates the differential sympathetic-vagal activation.
Data analysis
After assessing the normality of the data using the Shapiro–Wilk test (p < .05), non-normal data were log10 transformed. T-tests were performed with “group” (caregivers and noncaregivers) as the between-subject factor for anthropometric data (age and body mass index or BMI) and GHQ-28 severe depression score stratifying by gender. Chi-square statistics were calculated for analyzing the frequencies of the demographic variables.
HR and HRV (LF/HF ratio, LFnu and HFnu) changes in response to cognitive tasks were examined by repeated-measures analyses of variance (ANOVAs). Further, to explore “group” effects, repeated-measures ANCOVAs were performed with “time” (Baseline, Preparatory, Stressor and Recovery) as the within-subject factor, “group” as the between-subject factor and selected variables which differ between groups as covariates. Greenhouse-Geisser corrections for degrees of freedom were applied where appropriate. For significant results, partial eta-squared (ηp2) was reported as a measure of effect size.
For psychological states, ANOVAs for repeated measures (pre- and poststressor) were used with “period” as the within-subject factor and “group” as the between-subject factors. Moreover, to explore “group” effects, repeated-measures ANCOVAs were performed with “time” (pre- and poststressor) as the within-subject factor, “group” as the between-subject factor and selected variables which differ between groups as covariates.
The magnitude of the cardiovascular response was estimated by calculating the area under the curve with respect to the ground (AUCg), using formulae derived from the trapezoidal rule, as previously described (Pruessner, Kirschbaum, Meinlschmidt, & Hellhammer, 2003). These formulae are simple additions of triangular and rectangular areas.
Spearman’s or Pearson’s correlation coefficients were calculated to assess relationships between variables when appropriate for each group (caregivers and noncaregivers).
Data analyzes were carried out using IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY). p values < .05 were considered statistically significant, while p values >.05 but ≤.07 were considered to be close to significance. Average values are reported in tables as mean ± SD.
Results
Participant characteristics
Caregivers did not differ from noncaregivers in BMI or sociodemographic characteristics. In contrast, differences were observed in age t (45.83) = 4.23, p < .001, d = 1.22, with caregivers being older than noncaregivers. For this reason, this variable was included as covariate in the subsequent analysis.
Regarding depressive symptoms, ED caregivers obtained higher scores in the severe depression subscale of the GHQ-28 than noncaregivers, t (43.18) = 1.61, p = .05, d =1.27 ().
Table 1. Mean ± SD of age and body mass index and demographic variables for caregivers and non-caregivers and care recipient characteristics.
Appraisal scores
Caregivers had similar appraisal scores to controls for perceived stress (4.92 ± 2.60 and 5.04 ± 2.04, respectively), satisfaction (4.38 ± 2.01 and 5.23 ± 1.18, respectively) and internal (7.00 ± 2.72 and 6.58 ± 2.12, respectively) and external (3.42 ± 2.93 and 3.27 ± 2.20, respectively) control.
Effectiveness of the stressor in eliciting cardiovascular and psychological responses
The psychosocial stressor employed in this study was found to be effective, as indicated by the significant “time” effect on HR in the total sample, ɛ = .62; F(1.86, 91.21) = 28.88, p < .001, ηp2 = .37. Dividing the sample into groups, intragroup comparisons revealed a significant “time” effect in both caregivers, ɛ = .54, F(1.61, 37.17) = 11.50, p < .001, ηp2 = .33 and noncaregivers, ɛ = .77, F(2.31, 57.71) = 22.14, p < .001, ηp2 = .47. In caregivers, HR increased from resting to the preparatory period and from then to the tasks, subsequently decreasing until the recovery period (p < 0.05 for all comparisons). In noncaregivers, HR followed a similar pattern to that observed in the caregivers (p < .05 for all comparisons).
Further, the laboratory stressor was shown to be efficient in eliciting LFnu and HFnu alterations, the factor “time” being significant in the total sample, ɛ = .45, F(1.35, 66.16) = 94.31, p < .001, ηp2=.66; and ɛ =.46, F(1.35, 66.15) = 94.35, p < .001, ηp2 = .65, respectively. In all participants, LFnu decreased from baseline to the stressor period and increased from then to the recovery period. Additionally, in both groups, HFnu decreased from baseline to the preparatory period and increased from then to the recovery period.
Regarding the LF/HF ratio, a significant “time” effect was found in the total sample, ɛ = .77, F(2.30, 112.89) = 118.36, p < .001, ηp2 = .69, this remaining significant after dividing the sample into caregivers and noncaregivers, ɛ = .82, F(2.46, 56.68) = 31.87, p < .001, ηp2 = .58; and ɛ = .58, F(1.75, 46.62) = 105.93, p < .001, ηp2 = .81, respectively. The two groups followed a similar pattern: the LF/HF ratio decreased from baseline to the preparatory period, increased from then to the stressor period, and finally, decreased to the recovery period.
Significant differences were found between pre- and poststressor POMS depression scores, F(1,46) = 40.25, p < .001, ηp2 = .45, scores being higher after the stressor.
Differences between caregivers and controls in cardiovascular and psychological responses to acute stress
In the case of HR, a significant “time × group” interaction and “group” effects were found, ɛ = .64; F(1.94, 90.92) = 3.62, p = .032, ηp2 = .07; and F(1, 47) = 8.11, p = .007, ηp2 = .15, with caregivers having lower HR than non-caregivers at all time points assessed (p < .05) ().
Figure 1. Cardiovascular variables during resting, preparation, stressor and recovery times for groups (caregivers and non-caregivers). *p<.05.
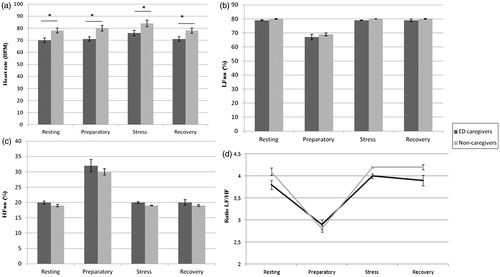
Analyzing powers in normalized units, a main effect of “group” was found for LFnu and HFnu, F(1, 47) = 5.34, p = .025, ηp2 = .10; and F(1, 47) = 5.33, p = .027, ηp2 = .10, respectively, caregivers showing lower LFnu () and higher HFnu () than noncaregivers. Further, a main effect of “group” was found in LF/HF ratio, F(1, 47) = 7.31, p= .010, ηp2 = .14, caregivers having a smaller ratio than noncaregivers ().
Concerning the magnitude of the response, a significant “group” effect was found in AUCg for HR, F(1, 47) = 7.57, p = .008, ηp2 = .14; LFnu, F(1, 47) = 6.72, p = .013, ηp2 = .13; HFnu, F(1, 47) = 8.53, p = .005, ηp2 = .15; and LF/HF ratio F(1, 47) = 8.87, p = .005, ηp2 = .16; with caregivers having lower HRs, LF/HF ratios and LFnu values but higher HFnu values than noncaregivers.
A significant “group” effect was found for POMS depression, F(1, 47) = 3.93, p = .05, ηp2 = .08, with caregivers (3.28 ± 0.74) obtaining higher depression scores than noncaregivers (1.10 ± 0.70). However, there was no difference between groups in the change in POMS depression scores.
Relationships of resting HR and AUC for cardiovascular variables with GHQ-28 severe depression and POMS depression scores
In caregivers, low resting HR and AUC were related to high GHQ-28 severe depression subscale scores, r= −.404, p < .05 and r = −.441, p < .05, respectively. In contrast, the rest of the cardiovascular variables were unrelated to GHQ-28 severe depression and POMS depression scores.
Regarding noncaregivers, none of the cardiovascular variables were related to GHQ-28 severe depression or POMS depression scores ().
Table 2. Relationships between cardiovascular parameters (HR, LF, HF and LF/HF ratio) with depression scores (GHQ-28 and POMS) in caregivers and noncaregivers. *p<.05.
Discussion
Informal caregivers of individuals with EDs had lower HRs, AUC HRs, LF HRV power (LFnu values) and LF/HF ratios but higher HF HRV powers (HFnu values) than non-caregivers. With respect to depressive state and trait, caregivers had higher depressive mood and symptoms than noncaregivers. Finally, comparing cardiovascular measures and depressive traits revealed that low resting HR and AUC were associated with high depressive symptom scores in caregivers but not in controls.
Several studies have reported that HR tends to increase with increased sympathetic input (Carrillo et al., Citation2001; Cyr et al., Citation2009; Hjortskov et al., Citation2004; Lucini et al., Citation2008; Teixeira & Pereira, Citation2014). Hence, low HR reactivity could be partially explained by low sympathetic and high parasympathetic inputs (Brindle et al., Citation2017; Kemp et al., 2010). In our study, caregivers had higher HFnu values, and lower LFnu values and LF/HF ratios than noncaregivers, which may indicate elevated vagal tone. The LF/HF ratio being lower in caregivers than controls suggests that caregivers have a higher than normal level of parasympathetic activity and reciprocally lower sympathetic activity (and hence, overall lower HRV). This may indicate a loss of complexity in HR dynamics and a more deterministic control of HRV by the parasympathetic branch in reaction to chronic stress. Hence, chronic stress might be associated with reductions in sympathetic-mediated cardiovascular control and disinhibition of parasympathetic influences that are mediated by alterations in central autonomic function, leading to reduced flexibility in responding to environmental demands and appropriate responsiveness.
The above-described cardiovascular profile could be considered to be a result of stress habituation, which supports the hypothesis that caregivers have a blunted psychobiological response to stress (De Andrés-García, Moya-Albiol, & González-Bono, Citation2012; Ruiz-Robledillo et al., Citation2015; Citation2016). In contrast, several other studies have suggested a sympathetic predominance and an absence of a blunted cardiovascular response to stress (Lucini et al., Citation2008; Teixeira & Pereira, Citation2014; Von Känel et al., Citation2003), and the differences could be explained by caregivers’ age, the number of years they have been caregiving, the characteristics of care recipients or even the type of laboratory task. Notably, our results are congruent with two previous studies, which employed the same type of laboratory task (Ruiz-Robledillo et al., Citation2015; Citation2016).
Blunted stress reactivity has been linked cross-sectionally and prospectively to increased depressive symptomatology (Carroll et al., 2007; de Rooij, Schene, Phillips, & Roseboom, Citation2010; Phillips, Hunt, Der, & Carroll, Citation2011; Salomon, Bylsma, White, Panaite, & Rottenberg, 2013) and poor self-reported health (de Rooij & Roseboom, Citation2010; Phillips, Der, & Carroll, Citation2009). In congruence with these findings, in our study, caregivers of people with EDs obtained higher depressive mood and trait scores than noncaregivers. Moreover, high depressive mood and symptoms were associated with a low HR and HR response to stress. These abnormalities may lead to cardiovascular somatic symptoms such as tachycardia, blood pressure lability and tendencies toward hypertension, which put caregivers at a markedly higher risk of developing cardiovascular diseases with the associated higher mortality.
The current study is not without limitations. First, it is cross-sectional research, and hence causality cannot be inferred. Second, it could be argued that the small sample size of each group weakens the results. Nevertheless, the research design is strong, including a control group, which was matched with cases for the main demographic characteristics. However, our findings can’t distinguish among groups at individual testing time points. Hence, the results of our assays in this group of informal caregivers should be interpreted with caution. This fact should be considered in relation to the measurement of HRV in future studies. Third, depression has been assessed through a self-report measure, which is a possible source of bias, as results are based on participants' subjective perception. Furthermore, other types of variables, especially relating to the care recipient, should be analyzed to evaluate their possible influence on cardiovascular response to stress in caregivers. Future studies should attempt to replicate the results of this study with other samples of informal caregivers, and as this could add to the body of evidence to support the idea of blunted cardiovascular response or hyporeactivity to stress in chronically stressed populations.
The findings advance our understanding of the chronic stress response in this population. Hyporeactivity to stress could directly affect the adaptive stress response of caregivers and alter their ability to cope with every day challenges. It could also compromise the physiological ability of caregivers to cope with stressors, both associated with and independent of the care context. Developing and implementing psychotherapeutic interventions focused on stress management would help caregivers to reduce their stress levels and cope effectively with stressors, and studies should be conducted exploring potential changes in cardiovascular response in caregivers after this type of psychotherapeutic intervention. Such research should assess whether these types of interventions are helpful for restoring effective stress responses in caregivers of people with EDs.
Acknowledgments
The authors wish to thank the clinic for parents of people with anorexia (CTA) for their help with this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alda, I., Espina, A., & Ortego, M.A. (2006). Un estudio sobre personalidad, ansiedad y depresión en padres de hijas con un trastorno alimentario. Clinica y Salud, 17, 151–170.
- Appelhans, B.M., & Luecken, L.J. (2008). Heart rate variability and pain: Associations of two interrelated homeostatic processes. Biological Psychology, 77, 174–182.doi:10.1016/j.biopsycho.2007.10.004
- Balaguer, I., Fuentes, I., Meliá, J.L., García-Merita, M.L., & Pérez Recio, G. (1993). El perfil de los estados de ánimo (POMS): Baremo para estudiantes valencianos y su aplicación en el contexto deportivo. Revista De Psicología Del Deporte, 4, 39–52.
- Brindle, R.C., Whittaker, A.C., Bibbey, A., Carroll, D., & Ginty, A.T. (2017). Exploring the possible mechanisms of blunted cardiac reactivity to acute psychological stress. International Journal of Psychophysiology, 113, 1–7. doi:10.1016/j.ijpsycho.2016.12.011
- Burr, R.L. (2007). Interpretation of normalized spectral heart rate variability indices in sleep research: A critical review. Sleep, 30, 913–919.
- Carrillo, E., Moya-Albiol, L., González-Bono, E., Salvador, A., Ricarte, J., & Gómez-Amor, J. (2001). Gender differences in cardiovascular and electrodermal responses to public speaking task: The role of anxiety and mood states. International Journal of Psychophysiology, 42, 253–264.doi: 10.1016/S0167-8760(01)00147-7
- Carroll, D., Phillips, A.C., Ring, C., Der, G., & Hunt, K. (2005). Life events and hemodynamic stress reactivity in the middle-aged and elderly. Psychophysiology, 42, 269–276. doi:10.1111/j.1469-8986.2005.00282.x
- Chadda, R.K. (2014). Caring for the family caregivers of persons with mental illness. Indian Journal of Psychiatry, 56, 221–227. doi:10.4103/0019-5545.140616
- Corrêa, M.S., Vedovelli, K., Giacobbo, B.L., de Souza, C.E., Ferrari, P., de Lima-Argimon, I.I., … Bromberg, E. (2015). Psychophysiological correlates of cognitive deficits in family caregivers of patients with Alzheimer disease. Neuroscience, 286, 371–382.doi:10.1016/j.neuroscience.2014.11.052
- Cyr, N.E., Dickens, M.J., & Romero, L.M. (2009). Heart rate and heart-rate variability responses to acute and chronic stress in a wild-caught passerine bird. Physiological and Biochemical Zoology : Pbz, 82, 332–344. doi:10.1086/589839
- De Andrés-García, S., Moya-Albiol, L., & González-Bono, E. (2012). Salivary cortisol and immunoglobulin A: Responses to stress as predictors of health complaints reported by caregivers of offspring with autistic spectrum disorder. Hormones and behavior, 62, 464–474. doi:10.1016/j.yhbeh.2012.08.003
- de Rooij, S. R., & Roseboom, T. J. (2010). Brief reports: Further evidence for an association between self?reported health and cardiovascular as well as cortisol reactions to acute psychological stress. Psychophysiology, 47, 1172–1175. doi:10.1111/j.1469-8986.2010.01023.x
- de Rooij, S.R., Schene, A.H., Phillips, D.I., & Roseboom, T.J. (2010). Depression and anxiety: Associations with biological and perceived stress reactivity to a psychological stress protocol in a middle-aged population. Psychoneuroendocrinology, 35, 866–877. doi:10.1016/j.psyneuen.2009.11.011
- Dickerson, S.S., Gruenewald, T.L., & Kemeny, M.E. (2004). When the social self is threatened: Shame, physiology, and health. Journal of Personality, 72, 1191–1216. doi:10.1111/j.1467-6494.2004.00295.x
- Endicott, J., Spitzer, R.L., Fleiss, J.L., & Cohen, J. (1976). The global assessment scale: A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry, 33, 766–771.doi:10.1001/archpsyc.1976.01770060086012
- Fatisson, J., Oswald, V., & Lalonde, F. (2016). Influence diagram of physiological and environmental factors affecting heart rate variability: An extended literature overview. Heart International, 11, e32–e40. doi:10.5301/heartint.5000232
- Goldberg, D.P., & Hillier, V.F. (1979). A scaled version of the General Health Questionnaire. Psychological Medicine, 9, 139–145.
- Gotlib, I.H., & Joormann, J. (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. doi:10.1146/annurev.clinpsy.121208.131305
- Hamilton, J.L., & Alloy, L.B. (2016). Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clin Psychol Rev, 50, 67–79. doi:10.1016/j.cpr.2016.09.003
- Highet, N., Thompson, M., & King, R.M. (2005). The experience of living with a person with an eating disorder: The impact on the carers. Eating Disorders: The Journal of Treatment and Prevention, 13, 327–344.doi:10.1080/10640260591005227
- Hjortskov, N., Rissén, D., Blangsted, A.K., Fallentin, N., Lundberg, U., & Søgaard, K. (2004). The effect of mental stress on heart rate variability and blood pressure during computer work. European Journal of Applied Physiology, 92, 84–89. doi:10.1007/s00421-004-1055-z
- Jandackova, V.K., Britton, A., Malik, M., & Steptoe, A. (2016). Heart rate variability and depressive symptoms: A cross-lagged analysis over a 10-year period in the Whitehall II study. Psychological Medicine, 46, 2121–2131. doi:10.1017/S003329171600060X
- Krassas, G.E. (2003). Endocrine abnormalities in anorexia nervosa. Psychological Medicine, 1, 46–54.
- Lucini, D., Cannone, V., Malacarne, M., Bruno, D., Beltrami, S., Pizzinelli, P., … Pagani, M. (2008). Evidence of autonomic dysregulation in otherwise healthy cancer caregivers: A possible link with health hazard. European Journal of Cancer, 44, 2437–2443. doi:10.1016/j.ejca.2008.08.006
- Mahoney, F.I., & Barthel, D.W. (1965). Functional evaluation: The Barthel Index. Maryland State Medical Journal, 14, 61–65.
- McEwen, B., & Gianaros, P. (2011). Stress and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. doi:10.1146/annurev-med-052209-100430
- Phillips, A.C., Der, G., & Carroll, D. (2009). Self?reported health and cardiovascular reactions to psychological stress in a large community sample: Cross?sectional and prospective associations. Psychophysiology, 46, 1020–1027. doi:10.1111/j.1469-8986.2009.00843.x
- Phillips, A.C., Hunt, K., Der, G., & Carroll, D. (2011). Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: Evidence from a large community study. Psychophysiology, 48, 142–148. doi:10.1111/j.1469-8986.2010.01045.x.
- Pruessner, J.C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D.H. (2003). Two formulas for the computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28, 916–931. doi:10.1016/S0306-4530(02)00108-7
- Romero-Martínez, Á., Ruiz-Robledillo, N., & Moya-Albiol, L. (2016). Depressive mood and testosterone related to declarative verbal memory decline in middle-aged caregivers of children with eating disorders. International Journal of Environmental Research and Public Health, 4, 13. doi:10.3390/ijerph13030286
- Rottenberg, J. (2005). Mood and emotion in major depression. Current Directions in Psychological Science, 14, 167–170.
- Ruiz-Robledillo, N., Arce-Carmona, M., Vitoria-Estruch, S., & Moya-Albiol, L. (2015). Respuesta cardíaca al estrés cognitivo en cuidadores informales de personas con trastorno del espectro autista. Revista Electrónica De Psicología Iztacala, 18, 48–67.
- Ruiz-Robledillo, N., Bellosta-Batalla, M., & Moya-Albiol, L. (2015). Lower cardiovascular reactivity to acute stress in informal caregivers of people with autism spectrum disorder than in non-caregivers: Implications for the health outcomes. International Journal of Psychophysiology, 98, 143–150.doi: 10.1016/j.ijpsycho.2015.07.011
- Ruiz-Robledillo, N., Romero-Martínez, Á., & Moya-Albiol, L. (2016). Blunted electrodermal and psychological response to acute stress in family caregivers of people with eating disorders. The Spanish journal of psychology, 19, E20. doi:10.1017/sjp.2016.24
- Seeman, T.E., McEwen, B.S., Rowe, J.W., & Singer, B.H. (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences, 98, 4770–4775. doi:10.1073/pnas.081072698
- Sepúlveda, A.R., López, C., Todd, G., Whitaker, W., & Treasure, J. (2008). An examination of the impact of Maudsley eating disorder collaborative care workshops on the well-being of family members: A pilot study. Social Psychiatry and Psychiatric Epidemiology, 43, 584–591.doi: 10.1007/s00127-008-0336-y
- Salomon, K., Bylsma, L.M., White, K.E., Panaite, V., & Rottenberg, J. (2013). Is blunted cardiovascular reactivity in depression mood-state dependent? A comparison of major depressive disorder remitted depression and healthy controls. International Journal of Psychophysiology, 90, 50–57. doi:10.1016/j.ijpsycho.2013.05.018
- Su, X., Liang, H., Yuan, W., Olsen, J., Cnattingius, S., & Li, J. (2016). Prenatal and early life stress and risk of eating disorders in adolescent girls and young women. European Child & Adolescent Psychiatry, 25, 1245–1253. doi:10.1007/s00787-016-0848-z
- Thayer, J.F., Ahs, F., Fredrikson, M., Sollers, J.J., 3rd., & Wager, T.D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience Biobehavioral Review, 36, 747–756. doi:10.1016/j.neubiorev.2011.11.009
- Thayer, J.F., & Brosschot, J.F. (2005). Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology, 30, 1050–1058. doi:10.1016/j.psyneuen.2005.04.014
- Task Force of the European Socierty of the North America Society of Pacing. (1996). Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation, 93, 1043–1065.doi:10.1161/01.CIR.93.5.1043
- Teixeira, R.J., & Pereira, M.G. (2014). Psychological morbidity and autonomic reactivity to emotional stimulus in parental cancer: A study with adult children caregivers. European Journal of Cancer Care, 23, 129–139. doi:10.1111/ecc.12102
- Treasure, J., Murphy, T., Szmukler, T., Todd, G., Gavan, K., & Joyce, J. (2001). The experience of caregiving for severe mental illness: A comparison between anorexia nervosa and psychosis. Social Psychiatry and Psychiatric Epidemiology, 36, 343–347.
- Tobaldini, E., Costantino, G., Solbiati, M., Cogliati, C., Kara, T., Nobili, L., & Montano, N. (2017). Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neuroscience and Biobehavioral Reviews, 74, 321–329. doi:10.1016/j.neubiorev.2016.07.004
- Von Känel, R., Dimsdale, J.E., Patterson, T.L., & Grant, I. (2003). Acute procoagulant stress response as a dynamic measure of allostatic load in Alzheimer caregivers. Annals of Behavioral Medicine, 26, 42–48. doi:10.1207/S15324796ABM2601_06
