Abstract
Much of the extant cortisol awakening response (CAR) literature posits that CAR is an anticipatory response to perceived demands later that same day. However, expanding and switching the temporal order of cortisol and psychosocial influences may motivate more flexible approaches to understanding the dynamic relationship between mind and body, including cumulative strain on the HPA axis. This study was novel because we used two models to explore the effects of one day’s emotion regulation and cortisol levels on cortisol and CAR the following day in 100 mildly stressed adults aged 50–81 years old, which contrasts with the more common CAR-anticipatory-response design. In the first model, High negative-affect-variation on day 1 predicted a higher risk of having a flat CAR the next day, relative to the moderate-affect-variation group (RR = 10.10, p < .05). In the second model, higher bedtime cortisol on day 1 was positively associated with waking cortisol (β = .293, p < .01) and flatter CAR slopes on day 2 (β = −.422, p < .001). These results show that morning cortisol intercepts and slopes may be associated with previous days’ affect variability and levels of bedtime cortisol. These results also suggest that anticipation of demands may extend to the previous day, rather than just the morning of the demand, indicating a broader temporal framework for the study of CAR.
Expanding the time frame for assessing predictors of the cortisol awakening response: what is possible?
The brief window (30–45 minutes) of the cortisol awakening response (CAR) provides researchers with a unique picture of the health of the hypothalamic–pituitary–adrenal (HPA) axis (for a review, see Stalder et al., Citation2015). The 30–60% increase in cortisol during the CAR period can be considered “normative,” whereas “flat” cortisol slopes are characterized by a less-pronounced CAR increase. Flatter slopes are important to understand because flat daily cortisol slopes (Human et al., Citation2013) in general, including flatter CAR in particular (Neylan et al., Citation2005), are indicative of poor psychological and physiologic conditions.
Much of the extant CAR literature supports the hypothesis that waking levels of cortisol and the subsequent rise of the CAR reflect anticipation of upcoming demands or challenges later the same day (Rohleder, Beulen, Chen, Wolf, & Kirschbaum, Citation2007; for a review see, Stalder et al., Citation2015). For example, Wetherell, Lovell, and Smith (Citation2015) used cortisol sampling over two days to show that measures of CAR (i.e., AUCg, peak levels, higher increase in cortisol) were higher on the second day of sampling, when participants expected a laboratory challenge. On the other hand, Powell & Schlotz (Citation2012) showed that there was no relationship between CAR and anticipation of demands later in the day.
Higher positive affect (PA) is generally associated with lower cortisol and higher negative affect (NA) is generally associated with higher cortisol levels (Polk, Cohen, Doyle, Skoner, & Kirschbaum, Citation2005). However, mean (trait) levels of affect may fail to account for momentary fluctuations (state) of affect on physiologic markers of stress, which is important because greater variability may be associated with physical and mental stress (Human et al., Citation2013). Furthermore, previous evidence suggests that emotional instability or higher variation, is associated with flatter cortisol slopes across the day (Human, Whillans, Hoppmann, Klumb, Dickerson, & Dunn, Citation2015).
The current study used two models to explore the hypothesis that CAR is not simply an anticipatory response. One model is built upon prior research showing that emotions on one day (Naragon-Gainey, Watson, & Markon, Citation2009) are related to CAR on the next day (Gartland, O’Connor, Lawton, & Bristow, Citation2014). However, we expand on the previous research by incorporating a model from Human et al. (Citation2015), which predicts that too much or too little affect variability is associated with poor cortisol outcomes (too much or too little cortisol reactivity) and that moderate affect variability predicts more normative patterns of cortisol (with the uncertainty of what is considered normative cortisol levels in naturalistic settings) (see, Miller, Chen, & Zhao, Citation2007).
We also wanted to account for cortisol levels at bedtime one day and waking cortisol levels and CAR the following day. Studies have indicated that high levels of waking cortisol are associated with flatter CAR (Dahlgren, Kecklund, Theorell, & Akerstedt, Citation2009). However, the relationship between bedtime cortisol on one day and CAR the following day is rarely explored. Studies also suggest that previous days’ stressors are associated with higher bedtime cortisol (Zoccola & Dickerson, Citation2015) and the next morning’s cortisol levels (Wong et al., Citation2014), and that these higher waking cortisol levels are associated with flatter CAR slopes (Adam et al., Citation2006).
This report focused on expanding the time frame of and temporal order of variables associated with CAR. This study was novel because we used two models to explore the effects of one day’s emotion regulation and cortisol levels on cortisol and CAR the following day in 100 mildly stressed adults aged 50–81 years old, which contrasts with the more common CAR-anticipatory-response design. We hoped that our work might help to better understand the processes associated with cumulative strain on the HPA axis. In our first analysis, we hypothesized that (1) high variability in affect (PA and NA) would be associated with a higher log-odds likelihood of a flatter CAR pattern (that is, a flatter cortisol slope the following day for the High-Variability-Affect group relative to the Moderate-Affect-Variability reference group). We used multinomial logistic regression to assess the likelihood of falling into one of the three CAR categories based on model predictors (Dmitrieva, Almeida, Dmitrieva, Loken, & Pieper, Citation2013). In our second analysis, we hypothesized that a path model would show that higher levels of bedtime cortisol would be associated with higher waking cortisol (Wong et al., Citation2014) and flatter CAR the next day (Dahlgren et al., Citation2009).
Methods
Participants
Participants in this study consisted of generally healthy older adults (mean age = 59.81; range = 50–85 years) who participated in a mindfulness meditation study at Oregon Health & Science University (OHSU). The upper age cutoff helped to control for multiple brain pathologies contributing to age-related cognitive alterations. All data described were from the baseline pre-randomization visit. Participants were recruited from the Portland, Oregon metropolitan area from June 2011 to January 2015 using paper and/or digital postings. This study was approved by the OHSU IRB and all participants provided written informed consent. Participant identifiers were kept anonymous, including in this manuscript.
The sample for these analyzes included 100 participants with full data. There were 78 women and 22 men in this sample. This sample size was less than the 134 for the intervention study (Oken et al., Citation2016) due to removal of participants that did not have reliable measures of PA and NA in the ecological momentary assessment (EMA). The majority of participants were white (n = 92).
Pre-study self-rated measures (stress, personality)
Personality. Neuroticism is known to have an effect on cortisol outcomes and to be closely associated with NA, and thus we accounted for neuroticism. Neuroticism was obtained as a subscale of the 60-item NEO-FFI (Scandell, Citation2000) (neuroticism alpha = .79) and collected via paper survey prior to the baseline visit of the larger randomization study.
Daily and ecological momentary assessment measures (cortisol, affect)
Cortisol. Cortisol was collected up to 4 times per day following the in-lab testing visit. These times included waking, 30 minutes after waking, afternoon (around 4:00 p.m.), and before bedtime. Only bedtime cortisol from the first day and the first two cortisol measures of the second day were utilized in the current study. Cortisol values (μg/dl) were quantified by the Oregon Clinical and Translational Research Institute lab in duplicate with enzyme-linked immunoassay. Participants practiced using Sarstedt-brand salivettes during the in-lab testing visit in order to minimize at-home collection errors. Experimenters specifically instructed individuals to refrigerate samples and to refrain from using tobacco, brushing their teeth, or eating or drinking anything other than water prior to collection. Participants were given duplicate instructions to take home and instructed to record collections times for each sample on a form provided by the research team.
Cortisol awakening response. CAR was assessed by subtracting the second cortisol value (30 minutes after waking) from the cortisol value at waking and dividing the difference by the time delay between time 1 and time 2. On average, this time difference was 30 minutes.
CAR groups. For our first model, we calculated and defined three distinct CAR groups: Low CAR, Moderate CAR, and High CAR. Participants were divided into these groups based on statistically assessed tertiles of CAR.
Affect. This study used the Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, Citation1988). The standard 20-question trait version of the PANAS was filled out at home prior to the baseline study visit, and the 10-question state-version was filled out at home after the visit using ecological momentary assessment (EMA) technology (Leue & Beauducel, Citation2011; state PA alpha = .89; state NA alpha = .85).
Ecological momentary assessment. Participants completed up to four 5-minute EMA sessions (Leue & Beauducel, Citation2011) to assess PA and NA on the day prior to measure CAR. The EMA software was administered using repurposed smart phones with no cellular or internet connectivity.
Affect mean and variability. Mean affect scores were the average of individual PA and NA measures across the day. PA and NA variability were assessed using intra-individual standard deviation (iSD; Ram & Gerstorf, Citation2009). iSD was computed by assessing the within-person SDs of PA and NA across the day of the EMA assessment.
Affect variability groups. We calculated PA and NA variability groups in a similar manner to the CAR groups. That is, participants were divided into statistically calculated tertiles of the iSD data (Low-Affect-Variability, Moderate-Affect-Variability, and High-Affect-Variability).
Analysis
Data were first inspected to ensure there were no outliers; extreme outliers (e.g. more than 3 SDs above the mean cortisol levels at any given point of cortisol collection) were deleted. Non-transformed data showed similar results to log-transformed data, so we report the non-transformed outcomes for interpretability. Waking time and medications may affect CAR, so we used random effects models with only self-reported waking time and medications as predictors of CAR to assess influence of these variables in our data. There was no significant effect of waking time and medications, so we did not include them as covariates in subsequent models.
Analysis 1: Multinomial logistic regression with CAR as our outcome variable. Multinomial logistic regression is a generalization of logistic regression, but can handle data that may have more than one categorical outcome (Long & Freese, Citation2014), where,
(1)
EquationEquation 1(1) shows that the categorical variable CAR Group for person i on day j was the outcome (note: “j” in this equation is simply to illustrate a model that assesses day-to-day effects). This indicates that the given set of independent variables predict the probability of different possible outcomes of the categorical variable CAR Group (Low CAR, Moderate CAR, and High CAR). The α in this model is the slope for the reference CAR Group, which we chose as the Moderate-CAR group. The PA iSD and NA iSD groups for person i on day j were used as the categorical predictors of affect variation (Low-Affect-Variability, Moderate-Affect-Variability, and High-Affect-Variability). The outcomes of the Low-Affect-Variability and High-Affect-Variability were reported in relation to the Moderate-Affect-Variability reference group. We controlled for age, neuroticism, and mean PA and NA due to their possible influence on CAR outcomes. The data in this model are unstandardized.
Analysis 2: The assessment of the relationship between bedtime cortisol, waking cortisol, and CAR slopes. Path analysis illustrates the direct effects of an exogenous variable (e.g. bedtime cortisol) on a dependent, or endogenous, variable (Streiner, Citation2005), such as CAR. Path analysis can also provide information on how the exogenous variable has an indirect effect on the final endogenous variable through another independent variable (e.g., waking cortisol). It is common that endogenous variables will be affected by factors not included in the model, which are represented by error terms. The model for this study can be represented by,
(2)
(3)
The regression path in EquationEquation 2(2) shows the full indirect regression path for the model in this study. EquationEquation 3
(3) models the direct path between bedtime cortisol on the next day’s CAR. The terms e1 and e2 represent the unexplained variance in this model. We controlled for age, neuroticism, and mean PA and NA due to their possible influence on CAR outcomes. This path model included standardized regression coefficients.
Results
Analysis 1
shows that being in the High-Affect-Variability group for NA was associated with an approximate 10-fold logged increased risk of being in the Low-CAR group, or having a flatter CAR slope, relative to the NA Moderate-Affect-Variability reference group, RR = 10.196, p < .05. Age also predicted a greater risk of being in the Low-CAR group, RR = 1.110, p < .05. Mean NA predicted a marginally significant amount of variance in the Low-CAR group, RR = .641, p < .10, which suggested a trend toward a lower risk of being in the Low-CAR group compared to the reference Moderate-CAR group.
Table 1. Results of hypothesis testing of the risk of being in a specific CAR group in comparison to the baseline CAR.
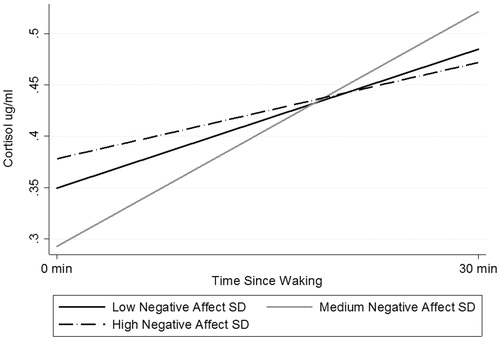
shows the individual slopes for each category of NA affect variability (there were no significant relationships between PA variability and CAR). The Moderate-Affect-Variability group has a lower intercept than the other two groups (low, high iSD). Furthermore, this graph illustrates how higher variation in NA is associated with not only higher waking levels of cortisol, but also a flatter CAR slope.
Figure 1. CAR slopes as predicted by negative affect SD on previous day. The Moderate-Affect-Variability group has a lower intercept than the other two groups (low, high iSD). Further, this graph illustrates how higher variation in NA is associated with not only higher waking levels of cortisol, but also a flatter CAR slope.
Analysis 2
and show the results of the path analysis of the relationship between bedtime cortisol and waking cortisol and CAR slopes the following day. Bedtime cortisol had a significant effect on the following day’s waking cortisol measure, β = .293, p < .01, indicating that higher bedtime cortisol was associated with higher waking cortisol. Furthermore, the path from waking cortisol to CAR on the same day indicated that waking cortisol had a significant and negative relationship with CAR,
Figure 2. Path analysis examining the relationship between previous day's bedtime cortisol levels and the next day's waking cortisol and CAR. Higher bedtime cortisol was associated with higher waking cortisol the next day. Further, the path from waking cortisol to CAR on the same day indicated that waking cortisol had a significant and negative relationship with CAR, suggesting that higher waking levels of cortisol were associated with flatter CAR. Note: ***p <.001; **p <.01; †p <.10; CAR: cortisol awakening response; mean_PA: mean positive affect; mean_NA: mean negative affect; all coefficients standardized.
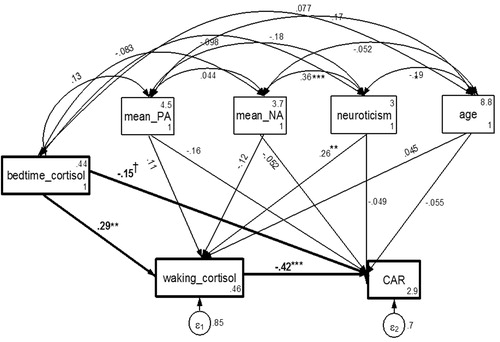
Table 2 Path models of bedtime cortisol to waking cortisol to CAR (n = 98).
β = −.422, p < .001, suggesting that higher waking levels of cortisol were associated with flatter CAR. Bedtime cortisol also had a moderate direct effect on the next day’s CAR, β = −.152, p < .10, suggesting that higher bedtime cortisol was marginally associated with flatter CAR the next day. Neuroticism also had a significant direct effect on waking cortisol levels, β = .262, p < .01. The relationship between mean PA and CAR was marginally significant, β = −.160, p < .10.
Discussion
This study explored the relationship between prior days’ psychosocial and physiologic variables on the following day’s waking cortisol and CAR. Our intention was not to dispute the interpretation that CAR is a predictor of the same day’s stressors (see Clow et al., Citation2004; Stalder et al., Citation2015), but was instead intended to highlight the possible connections between day-to-day psychosocial and physiologic activity on cortisol responses. We understand that the limitations of this data (e.g., number of days, participants) prevent us from making broad statements in regards to CAR modeling. However, we noted that increasing numbers of studies are looking at CAR effects over several days, and yet there is still a strong emphasis on how CAR predicts events later in the same day.
Regarding the hypothesis that previous days’ affect variation was related to morning cortisol levels the following day, the results showed that high variability in NA on day 1, relative to moderate variability, predicted a higher relative risk for flatter CAR on day 2. Affect variability has been previously observed to have an effect on the following day’s CAR slopes, with high PA variability being associated with both higher overall cortisol levels and flatter cortisol slopes (Human et al., Citation2015). We partially supported these findings, except that our results showed NA variability, rather than PA variability, to be the primary predictor of CAR slope category. However, these findings suggest that neuroendocrine drive in the morning may be as much a reflection of the previous day as it is anticipation of the upcoming day. A range of variables may need to be examined in order to better understand how our neuroendocrine system transacts with psychological variation, and perhaps memory, as the transition from sleep cortisol levels to waking cortisol levels are moderated by the hippocampus.
Notably, we went further in this examination by piecing together findings from studies that showed that a higher stress was associated with higher bedtime cortisol levels (Zoccola & Dickerson, Citation2015) with studies that showed that some of these same variables are associated with higher waking cortisol the following day (Wong et al., Citation2014). These data support our previous statement that exploring day-to-day transaction between psychosocial and physiologic effects may provide a new avenue for assessing CAR, and perhaps the cortisol diurnal rhythm in general. However, the results of the current study may also suggest that people begin to anticipate a demand the day before the demand is to occur. This possibility is still consistent with our hypotheses that the previous days’ psychosocial activity and cortisol levels are associated with the following days’ morning cortisol activity, but that understanding potential causes of cumulative strain on the HPA axis requires that we consider expanding our frame from which to look at these relationships.
Limitations
The sample size in this study was relatively low (n = 100), and data were collected only on two days. Furthermore, the study design prevented us from having two full days of CAR and EMA data, thereby preventing day-to-day comparisons of these measures. We were also unable to meet the criteria for measuring CAR as set out by Stalder et al. (Citation2015). Low sample size is likely associated with high standard errors in the outcomes. Furthermore, EMA assessments were only collected four times, at most, on each day of measurement. The range of ages, ethnicity, and gender in this study was limited. There was no validated measure of daily stress (such as a daily diary or stressor reports during the EMA) to pair with the affective responses reported in the EMA.
Future studies
Future studies may also look at potential differential effects of affect variability on cortisol function, both within and across days. Such a study requires more measures of cortisol and EMA across more days. Furthermore, future studies should rely on the cortisol sampling recommendations, particularly around CAR measures, as outlined by Stalder et al. (Citation2015). An exploration of cortisol measures during sleep hours may help researchers to better understand how overnight HPA activity is related to the next day’s CAR.
Acknowledgements
Authors thank Roger Ellingson, MSEE, Trial Registration: NCT0138606.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Refrences
- Adam, E.K., Hawkley, L.C., Kudielka, B.M., & Cacioppo, J.T. (2006). Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America, 103, 17058–17063. doi: 10.1073/pnas.0605053103
- Clow, A., Thorn, L., Evans, P., & Huckelbridge, F. (2004). The awakening cortisol response: methodological issues and significance. Stress, 7, 29–37. doi: 10.1080/102538904/1000166725
- Dahlgren, A., Kecklund, G., Theorell, T., & Akerstedt, T. (2009). Day-to-day variation in saliva cortisol—Relation with stress and self-rated health. Biological Psychology, 82, 149–155. doi: 10.1016/j.biopsycho.2009.07.001
- Dmitrieva, N.O., Almeida, D.M., Dmitrieva, J., Loken, E., & Pieper, C.F. (2013). A day-centered approach to modeling cortisol: Diurnal cortisol profiles and their associations among U.S. adults. Psychoneuroendo-crinology, 38, 2354–2365. doi: 10.1016/j.psyneuen.2013.05.003
- Gartland, N., O’connor, D.B., Lawton, R., & Bristow, M. (2014). Exploring day-to-day dynamics of daily stressor appraisals, physical symptoms and the cortisol awakening response. Psychoneuroendocrinology, 50, 130–138. doi: 10.1016/j.psyneuen.2014.08.006
- Human, L.J., Whillans, A.V., Hoppmann, C.A., Klumb, P., Dickerson, S.S., & Dunn, E.W. (2015). Finding the middle ground: curvilinear associations between positive affect variability and daily cortisol profiles. Emotion, 15, 705–720. doi: 10.1037/emo0000071
- Human, L.J., Biesanz, J.C., Miller, G.E., Chen, E., Lachman, M.E., & Seeman, T.E. (2013). Is change bad? Personality change is associated with poorer psychological health and greater metabolic syndrome in midlife. Journal of Personality, 81, 249–260. http://dx.doi.org/10.1111/jopy.12002
- Leue, A., & Beauducel, A. (2011). The PANAS structure revisited: on the validity of a bifactor model in community and forensic samples. Psychological Assessment, 23, 215–225. doi: 10.1037/a0021400
- Long, J.S., & Freese, J. (2014). Regression models for categorical dependent variables using Stata. College Station, TX: Stata Press.
- Miller, G.E., Chen, E., & Zhou, E.S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi: 10.1037/0033-2909.133.1.25
- Naragon-Gainey, K., Watson, D., & Markon, K.E. (2009). Differential relations of depression and social anxiety symptoms to the facets of extraversion/positive emotionality. Journal of Abnormal Psychology, 118, 299–310.
- Neylan, T.C., Brunet, A., Pole, N., Best, S.R., Metzler, T.J., Yehuda, R., & Marmar, C.R. (2005). PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology, 30, 373–381. doi: 10.1016/psyneuen.2004.10.005
- Oken, B.S., Wahbeh, H., Goodrich, E., Klee, D., Memmott, T., Miller, M., & Fu, R. (2016). Meditation in Stressed Older Adults: Improvements in self-rated mental health not paralleled by improvements in cognitive function or physiological measures. Mindfulness, 3, 627–638. doi:10.1007/s12671-016-06407.
- Polk, D.E., Cohen, S., Doyle, W.J., Skoner, D.P., & Kirschbaum, C. (2005). State and trait predictors of salivary cortisol in healthy adults. Psychoneuro-endocrinology, 30, 261–272. doi:10.1016/j.psyneuen.2004.08.004
- Powell, D.J., & Schlotz, W. (2012). Daily life stress and the cortisol awakening response: Testing the Anticipation Hypothesis. PLoS One, 7, e52067. doi: 10.1371/journal.pone.0052067
- Ram, N., & Gerstorf, D. (2009). Time-structured and net intraindividual variability: tools for examining the development of dynamic characteristics and processes. Psychology and Aging, 24, 778–791. doi: 10.1037/a0017915
- Rohleder, N., Beulen, S.E., Chen, E., Wolf, J.M., & Kirschbaum, C. (2007). Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancers. Personality and Social Psychology Bulletin, 33, 69–84. doi: 10.1177/0146167206293986
- Scandell, D. (2000). Development and initial validation of validity scales for the NEO-Five Factor Inventory. Personality and Individual Differences, 29, 1153–1162. doi: 10.1016/S0191-8869(99)00262-7
- Stalder, T., Kirschbaum, C., Kudielka, B.M., Adam, E.K., Pruessner, J.C., Wüst, S., …Clow, A. (2015). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. doi: 10.1016/j.psyneuen.2015.10.010.
- Streiner, D.L. (2005). Finding our way: an introduction to path analysis. Can J Psychiatry, 50, 115–122. doi: 10.1177/070674370505000207
- Watson, D., Clark, L.A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063
- Wetherell, M., Lovell, B., & Smith, M. (2015). The effects of an anticipated challenge on diurnal cortisol secretion. Stress (Amsterdam, Netherlands), 18, 42–48. doi: 10.3109/10253890.2014.993967.
- Wong, J.D., Mailick, M.R., Greenberg, J.S., Hong, J., & Coe, C.L. (2014). Daily work stress and awakening cortisol in mothers of individuals with autism spectrum disorders or Fragile X Syndrome. Family Relations, 63, 135–147. doi: 10.1111/fare.12055
- Zoccola, P.M., & Dickerson, S.S. (2015). Extending the recovery window: Effects of trait rumination on subsequent evening cortisol following a laboratory performance stressor. Psychoneuroendocrinology, 58, 67–78. doi: 10.1016/j.psyneuen.2015.04.014
