Abstract
The late adolescent period is characterized by marked neurodevelopmental and endocrine fluctuations in the transition to early adulthood. Adolescents are highly responsive to the external environment, which enhances their ability to adapt and recover from challenges when given nurturing influences, but also makes them vulnerable to aberrant development when exposed to prolonged adverse situations. Female rats are particularly sensitive to the effects of chronic stress in adolescence, which manifests as passive coping strategies and blunted hypothalamo-pituitary adrenocortical (HPA) stress responses in adulthood. We sought to intervene by exposing adolescent rats to environmental enrichment (EE) immediately prior to and during chronic stress, hypothesizing that EE would minimize or prevent the long-term effects of stress that emerge in adult females. To test this, we exposed male and female rats to EE on postnatal days (PND) 33–60 and implemented chronic variable stress (CVS) on PND 40–60. CVS consisted of twice-daily unpredictable stressors. Experimental groups included: CVS/unenriched, unstressed/EE, CVS/EE and unstressed/unenriched (n = 10 of each sex/group). In adulthood, we measured behavior in the open field test and forced swim test (FST) and collected blood samples following the FST. We found that environmental enrichment given during the adolescent period prevented the chronic stress-induced transition to passive coping in the FST and reversed decreases in peak adrenocortical responsiveness observed in adult females. Adolescent enrichment had little to no effect on males or unstressed females tested in adulthood, indicating that beneficial effects are specific to females that were exposed to chronic stress.
Introduction
Adolescence is a period of heightened neuroplasticity and hormonal variation, which influence the brain’s cognitive-emotional circuits as they reach the chronological culmination of limbic system development (Andersen, Citation2003; Andersen & Teicher, Citation2008; Eiland & Romeo, Citation2013). This sensitive window increases the capacity for favorable adaptations, but simultaneously escalates the risk for dysfunctional development and later psychopathology (Andersen, Citation2003). Understanding both positive and negative influences during adolescence will facilitate design of focused interventions to shape the neurodevelopment of effective coping strategies.
During adolescence, brain circuits that integrate the emotion, cognition, and stress are still undergoing significant remodeling and development, particularly the prefrontal cortex and amygdala (Koss, Belden, Hristov, & Juraska, Citation2014; Willing & Juraska, Citation2015). Because these limbic brain regions are still maturing, stress exposure in adolescence can set the stage for psychological disorders such as anxiety and depression (Eiland & Romeo, Citation2013; Ge, Natsuaki, & Conger, Citation2006; Goodyer, Herbert, & Altham, Citation1998). In rodent models, adolescent stress causes long lasting dendritic modifications in the prefrontal cortex (Leussis, Lawson, Stone, & Andersen, Citation2008), a key regulator of stress and emotion. However, adolescent males may be resistant to the negative impact of chronic stress on coping behaviors and even display increased levels of brain-derived neurotrophic factor following chronic mild stress, which may enhance neuroplasticity and adaptation (Toth et al., Citation2008). In contrast, adolescent chronic stress in females promotes passive coping behaviors often associated with depression, and constrains corticosteroid responses to subsequent novel stressors (Bourke & Neigh, Citation2011; Wulsin, Wick-Carlson, Packard, Morano, & Herman, Citation2016).
By consensus, in rats, adolescence is divided into three distinct stages: pre-pubertal (early, postnatal day (PND) 27–34), pubertal (mid, PND 34–46), and post-pubertal (late, PND 47–59) (Eiland & Romeo, Citation2013; Lupien, McEwen, Gunnar, & Heim, Citation2009; Wulsin et al., Citation2016). In particular, females are especially vulnerable to chronic stress in late adolescence and the effects either persist or emerge in adulthood (Bourke & Neigh, Citation2011; Wulsin et al., Citation2016). In humans, late adolescent females have complicated patterns of hormonal secretion (Schulz & Sisk, Citation2016; Veldhuis, Roemmich, & Rogol, Citation2000), increased emotional stress reactivity, and coping strategies that may lead to an increased risk of depression (Nolen-Hoeksema, Citation2001). In identifying late adolescent females as an at-risk demographic, there is a serious need for preventative and curative strategies to optimize psychological development for this group.
Enrichment early in life (PND 10–24) in rats improves cognitive function throughout adulthood, suggesting that the neuroprotective properties of enrichment are maintained (Passig et al., Citation1996). In combating the effects of stress applied early in the postnatal period, later adolescent exposure to environmental enrichment is largely successful in restoring behavioral and hormonal function (do Prado et al., Citation2016; Francis, Diorio, Plotsky, & Meaney, Citation2002; Hui et al., Citation2011; Morley-Fletcher, Rea, Maccari, & Laviola, Citation2003). As environmental enrichment in adolescence can ameliorate the effects of prior stress in early postnatal life, we predicted that it may also prevent the enduring effects of stress occurring in late adolescence. To this end, we hypothesized that adolescent environmental enrichment would protect females from the adulthood behavioral and endocrine manifestations of adolescent chronic stress.
Methods
Animals
Female and male Sprague Dawley rats from Harlan (Indianapolis, IN) were acclimated to the colony room and then paired for in-house breeding. The colony room was temperature (23 °C) and humidity-controlled with a 12-h light–dark cycle (06:00 h lights-on, 18:00 h lights-off). All rats were housed in translucent polycarbonate shoebox cages, with corncob bedding, with food and water available ad libitum. Offspring of breeding pairs were maintained in colony rooms with the same conditions (except for the timing of the light cycle, see below). All experiments complied with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Weaning and experimental groups
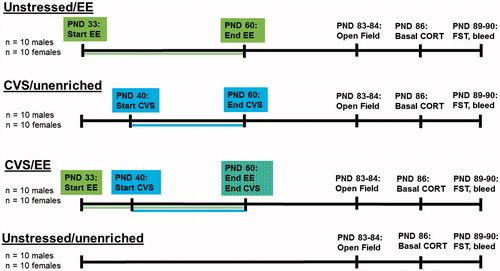
From 10 litters (ranging from 3 to 14 pups), 40 female and 40 male offspring were weaned into same sex pair housing at postnatal day (PND) 25–27, based on previous work (Wulsin et al., Citation2016). Offspring were born over a period of 3 days (day of birth = PND 0), but we will report median age for the remainder of the experimental manipulations. Male and female rats were housed in separate colony rooms, but both on a 12 h light cycle (09:00 h lights-on and 21:00 h lights-off). On post-natal day (PND 28), rats were assigned a non-littermate same-sex cage mate and maintained this same cage mate for the duration of the study. For each sex, rats were divided into 4 groups with equal representation from all litters, utilizing only one rat per litter per experimental group. Each rat was housed with a same-sex cage mate of the same experimental group. The experimental groups included: unstressed/unenriched (n = 10 males, n = 10 females); unstressed/enriched (unstressed/EE, n = 10 males, n = 10 females); chronic variable stress/unenriched (CVS/unenriched, n = 10 males, n = 10 females); chronic variable stress/enriched (CVS/EE, n = 10 males, n = 10 females). See for experimental timeline.
Figure 1. Experimental timeline Experimental manipulations and timeline for the four experimental groups, with males and females run concurrently for a total of eight groups (n = 10/group; CVS: chronic variable stress; CORT: corticosterone; EE: environmental enrichment; FST: forced swim test; PND: postnatal day.
Environmental enrichment
For the selected experimental groups, rats received environmental enrichment from PND 33 to 60, to encapsulate the late adolescent period. Enrichment was initiated 1 week prior to chronic stress to allow habituation to the enriched environment and avoid any compounding effects of initiating the two manipulations simultaneously (see Johnson, Traver, Hoffman, Harrison, & Herman, Citation2013 for photograph of the enrichment chambers). Rats were exposed to active cycle environmental enrichment as previously published (Smith et al., Citation2017). Briefly, rats were enriched only during the active cycle (21:00–09:00 h) in a neighboring colony room to the unenriched rats. During the non-active cycle (09:00–21:00 h), rats were pair housed in standard shoebox cages (20 cm height × 22 cm width × 43 cm length) in the same colony room as the unenriched rats. Enrichment consisted of 10 rats (same sex) per 1 m3 cage with assorted toys, wire mesh walls for climbing access and a wire mesh platform placed 0.5 m from the ground with a dimension of 1 m × 0.5 m. Enrichment cages and toys were cleaned and sanitized once per week, with the toy selection changing every week. Rats did not sustain any wounds and no aggressive behaviors were observed throughout the course of the study.
Chronic variable stress
For the selected experimental groups, rats received CVS during late adolescence from PND 40 to 60. CVS consisted of two stressors per day, with each stressor lasting approximately 5 min–1 h. The morning stressor was administered between the hours of 09:00–14:00 h, the evening stressor was administered between the hours of 14:00 and 20:00 h, the rats were given a minimum of 2 h between stressors, and no stressors were repeated within the same day. Stressors were delivered in an unpredictable manner and included: hypoxia (30 min with 92% N2 and 8% O2), cold room (2 per cage; 1 h at 4 °C; no bedding), orbital shaker (5–6 per cage; 1 h at 100 rpm; 1.9 cm orbit), restraint (30 min in a Plexiglas® restraint cylinder in home cage; adjustable notches for 10, 12.5, or 15 cm length; 5.5 cm diameter; 0.5 and 1 cm openings for sufficient ventilation), pedestal (5 min on a 20 cm2 platform; elevated 45 cm off the ground), and wet bedding (5–6 per cage; 1 h; sufficient to moisten bedding without producing standing water) (Smith et al., Citation2017).
Open field test
On PND 83–84, experimental rats were individually exposed to the open field test (OFT) for 5 min (Smith et al., Citation2017). Each rat started the OFT in the corner of the 1 m2 arena with 30 cm walls and their behavior was video recorded with Clever Capture Star software (CleverSys, Reston, VA). Lighting conditions were the same as in the colony rooms. One cage mate was exposed to the OFT on PND 83 and the other cage mate received the test on PND 84 the following day. On PND 83, one male cage mate from each of the 20 male cages was exposed to the OFT, followed by one female cage mate from each of the 20 female cages. On PND 84, the order was reversed and the remaining 20 female cage mates were exposed to the OFT, followed by the remaining 20 male cage mates. The arena was wiped clean with 20% ethanol in between every rat, to limit odor detection between subjects. Videos were automatically scored using Clever Top Scan software (CleverSys, Reston, VA).
Forced swim test
On PND 89–90, experimental rats were exposed to the forced swim test (FST) for 6 min on a single day, to replicate previous studies with enrichment removal in adult rats (Smith et al., Citation2017). We utilized a one-day exposure to the FST because this version detects the effects of antidepressants and validates animal models of depression-like behavior (Cryan, Page, & Lucki, Citation2005; Cryan, Valentino, & Lucki, Citation2005; Overstreet, Citation2002; Overstreet, Keeney, & Hogg, Citation2004; Smith et al., Citation2017; Wulsin, Herman, & Solomon, Citation2010). We elected to use the one day protocol to avoid the potential confound of learning, as we were interested in using the procedure as a stress provocation. The FST tanks (20 cm diameter, 45 cm high) were filled to a depth of 31 ± 3 cm with 24 ± 2 °C water. Behavior was recorded from the side with Clever Capture Star software (CleverSys, Reston, VA). A black divider was placed between the two tanks, with one rat per tank and water changed between every rat. Half of the rats were exposed to the FST on PND 89 (males first) and the other half on PND 90 (females first). Both cage mates were exposed to the test on the same day, and groups were equally represented per day. Active behaviors quantified included: swimming, diving, and climbing. Swimming was defined as active circular motion around the tank, diving was defined as immersing the entire head under the surface of the water, and climbing was defined as active vertical motion up the sides of the tank. Immobility duration was a measure of passive behavior and was defined as either no movement or the minimum movements required to stay afloat. Videos were scored manually using Hindsight (behavioral scoring program developed by Scott Weiss, PhD) (Markham, Norvelle, & Huhman, Citation2009), and the individual was blind to the experimental groups.
Blood sampling
On PND 86, basal tail vein samples were taken by tail clip, without anesthetic, at 11:00 h (2 h after lights-on) (Vahl et al., Citation2005). The tip of the tail was clipped with a razor blade, removing just enough to access the blood supply (<2 mm). Then on PND 89–90, tail vein blood samples were collected at 15, 30, 60, and 120 min after FST initiation. Samples (approximately 250 µL) were collected in tubes containing 10 µL of 100 mM EDTA and kept on ice. Blood was centrifuged at 4 °C for 15 min at 3500 × g. Plasma was obtained and kept at −20 °C until use for radioimmunoassay.
Radioimmunoassay
Plasma adrenocorticotropic hormone (ACTH) concentration was determined from samples after FST by RIA with a specific antiserum (diluted 1:120,000; graciously provided by Dr. William Engeland – University of Minnesota, Minneapolis, MN) with 125I ACTH as a tracer label (Amersham Biosciences, Piscataway, NJ) (Jasper & Engeland, Citation1991). Plasma samples were diluted in steroid diluent and run in duplicate for determination of plasma corticosterone concentrations with an 125I kit (MP Biomedicals., Orangeburg, NY). The intra-assay coefficient of variation was <10% for both the ACTH and corticosterone assays, and sensitivities (minimum detectable values) were 3.13 pg/ml and 25 ng/ml, respectively.
Food intake and body weight
Upon weaning, body weights were collected weekly from PND 28 to 84. To study the effects of EE and CVS, body weight was analyzed as percent change from initial body weight (Smith et al., Citation2017). The percent change in body weight was calculated versus PND 28 for PND 28–56, representing the duration of EE/CVS exposure () (Smith et al., Citation2017). The percent change was taken versus PND 56 body weight for PND 56–84, to examine the specific effects of EE removal and CVS recovery (Smith et al., Citation2017) (). Food intake was measured weekly once enrichment and stress paradigms ended, on PND 61–84. Over the time bins represented in , raw food intake was calculated for each pair housed rat as g/rat/day.
Somatic markers of chronic stress
On PND 89–90, rats were euthanized by injection intraperitoneally of an overdose of sodium pentobarbital (117 mg dissolved in 0.3 ml vehicle, injected at 120 min after FST exposure). Heart, thymus, and adrenal glands were collected, cleaned, and weighed for each rat. Adrenal weight was calculated as the sum of the left and right adrenal glands. Organ weights were normalized to each rat’s final bodyweight on PND 89–90 and analyzed as weight in mg/g.
Statistics
All data are shown as mean ± SEM. Data were analyzed by three-way ANOVA with sex, stress, and enrichment as between-subjects factors. For endpoints with repeated sampling (body weight, food intake, and serial blood sampling), data were analyzed by four-way repeated measures ANOVA with time as a within-subjects factor and sex, stress, and enrichment as between-subjects factors. SigmaPlot 12.5 (Systat Software Inc) and IBM SPSS Statistics 23 (SPSS Inc.) were used for analysis. Data analyzed by four-way repeated measures ANOVA are graphed separately by sex for clarity ( and ). For three-way ANOVAs, Fisher’s LSD post hoc testing was used to determine singular effects of either sex, enrichment, or stress, and the Sidak correction for multiple comparisons was used a posteriori to determine combined effects of sex × enrichment × stress. For four-way ANOVAs, a generalized linear model was employed (SPSS) with Greenhouse–Geisser correction for the omnibus F statement and Sidak correction for post hoc testing. Initially, we analyzed the data separately for males and females and we did not find any notable differences in the results. We decided to analyze males and females together because we sought to directly compare effects in one sex versus the other, so we used sex as a factor in addition to enrichment and stress.
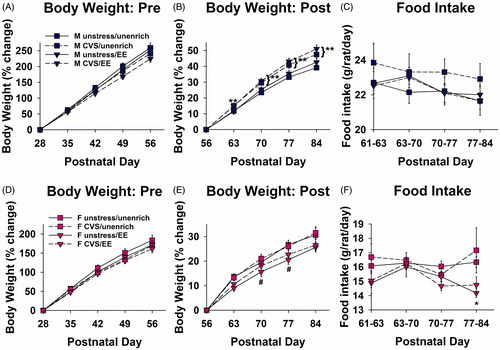
Figure 2. Adolescent enrichment loss does not increase body weight gain. (A) No differences in body weight change due to enrichment or stress during adolescent EE/CVS in males. (B) Adolescent CVS increased male body weight gain in adulthood (**applies to both EE and unenriched CVS male groups: p < 0.05 vs. unstressed male group, Sidak). (C) No differences in male food intake after EE/CVS were removed. (D) No differences in body weight change due to enrichment or stress during adolescent EE/CVS in females. (E) Adolescent EE decreased female weight gain in adulthood (#p < 0.05 vs. unenriched/unstressed females, Sidak). (F) Females previously exposed to enrichment and CVS had decreased food intake on the fourth week after these stimuli were removed (*p < 0.05 vs. unenriched CVS females, Sidak) (n = 10/group; four-way repeated measures ANOVAs). Data are mean ± SEM. (CVS: chronic variable stress; EE: environmental enrichment; Pre: prior to cessation of EE/CVS; Post: after cessation of EE/CVS).
Results
Body weight, food intake, and somatic measures
depicts the experimental timeline. For body weight on PND 28–56 (during EE and CVS exposure), there were significant within-subjects interactions of day × stress [F(4, 288) = 4.45; p = .036] and day × sex [F(4, 288) = 61.41; p < .001]. There were also significant between-subjects main effects of stress [F(1, 72) = 4.021; p = .049] and sex [F(1, 72) = 48.83; p < .001]. Starting at PND 35 in the enriched groups and at PND 42 in the unenriched groups, females gained less body weight than their male counterparts (p < .05, Sidak) (). Rats exposed to CVS gained less weight than the unstressed rats, but post hoc analysis did not reveal any further significant effects between groups. For raw body weight, see Supplemental Table 1 (males) and Supplemental Table 2 (females).
For body weight on PND 56–84 (after EE and CVS exposure), there were significant within-subjects interactions of day × enrichment × sex [F(4, 288) = 5.22; p = .01] and day × stress × sex [F(4, 288) = 4.61; p = .016]. There were also significant between-subjects interactions of enrichment × sex [F(1, 72) = 6.95; p = .01] and stress × sex [F(1, 72) = 5.38; p = .023]. Females gained less body weight than their respective male counterparts on PND 77–84 in all groups (p < .05, Sidak) (). Post hoc tests revealed significant EE effects in the females and significant CVS effects in the males. On PND 70 and 77 in the unstressed rats only, females previously exposed to EE had decreased body weight gain compared to unenriched females (p < .05, Sidak) (). This effect of EE was not observed in the males. The males previously exposed to CVS had increased body weight gain on PND 63–84 compared to the unstressed males, both in the enriched and unenriched groups (p < .05, Sidak) (). This effect of CVS was not observed in the females. For raw body weight in g, see Supplemental Table 1 (males) and Supplemental Table 2 (females).
For food intake on PND 63–84, there was a significant within-subjects interaction between day × enrichment [F(3, 96) = 5.71; p = .002]. There was also a significant between-subjects main effect of sex [F(1, 32) = 259; p < .001]. Females consumed less chow than males in all groups (p < .05, Sidak) (). For the stressed females on PND 77–84, CVS EE rats consumed less chow than the CVS unenriched females (p < .05, Sidak) (). This effect did not reach significance in the unstressed females (p = .063, Sidak).
There were no significant differences due to sex, stress, or enrichment on normalized heart weight (Supplemental Figure 1(A)). For normalized adrenal weight, there was a significant effect of sex [F(1, 72) = 664; p < .001]. Females had greater adrenal weight compared to males in all respective experimental groups (p < .05, Sidak) (Supplemental Figure 1(B)). See Supplemental Table 3 for raw adrenal weights (mean total right and left adrenal gland per group). For normalized thymus weight, there was also a significant effect of sex [F(1, 72) = 23.7; p < .001]. Females again had increased thymus weight compared to the males in the unenriched/unstressed, unenriched/CVS, and EE/unstressed groups (p < .05, Sidak) (Supplemental Figure 1(C)). This sex effect did not reach significance in the EE/CVS group (p > 0.05).
Open field test
In the open field, there were significant main effects of sex [F(1, 72) = 11.9; p = .001] and stress [F(1, 72) = 10.7; p = .002] on duration of time spent in the center of the open field. Females spent a greater duration of time in the center than males in the unstressed groups, both in enriched and unenriched conditions (p < .05, Sidak) (). There were no significant sex differences in the groups that received adolescent CVS. Adolescent CVS exposure decreased center duration, reaching significance in the females that did not receive adolescent enrichment (p < .05, Sidak) ().
Figure 3. Cautious behavior in the open field test (OFT) following chronic stress persists despite enrichment exposure. (A) Adolescent CVS decreased center time in the open field (potentially cautious behavior) in females without access to enrichment (*p < 0.05 vs. unenriched/unstressed females, #p < 0.05 vs. respective male group, Sidak). (B) Adolescent CVS decreased total distance traveled in males without access to enrichment (*p < 0.05 vs. unenriched/unstressed males, #p < 0.05 vs. males, Sidak). (C) Adolescent CVS decreased total number of center entries in the females (potentially cautious behavior), both in enriched and unenriched rats (*p < 0.05 vs. respective unstressed female group, #p < 0.05 vs. males, Sidak). (D) No significant differences in latency to travel to the center (n = 10/group; three-way ANOVAs). Data are mean ± SEM. (CVS: chronic variable stress; EE: environmental enrichment).
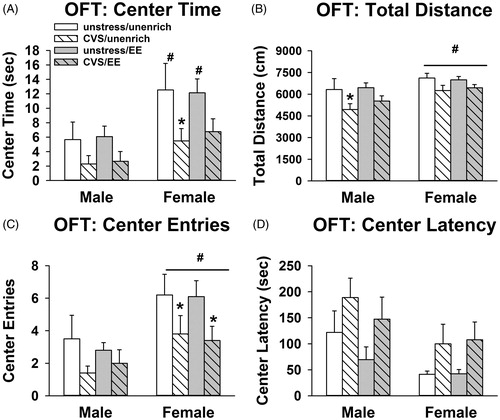
There were also effects of sex [F(1, 72) = 9.73; p = .003] and stress [F(1, 72) = 10.6; p = .002] on total distance traveled in the open field. Females traveled a greater distance and stress decreased distance traveled (p < .05, Fisher’s LSD) (). Specifically, CVS decreased distance traveled in males that did not receive enrichment (p < .05, Sidak) (). There were again significant main effects of sex [F(1, 72) = 12.3; p = .001] and stress [F(1, 72) = 8.17; p = .006] on number of entries into the center of the open field. Females made significantly more center entries than males and CVS exposure decreased center entries (p < .05, Fisher’s LSD) (). Exposure to CVS decreased center entries in the females (p < .05, Sidak) (). Finally, there were no significant differences in latency to enter the center of the open field (after log10 transforming the data to correct for non-homogenous variance) ().
Forced swim test
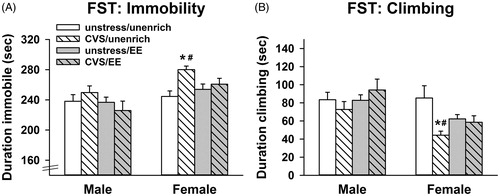
For immobility duration in the FST, there was a significant main effect of sex [F(1, 68) = 13.8; p < .001] and an interaction between enrichment x stress [F(1, 68) = 4.58; p = .036]. CVS increased immobility only in the females that did not receive enrichment (p < .05, Sidak) (). Females also had increased immobility compared to males in the CVS rats that did not receive enrichment (p < .05, Sidak) (). For climbing duration in the FST, there was a significant main effect of sex [F(1, 70) = 11.2; p = .001] and an interaction between enrichment × stress [F(1, 70) = 5.84; p = .018]. Stress decreased climbing behavior specifically in the females without enrichment (p < .05, Sidak) (). Only CVS-unenriched females had decreased climbing compared to males (p < .05, Sidak) (). There were no significant differences in swimming behavior.
Figure 4. Adolescent enrichment blocks adulthood emergence of passive coping behavior (immobility) caused by adolescent chronic stress in females. (A) Females stressed in adolescence without access to environmental enrichment (EE) had increased immobility and B.) decreased climbing in the forced swim test (FST; *p < 0.05 vs. unenriched/unstressed females, Sidak, #p < 0.05 vs. unenriched/CVS males, Sidak) (n = 10/group; three-way ANOVAs), Data are mean ± SEM. (CVS: chronic variable stress).
HPA axis response to FST
Analysis of plasma ACTH concentrations after FST revealed a within-subjects time effect [F(3, 216) = 301; p < .001] and time × sex interaction [F(3, 216) = 32.5; p < .001]. There was also a between-subjects effect of sex [F(1, 72) = 65.2; p < .001]. Females had significantly elevated plasma ACTH concentrations in comparison to males at the 15 and 30 min time points in all experimental groups (p < .05, Sidak) (). In the unenriched CVS group, females also differed from males at the 60 min time point (p < .05, Sidak) (). Differences between CVS and unstressed EE females at the 30 and 60 min time points did not reach statistical significance (p = .09 and p = .07, respectively) ().
Figure 5. Adolescent enrichment blocks adulthood emergence of acute stress-induced adrenal hypo-responsivity caused by adolescent chronic stress in females. (A) There were no significant effects of enrichment or CVS on plasma ACTH or (B) corticosterone concentrations in males (four-way repeated measures ANOVAs). (C) Adolescent stress decreased peak adrenal responsivity in females that were unenriched, while enrichment increased peak adrenal responsivity in females that were stressed (three-way ANOVA, Sidak; *p < 0.05 vs. unenriched/unstressed females, ∧p < 0.05 vs. unenriched/CVS females). (D) There were no significant effects of enrichment or CVS on plasma ACTH or (E) corticosterone concentrations in females (four-way repeated measures ANOVAs) (n = 10/group; Data are mean ± SEM; ACTH: adrenocorticotropic hormone; CORT: corticosterone); CVS: chronic variable stress; EE: environmental enrichment; FST: forced swim test.
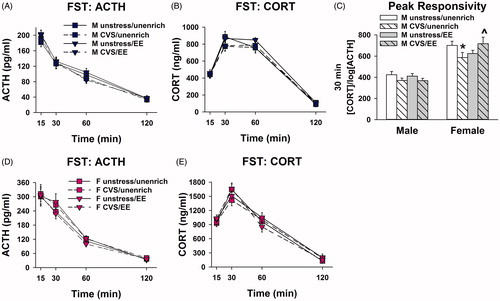
Analysis of plasma corticosterone concentrations after FST revealed a significant within-subjects time × sex interaction [F(3, 216) = 52.2; p < .001] and within-subjects time × enrichment × stress interaction [F(3, 216) = 3.09; p = .04]. Females had significantly higher corticosterone concentrations than their respective male counterparts at the 15 and 30 min time points (p < .05, Sidak) (). At the 30 min time point, stress tended to decrease corticosterone concentration specifically in the unenriched rats, but this effect did not reach significance (p = .055, Sidak) (). For the plasma corticosterone area under the curve (AUC), there was a significant effect of sex [F(1, 72) = 95.2; p < .001]. Females had significantly higher AUC than the males in all groups (p < .05, Sidak) (Supplemental Figure 2).
Because the data indicated a possible discordance between ACTH and corticosterone concentrations at the peak corticosterone response, peak adrenal responsivity was approximated for the 30-min time point by calculating [corticosterone]/log[ACTH] (Engeland, Byrnes, Presnell, & Gann, Citation1981; Jones, Myers, & Herman, Citation2011). This analysis revealed a significant effect of sex [F(1, 72) = 100; p < .001] and a significant interaction between enrichment × stress [F(1, 72) = 4.30; p = .042]. Adolescent exposure to CVS decreased peak adrenal responsivity to the FST challenge in adulthood, but only in females that did not receive enrichment (p < .05, Sidak) (). Adolescent enrichment increased adult peak adrenal responsivity specifically in females that were exposed to CVS, but did not increase it past the level of the unstressed unenriched rats (p < .05, Sidak) ().
Basal plasma corticosterone concentrations
For corticosterone concentrations measured from plasma taken on PND 86 at 10:00–12:00 h, there was a main effect of sex [F(1, 69) = 60.3; p < .001]. Females had higher corticosterone concentrations compared to their respective male counterparts (p < .05, Fisher’s LSD). Additionally, enrichment increased corticosterone concentrations specifically in the females but not in the males (p < .05, Sidak) (Supplemental Figure 3).
Discussion
Our results demonstrate that chronic stress in late adolescence causes expression of behavioral passive coping and decrease exploration in adulthood, and decreases HPA axis excitability (adrenal responsiveness to ACTH) following acute stress, specifically in females but not males. These behavioral shifts may allude to depression-like or anxiety-like characteristics. Importantly, exposure to environmental enrichment concurrently with chronic stress attenuates the emergence of passive coping (immobility), and blocks the effect of stress on adrenal responsivity. This indicates that an enrichment experience may offer protection from the long-term/delayed effects of concurrent stress in adolescent females.
Late adolescence is a critical period during which individuals can be hypersensitive to chronic stress, manifesting as exaggerated somatic, behavioral, and hormonal changes (Jankord et al., Citation2011; Wulsin et al., Citation2016). Females are especially sensitive to the long-term effects of adolescent chronic stress, as evidenced by the development of passive coping and blunted HPA responses in adulthood (Wulsin et al., Citation2016). Our study supports this finding and demonstrates that environmental interventions can be targeted to this at risk female population. Adolescent environmental enrichment specifically benefits adolescently stressed females, where males are relatively unaffected. Furthermore, female center entries in the open field are decreased by adolescent CVS and are not rescued by adolescent enrichment. This cautious, experience-dependent behavior may be adaptive (Del Giudice, Ellis, & Shirtcliff, Citation2011) and is preserved with enrichment, while the passive coping behavior and decrease in adrenal responsivity () are prevented. Overall, enrichment may buffer the effects of stress without blocking behaviors that could be necessary for survival.
In this study, enrichment exposure lasted for 4 weeks and encompassed late adolescence. Rats were removed from enrichment at the end of this period and assessed in adulthood. Previous work from our laboratory shows that exposing adult male rats to enrichment for 4 weeks and subsequently removing it causes marked physiological and behavioral changes (Smith et al., Citation2017). Adult males removed from enrichment have increased body weight gain, increased immobility in the FST, and blunted peak HPA responses (Smith et al., Citation2017). Instead, adolescent females removed from enrichment have a subsequent decrease in body weight gain. Future studies are needed to identify whether this is specific to adolescence or if enrichment removal in adult females will also decrease body weight gain. Although adult females have not yet been studied, our results indicate that removing enrichment from adolescent rats fails to produce effects seen with adult male enrichment removal. This indicates that adults may be more sensitive than adolescents to the effects of losing this rewarding stimulus. It is also possible that the beneficial effects of enrichment are more robust when exposure is initiated earlier in life, so they are preserved even after removal (Passig et al., Citation1996).
Females stressed in adolescence display passive coping strategy and blunted adrenal responses to stress in adulthood, as demonstrated by our current findings. Adolescent stress does not affect males in the same manner. Males stressed as adults exhibit depression-associated behavioral changes and exaggerated HPA axis responses immediately after stress (Choi et al., Citation2008; Wang, An, & Zhang, Citation2008; Willner, Citation1997), and enrichment removal in adult males induces passive coping strategies, possibly indicative of depression-like behavior (Smith et al., Citation2017). This suggests that males are more sensitive to the effects of stress and loss following exposure during adulthood (relative to adolescence), or alternatively, that the effects of chronic stress do not persist following discontinuation of stress exposure.
Whereas effects of concurrent enrichment and CVS are undetectable, it remains plausible that either manipulation may be of benefit in reducing exposure to chronic stress later in life, a possibility not tested here. According to the match/mismatch hypothesis and inoculation stress hypotheses, early life enrichment may serve as a mild stressor that causes biological adaptations to optimally prepare, or “inoculate” the organism for coping with subsequent stress exposure (Crofton, Zhang, & Green, Citation2015, Schmidt, Citation2011). Future studies are required to examine whether enrichment and/or concurrent stress and enrichment can block or attenuate chronic stress-related behavioral disruptions later in life.
Providing environmental enrichment superimposed with chronic stress to limit stress-induced changes in adulthood may improve stress coping in adolescence that is maintained throughout life. For example, the chronic stress-induced increase in FST immobility time that we report in females in our current study suggests deficits in stress coping (Commons, Cholanians, Babb, & Ehlinger, Citation2017), and adolescent enrichment blocks this effect. Future studies will identify changes in the brain that underlie the effects of adolescent stress in females and subsequent amelioration of these effects with adolescent enrichment.
Our current study sought to compare males and females simultaneously. Therefore, we were unable to include a large enough sample size to address any potential effects of estrous cycle on enrichment or stress exposure. Previous work from our laboratory shows that adolescent chronic stress causes physiological and behavioral changes in adult females without accounting for estrous cycle (Wulsin et al., Citation2016). We confirm these results and provide evidence that adolescent enrichment blocks these changes. However, future studies should take into account the potential influence of estrous cycle and gonadal steroids, especially since these hormones undergo dramatic changes during the adolescent period (Schulz & Sisk, Citation2016).
Taken together, our data demonstrate that an enriched environment can buffer against the long-term behavioral and hormonal manifestations of chronic stress in females. Environmental manipulations are immensely beneficial to the human population because they provide a safe therapeutic alternative to pharmacological interventions. Our data further suggest that adolescence is a possible critical period for enrichment to mitigate the impact of stress in females, underscoring the importance of early intervention. Understanding how to target environmental stimulation to at risk populations during sensitive developmental periods will enable us to optimize physical and mental health throughout the lifespan.
James_Herman_et_al_supplemental_content.zip
Download Zip (429.5 KB)Acknowledgements
We would like to thank our funding (to JPH R01 MH049698, R01 MH069860, R01 MH101729). We would also like to thank members of the Herman lab and Jody Caldwell from the Solomon lab for helping with the FST/blood and tissue collection.
Disclosure statement
The authors report no conflicts of interest.
References
- Andersen, S.L. (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews, 27, 3–18. doi:10.1016/S0149-7634(03)00005-8
- Andersen, S.L., & Teicher, M.H. (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences, 31, 183–191. doi:10.1016/j.tins.2008.01.004
- Bourke, C.H., & Neigh, G.N. (2011). Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior, 60, 112–120. doi:10.1016/j.yhbeh.2011.03.011
- Choi, D.C., Furay, A.R., Evanson, N.K., Ulrich-Lai, Y.M., Nguyen, M.M., Ostrander, M.M., & Herman, J.P. (2008). The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology, 33, 659–669. doi:10.1016/j.psyneuen.2008.02.006
- Commons, K.G., Cholanians, A.B., Babb, J.A., & Ehlinger, D.G. (2017). The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chemical Neuroscience, 8, 955–960. doi:10.1021/acschemneuro.7b00042
- Crofton, E.J., Zhang, Y., & Green, T.A. (2015). Inoculation stress hypothesis of environmental enrichment. Neuroscience and Biobehavioral Reviews, 49, 19–31. doi:10.1016/j.neubiorev.2014.11.017
- Cryan, J.F., Page, M.E., & Lucki, I. (2005a). Differential behavioral effects of the antidepressants reboxetine: fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology, 182, 335–344. doi:10.1007/s00213-005-0093-5
- Cryan, J.F., Valentino, R.J., & Lucki, I. (2005b). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and Biobehavioral Reviews, 29, 547–569. doi:10.1016/j.neubiorev.2005.03.008
- Del Giudice, M., Ellis, B.J., & Shirtcliff, E.A. (2011). The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35, 1562–1592. doi:10.1016/j.neubiorev.2010.11.007
- do Prado, C.H., Narahari, T., Holland, F.H., Lee, H.N., Murthy, S.K., & Brenhouse, H.C. (2016). Effects of early adolescent environmental enrichment on cognitive dysfunction, prefrontal cortex development, and inflammatory cytokines after early life stress. Developmental Psychobiology, 58, 482–491. doi:10.1002/dev.21390
- Eiland, L., & Romeo, R.D. (2013). Stress and the developing adolescent brain. Neuroscience, 249, 162–171. doi:10.1016/j.neuroscience.2012.10.048
- Engeland, W.C., Byrnes, G.J., Presnell, K., & Gann, D.S. (1981). Adrenocortical sensitivity to adrenocorticotropin (ACTH) in awake dogs changes as a function of the time of observation and after hemorrhage independently of changes in ACTH. Endocrinology, 108, 2149–2153. doi:10.1210/endo-108-6-2149
- Francis, D.D., Diorio, J., Plotsky, P.M., & Meaney, M.J. (2002). Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience, 22, 7840–7843.
- Ge, X., Natsuaki, M.N., & Conger, R.D. (2006). Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Development and Psychopathology, 18, 253–273. doi:10.1017/S0954579406060147
- Goodyer, I.M., Herbert, J., & Altham, P.M. (1998). Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychological Medicine, 28, 265–273.
- Hui, J.J., Zhang, Z.J., Liu, S.S., Xi, G.J., Zhang, X.R., Teng, G.J., … Reynolds, G.P. (2011). Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: a magnetic resonance study. Behavioural Brain Research, 217, 122–127. doi:10.1016/j.bbr.2010.10.014
- Jang, J.M., Park, J.I., Oh, K.Y., Lee, K.H., Kim, M.S., Yoon, M.S., … Chung, Y.C. (2014). Predictors of suicidal ideation in a community sample: roles of anger, self-esteem, and depression. Psychiatry Research, 216, 74–81.
- Jankord, R., Solomon, M.B., Albertz, J., Flak, J.N., Zhang, R., & Herman, J.P. (2011). Stress vulnerability during adolescent development in rats. Endocrinology, 152, 629–638. doi:10.1210/en.2010-0658
- Jasper, M.S., & Engeland, W.C. (1991). Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. The American Journal of Physiology, 261, (Pt 2):R1257–R1268.
- Johnson, E.M., Traver, K.L., Hoffman, S.W., Harrison, C.R., & Herman, J.P. (2013). Environmental enrichment protects against functional deficits caused by traumatic brain injury. Frontiers in Behavioural Neuroscience, 21, 44. doi:10.3389/fnbeh.2013.00044
- Jones, K.R., Myers, B., & Herman, J.P. (2011). Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiology & Behavior, 104, 266–271. doi:10.1016/j.physbeh.2011.03.021
- Koss, W.A., Belden, C.E., Hristov, A.D., & Juraska, J.M. (2014). Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse (New York, N.Y.), 68, 61–72. doi:10.1002/syn.21716
- Leussis, M.P., Lawson, K., Stone, K., & Andersen, S.L. (2008). The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse (New York, N.Y.), 62, 185–192. doi:10.1002/syn.20483
- Lupien, S.J., McEwen, B.S., Gunnar, M.R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience, 10, 434–445. doi:10.1038/nrn2639
- Markham, C.M., Norvelle, A., & Huhman, K.L. (2009). Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behavioural Brain Research, 198, 69–73. doi:10.1016/j.bbr.2008.10.022
- Morley-Fletcher, S., Rea, M., Maccari, S., & Laviola, G. (2003). Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. The European Journal of Neuroscience, 18, 3367–3374.
- Nolen-Hoeksema, S. (2001). Gender differences in depression. Current Directions in Psychological Science, 10, 173–176. doi:10.1111/1467-8721.00142
- Overstreet, D.H. (2002). Behavioral characteristics of rat lines selected for differential hypothermic responses to cholinergic or serotonergic agonists. Behavior Genetics, 32, 335–348.
- Overstreet, D.H., Keeney, A., & Hogg, S. (2004). Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. European Journal of Pharmacology, 492, 195–201. doi:10.1016/j.ejphar.2004.04.010
- Passig, C., Pinto-Hamuy, T., Moreno, J.P., Rodríguez, C., Rojas, C., & Rosas, R. (1996). Persistence of the cognitive effects of early stimulation assessed with an animal model. Revista Medica De Chile, 124, 409–416.
- Schmidt, M.V. (2011). Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology, 36, 330–338. doi:10.1016/j.psyneuen.2010.07.001
- Schulz, K.M., & Sisk, C.L. (2016). The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience and Biobehavioral Reviews, 70, 148–158. doi:10.1016/j.neubiorev.2016.07.036
- Smith, B.L., Lyons, C.E., Correa, F.G., Benoit, S.C., Myers, B., Solomon, M.B., & Herman, J.P. (2017). Behavioral and physiological consequences of enrichment loss in rats. Psychoneuroendocrinology, 77, 37–46. doi:10.1016/j.psyneuen.2016.11.040
- Toth, E., Gersner, R., Wilf-Yarkoni, A., Raizel, H., Dar, D.E., Richter-Levin, G., … Zangen, A. (2008). Age-dependent effects of chronic stress on brain plasticity and depressive behavior. Journal of Neurochemistry, 107, 522–532. doi:10.1111/j.1471-4159.2008.05642.x
- Vahl, T.P., Ulrich-Lai, Y.M., Ostrander, M.M., Dolgas, C.M., Elfers, E.E., Seeley, R.J., … Herman, J.P. (2005). Comparative analysis of ACTH and corticosterone sampling methods in rats. American Journal of Physiology Endocrinology and Metabolism, 289, E823–E828. doi:10.1152/ajpendo.00122.2005
- Veldhuis, J.D., Roemmich, J.N., & Rogol, A.D. (2000). Gender and sexual maturation-dependent contrasts in the neuroregulation of growth hormone secretion in prepubertal and late adolescent males and females-a general clinical research center-based study. The Journal of Clinical Endocrinology and Metabolism, 85, 2385–2394. doi:10.1210/jcem.85.7.6697
- Wang, D., An, S.C., & Zhang, X. (2008). Prevention of chronic stress-induced depression-like behavior by inducible nitric oxide inhibitor. Neuroscience Letters, 433, 59–64. doi:10.1016/j.neulet.2007.12.041
- Willing, J., & Juraska, J.M. (2015). The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience, 301, 268–275. doi:10.1016/j.neuroscience.2015.05.073
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology, 134, 319–329.
- Wulsin, A.C., Herman, J.P., & Solomon, M.B. (2010). Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology, 35, 1100–1112. doi:10.1016/j.psyneuen.2010.01.011
- Wulsin, A.C., Wick-Carlson, D., Packard, B.A., Morano, R., & Herman, J.P. (2016). Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology, 65, 109–117. doi:10.1016/j.psyneuen.2015.12.004
