Abstract
Pasireotide is a new-generation somatostatin analog that acts through binding to multiple somatostatin receptor subtypes. Studies have shown that pasireotide induces hyperglycemia, reduces glucocorticoid secretion, alters neurotransmission, and potentially affects stress responses typically manifested as hyperglycemia and increased corticosterone secretion. This study specifically aimed to evaluate whether pasireotide treatment modifies glucose and costicosterone secretion in response to acute restraint stress. Male Holtzman rats of 150–200 g were treated with pasireotide (10 µg/kg/day) twice-daily for two weeks or vehicle for the same period. Blood samples were collected at baseline and after 5, 10, 30, and 60 min of restraint stress. The three experimental groups comprised of vehicle + restraint (VEHR), pasireotide + restraint (PASR), and pasireotide + saline (PASNR). Following pasireotide treatment, no significant differences in baseline glucose and corticosterone levels were observed among the three groups. During restraint, hyperglycemia was observed at 10 min (p < .01 for both comparisons), peaked at 30 min (p < .01 for both comparisons) and showed higher 60 min areas under glucose curves in the VEHR and PASR stressed groups when compared to the non-stressed PASNR group (p < .05 for both comparisons). Restraint also increased corticosterone secretion in the VEHR and PASR stressed groups at 5 min (p < .01 for both comparisons), and peaked at 30 min (p < .01 for both comparisons) with corresponding higher 60 min areas under corticosterone curves when compared to the non-stressed PASNR group (p < .01 for both comparisons). In conclusion, pasireotide treatment does not modify hyperglycemic- and corticosterone-restraint stress responses, thus preserving acute stress regulation.
Introduction
Somatostatin is an inhibitory hormone widely distributed throughout the central nervous system and peripheral tissues, and plays an important regulatory role in neurotransmission (Csaba, Peineau, & Dournaud, Citation2012). Pasireotide (SOM230) is a multireceptor-targeted somatostatin analog with a cyclohexapeptide structure that exerts its pharmacologic activity through binding to multiple somatostatin receptor subtypes (sst) (Cuevas-Ramos & Fleseriu, Citation2016; Sun & Coy, Citation2016). It has recently been shown that pasireotide is effective in exerting inhibitory effects on pituitary adrenocorticotrophic hormone (ACTH) and adrenal corticosterone secretion (Silva, Schoeffter, Weckbecker, Bruns, & Schmid, Citation2005; Sun & Coy, Citation2016), as well as inducing hyperglycemia in both rats and humans (Schmid & Brueggen, Citation2012; Silverstein, Citation2016; Vergès, Citation2017).
Acute restraint stress activates the hypothalamic nuclei resulting in an autonomic discharge of catecholamines (Pacak et al., Citation1998). Specifically, restraint typically activates the paraventricular nucleus, medial preoptic nucleus, and the lateral hypothalamic area, all of which are known to be involved in metabolic stress regulation (Busnardo, Tavares, Resstel, Elias, & Correa, Citation2010). Furthermore, it has been commonly utilized as a model of stress-induced corticosterone secretion (Busnardo et al., Citation2010; Stewart, Roper, Young, O’Carroll, & Lolait, Citation2008).
In this study, we sought to evaluate whether pasireotide treatment would alter the hyperglycemic and corticosterone (the rodent equivalent of cortisol) secretion in rats subjected to acute restraint in order to address the possible role of pasireotide on acute stress regulation. We hypothesized that the switch on of stress-related pathways could enhance the hyperglycemic response and possibly reduce the stress-induced corticosterone response in pasireotide-treated stressed animals, as to the afore-mentioned known effects of pasireotide. To better address the stress-induced changes, we planned experiments to possibly start with similar baseline values among groups for both assessed variables. Therefore, experiments were based on a previous study from the co-author H.A.S (Schmid & Brueggen, Citation2012), which showed that the hyperglycemic effect of pasireotide in rats vanishes with prolongation of this treatment. Furthermore, experiments were started in the early morning, when corticosterone values are supposed to be very low in rats.
Materials and methods
Animals
Male Holtzman rats of 150–200 g weight provided from Federal University of Minas Gerais Medical School Central Animal Facility were maintained under temperature-controlled conditions with an artificial 12-hour light–dark cycle, and allowed standard chow and water ad libitum. All procedures, including anesthesia, were performed as recommended by the University Ethics Committee for Animal Experiments (protocol 1001/2007).
Compound and formulation
Pasireotide acetate (Novartis, Basel, Switzerland) was dissolved in 0.9% sterile saline, and stock solutions were stored frozen at −20 °C, under light protection, in 1 mg/mL concentration. Vials of stock solution were taken from the freezer every week, diluted with 0.9% sterile saline, and kept at 4 °C for daily treatment of the animals.
Experimental design
Rats were placed in individual cages and were treated with pasireotide acetate twice daily at 0800 h and 1700 h (10 µg/kg/day) injected subcutaneously for two weeks, or vehicle for the same period, as adapted from a previous established protocol (Silva et al., Citation2005).
Two days before the experiments, rats were weighed and then anesthetized with 0.075 mg/g intraperitoneal ketamine (Cristália, Itapira, Brazil) and 0.01 mg/g xylazine (Schering-Plough Coopers, Cotia, Brazil), followed by insertion of a silastic catheter (0.5 mm DI, 0.94 mm DE, N° 602-135, Dow Corning Corporation, Auburn, MI) through the jugular vein into the right atrium (Harms & Ojeda, Citation1974).
On the day of the experiment, rats had their venous catheter rinsed and connected to a polyethylene tube (PE 50) filled with heparinized saline (Liquemine, Roche, 10 IU/ml) after weighing and receiving their subcutaneous daily morning dose of pasireotide or 0.9% saline, accordingly. The animals were then returned to their home cages where they were freely moving and without access to food or drink for 60 min.
Blood samples (0.5 ml) were collected via the atrium catheter immediately before, and after 5, 10, 30, and 60 min of restraint stress or 0.9% saline infusion, in pre-chilled citrated eppendorf tubes, and were kept on ice until the end of the experiment. Plasma volume was restored by saline injection after each blood collection. All the experiments were carried out between 8:00 h and 12:00 h in a quiet equipped experimental room for accomplishment of stress experiments under temperature control. Plasma samples were separated by centrifugation (900 G, 20 min, 4 °C) and frozen at −80 °C until measurements.
The restraint stressor was made by a polyvinyl chloride (PVC) cylinder (21 cm length and 4.5 cm diameter), where they could not move, causing discomfort without apparent pain. The animals were divided into 3 experimental groups: control rats subjected to restraint stress (VEHR, n = 9), pasireotide-treated rats submitted to restraint stress (PASR, n = 8), and pasireotide-treated rats receiving a 0.9% saline infusion and not subjected to restraint stress (PASNR, n = 7). For the two groups of rats treated with pasireotide, the animals were randomly assigned to either stressed- or non-stressed group on the day of the experiment.
Assays
We have determined plasma glucose and corticosterone values as an index of sympathetic discharge and activation of hypothalamic–pituitary adrenal (HPA) axis, respectively. All parameters were assayed in duplicate. Glucose was assayed by glucose oxidize method (GOD-ANA, CENTERLAB, Santa Luzia, Minas Gerais, Brazil). Corticosterone was measured by an Elisa kit (Assay Designs Inc, Ann Arbor, MI) with average corticosterone intra- and inter-assay variations of 7% and 9%, respectively. The reported detection limit of the method is between 0.032 and 200 ng/mL. Measures higher than 200 ng/mL were diluted and then re-measured.
Statistical analysis
The statistical program GraphPad Prism 5 (La Jolla, CA) was used. The results were expressed as mean ± standard error of the mean (SEM). All data were first checked for normal distribution through Kolmogorov–Smirnov test. Baseline samples were then compared by a one-way ANOVA. Samples obtained during restraint stress were compared to respective baseline samples by Student’s paired t-test. The differences among groups during stress were determined by two-way repeated measures ANOVA and confirmed by Bonferroni's post hoc test. The areas under glucose and corticosterone curves were calculated by the trapezoidal rule and compared by one-way ANOVA followed by Bonferroni's post hoc test. p Values <.05 were considered as statistically significant.
Results
Effects of restraint stress on plasma glucose levels in pasireotide-treated rats
As illustrated in , the baseline plasma glucose levels compared through one-way ANOVA did not reach statistical difference among VEHR group and the two pasireotide-treated PASR and PASNR groups (n = 7–9 for each group, 88.11 ± 3.8, 102.6 ± 5.13, 96.29 ± 5.81 mg/dL, respectively, F (3,21) = 2.45, overall p > .05). In the VEHR group (n = 9), restraint induced an increase in plasma glucose levels at 5 min (99.28 ± 4.52 vs. 88.11 ± 3.8 mg/dL, t (8) = 3.59, p < .01), peaking at 30 min (119.0 ± 11.47 vs. 88.11 ± 3.8 mg/dL, t (8) = 3.52, p < .01), and persisting at elevated levels at 60 min (109.2 ± 3.4 vs. 88.11 ± 3.8 mg/dL, t (8) = 6.52, p < .01) although tending to return to baseline levels. In the PASR group (n = 8), plasma glucose levels increased at 10 min (116.0 ± 5.39 vs. 102.6 ± 5.13 mg/dL, t (7) = 4.02, p < .01), peaking at 30 min (121.9 ± 10.32 vs. 102.6 ± 5.13 mg/dL, t (7) = 2.37, p < .05), and persisting at elevated levels when compared to baseline at 60 min (116.2 ± 7.81 mg/dL vs. 102.6 ± 5.13 mg/dL, t (7) = 2.60, p < .05). In contrast, plasma glucose levels in PASNR (n = 7) did not otherwise vary significantly during the experiment (t (6) = 1.49, 1.38, 2.22, 2.17 for 5, 10, 30, and 60 min, respectively, p > .05 for all comparisons). The two-way repeated measures ANOVA did not reveal significant differences between stressed groups at any of the time points (F (8,84) = 3.83, p > .05 for all).
Figure 1. Effects of restraint stress upon plasma glucose levels in rats treated with pasireotide. VEH (N = 9): control rats submitted to restraint stress; PASR (N = 8): pasireotide-treated rats submitted to restraint stress. PASRN: pasireotide-treated rats receiving a 0.9% saline infusion and not submitted to restraint stress #p < .05 for comparisons with baseline; ##p < .001 for comparisons with baseline; **p < .01 for comparisons with baseline; Δp < .05 for comparisons with PASNR.
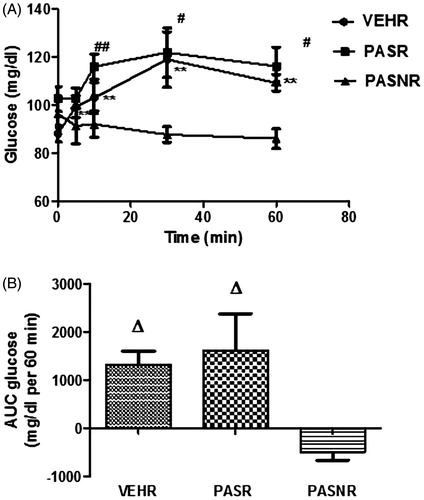
In addition, the VEHR and PASR 60 min areas under glucose curves, as analyzed through one-way ANOVA (F (3,21) = 4.98, overall p < .05), were significantly higher when compared to PASNR (p < .05 for both comparisons through Bonferroni's post hoc test), while there was no statistical difference for the comparison between stressed VEHR and PASR groups (p > .05 through Bonferroni's post hoc test, ).
Effects of restraint stress on plasma corticosterone levels in pasireotide-treated rats
As shown in , the baseline morning corticosterone values in rats with indwelling catheter were very similar in VEHR controls (n = 9, 8.19 ± 0.90 ng/mL) when compared through one-way ANOVA to the PASR (n = 8) and PASNR (n = 7) groups (9.13 ± 0.85 and 9.94 ± 2.16 ng/mL, respectively, F (3,21) = 0.43, overall p > .05). During restraint, there was a significant increase in corticosterone levels in both stressed VEHR and PASR groups at 5 min (149.10 ± 11.22 and 136.0 ± 25.0 ng/mL, t (8) = 12.51 and t (7) = 5.07, respectively, p < .01 for both comparisons to respective baseline values), peaking at 30 min (343.4 ± 61.38 and 279.7 ± 32.37 ng/mL, t (8) = 5.49 and t (7) = 8.3, respectively, p < .001 for both comparisons to respective baseline values), and remaining elevated at 60 min (145.7 ± 18.38 and 179.6 ± 18.45 ng/mL, t (8) = 7.52 and t (7) = 9.39, respectively, p < .001 for both comparisons to respective baseline values). On the other hand, PASNR showed a slight elevation in corticosterone levels which reached significant values at both 10 (21.84 ± 2.27 ng/mL, t (6) = 2.89, p < .05 for comparison with respective baseline), and at 30 min (26.80 ± 3.47 ng/mL, t (6) = 4.98, p < .01 for comparison with respective baseline). The two-way repeated measures ANOVA did not reveal significant differences between stressed groups for corticosterone secretion at any of the time points (F (8,84) = 7.97, p > .05 for all).
Figure 2. Effects of restraint stress upon plasma corticosterone levels in rats treated with pasireotide. VEH (N = 9): control rats submitted to restraint stress; PASR (N = 8): pasireotide-treated rats submitted to restraint stress. PASRN: pasireotide-treated rats receiving a 0.9% saline infusion and not submitted to restraint stress. **p < .01 for comparisons with baseline; ##p < .01 for comparisons with baseline; +p < .05 for comparisons with baseline; ++p < .01 for comparisons with baseline; ♦♦p < .01 for comparisons with PASNR.
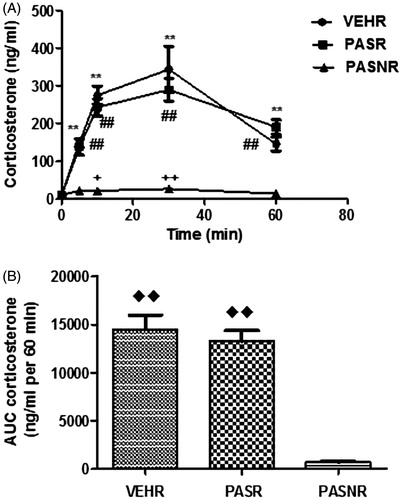
In addition, the analysis of the 60 min area under corticosterone curves (), as analyzed through one-way ANOVA F (3,21) = 4.98, overall p < .01), demonstrated similar corticosterone increases in response to restraint in the VEHR and PASR groups (p > .05 through Bonferroni's post hoc test), but otherwise much significantly higher values when compared to PASRN, p < .001 for both comparisons through Bonferroni's post hoc test.
Discussion
Pasireotide is a new-generation, multireceptor-targeted somatostatin analog targeting mainly sst5, that is currently approved for the treatment of patients with ACTH- and GH-secreting pituitary adenomas (Cuevas-Ramos & Fleseriu, Citation2016). The effects of chronic pasireotide treatment in inducing fasting and post-prandial hyperglycemia are well-known in humans (Breitschaft, Hu, Hermosillo Reséndiz, Darstein, & Golor, Citation2014; Silverstein, Citation2016; Vergès, Citation2017), but little is known about its in vivo effects on glucose and corticosterone metabolism in stressful situations. Our study demonstrates that acute restraint induces an early hyperglycemic response that is maintained throughout the 60 min experiment without differences as related to pasireotide treatment. In addition, we also noted that acute restraint elicited a strong and comparable increase in corticosterone levels in both stressed controls and pasireotide-treated stressed rats. Overall, we found that pasireotide treatment did not modify the acute restraint stress responses, as evaluated by the changes in glucose levels and corticosterone secretion.
Recently, there has been a greater understanding of the mechanisms that underlie the development of pasireotide-induced hyperglycemia that is primarily due to its direct effects in inhibiting insulin and incretin secretion (Henry et al., Citation2013; Vergès, Citation2017). Insulin secretion is mediated largely by sst2 and sst 5, whereas glucagon secretion is mediated mainly by sst2 (Henry et al., Citation2013; Schmid & Brueggen, Citation2012). Pasireotide binds to sst2 and sst5, with the greatest affinity to sst5, which is expressed not only by the pituitary but also by other cell types including pancreatic beta-cells (Henry et al., Citation2013; Schmid & Brueggen, Citation2012). However, the hyperglycemic effect of pasireotide in rats has been shown to be transient due to its minimal effects on glucagon secretion and neutral effects on insulin sensitivity (Schmid & Brueggen, Citation2012). In addition, IGFBP-2 levels have been shown to increase with pasireotide therapy (Schmid et al., Citation2016), which could attenuate the long-term effects of pasireotide. In our study, we have demonstrated that baseline plasma glucose levels of pasireotide-treated rats were not significantly higher than controls, suggesting that the afore-mentioned mechanisms may have been switched on to explain these results. Furthermore, prior studies from our group (Schmid & Brueggen, Citation2012) demonstrated that the rapid increase in plasma glucose in free-feeding unstressed rats using this dose of pasireotide dissipated over the course of long-term treatment, accounting for the failure to see significant effects in this study. In fact, studies in human subjects also show (Golor et al., Citation2012) transient elevations in blood glucose levels 2–6 hours after administration of pasireotide at doses between 200 μg and 1200 μg, but this resolved without intervention by 23 hours post-dosing. Our data, however, do not allow us to disregard the possibility of a hyperglycemic effect of pasireotide on baseline glucose for treatments prolonging for over two weeks in rats, as often observed in human diseases (Vergès, Citation2017). On the other hand, the restraint stress discharge of cathecolamines was such a potent stimulus of hyperglycemia (Kvetnansky et al., Citation1978; Pacak et al., Citation1998) that it possibly overcame the effects of pasireotide on pancreatic beta cells. Importantly, the hyperglycemic stress response was not changed by the pasireotide treatment as could be expected due to the effects of the somatostatin analog on the central neurotransmission (Csaba et al., Citation2012).
In addition, we did not observe a significant variation in baseline corticosterone secretion in our pasireotide-treated animals when compared to controls. These data have to be interpreted in the context of normal animals instead of those with tumors over-expressing sst5, when a decrease in corticosterone secretion might be expected (Castillo et al., Citation2011). Furthermore, our data also demonstrate that acute restraint stress exerted a rapid stimulatory effect on corticosterone secretion in both groups of stressed animals, implying that within a short time frame, this effect was not influenced by the pasireotide anti-secretory effect of ACTH from the pituitary and subsequently the corticosterone secretion from the adrenal.
Interestingly, we should keep in mind that the absence of significant differences in both baseline glucose and corticosterone levels among groups does not preclude the possibility of modifications of stress responses as related to previous treatments. Indeed, our group has disclosed some relevant stress-triggered effects (Carvalho Miranda et al., Citation2011; Reis, Ribeiro-de-Oliveira, Guerra, Reis, & Coimbra, Citation1996; Ribeiro-de-Oliveira et al., Citation1999), although the present study seems to preclude pasireotide effects in an acute stress time frame, However, due to the specificity of the stress responses, the extrapolation of these data to other stress subtypes should be seen with caution once each stressor has its own central neurochemical and peripheral neuroendocrine “signature” (Pacak et al., Citation1998). Further studies should address whether chronic stress exposure, when stress responses are expected to be lessened, would be able to disclose stress-induced changes in animals treated with pasireotide.
Somatostatin is known to inhibit hormone secretion by acting through modulation of the cAMP cascade and ion channel permeability. However, studies have shown that several other mechanisms may also be responsible, e.g. additional G-proteins, receptor subtype-specific phosphorylation and internalization, and activation of MAP kinase (Patel, Citation1999; Weckbecker et al., Citation2003). Furthermore, pasireotide can also act as an agonist on some signal transduction mechanisms and antagonist on others, and it is possible that intracellular trafficking is distinct from that activated by native somatostatin or analogs acting mainly upon sst2 (Cescato et al., Citation2010; Kao, Ghosh, & Schonbrunn, Citation2011; Lesche, Lehmann, Nagel, Schmid, & Schulz, Citation2009; Pöll et al., Citation2010). All that said, it is important to acknowledge through our data that pasireotide has not changed the responses to restraint.
In this study, a mild but significant increase in corticosterone could be detected in pasireotide-non-stressed controls at both 10 and 30 min after saline infusion. These changes could be related to the withdrawal of blood and restoration of volume throughout the experiment in freely moving non-anesthetized jugular-cannulated animals, with corticosterone peak at 30 min, as shown by others (Vachon & Moreau, Citation2001). Pecori Giraldi et al. (Citation2012) found that pasireotide has the capacity to induce a moderate stimulatory effect on adrenal corticosterone secretion in vitro without affecting ACTH-stimulated steroid secretion. In this regard, we have to acknowledge the limitation of not including a fourth control group of animals neither pasireotide-treated and nor submitted to restraint stress to better address the finding of increased corticosterone secretion after saline infusion in pasireotide-treated rats. It should be noted that fluid replacement was conducted to account for blood loss over time, so observed effects are unlikely to be due to hemodynamic stimuli. The experience of our group and others with stress experiments showing mild elevations of stress parameters in rats with indwelling catheters (Carvalho Miranda et al., Citation2011; Vachon & Moreau, Citation2001), as well as the well-known suppressive effects of corticosterone secretion by pasireotide treatment, turn the possibility of stimulation of corticosterone secretion by pasireotide quite improbable.
In conclusion, we have clearly demonstrated that two weeks of pasireotide treatment does not significantly modify baseline levels of glucose and corticosterone in rats. Furthermore, it does not change the hyperglycemic- and corticosterone-acute restraint stress responses, suggesting the preservation of metabolic and hormonal acute stress regulation. To the best of our knowledge, this is the first study evaluating the in vivo effects of pasireotide on stress, and our data indicate that the hyperglycemic-inducing effects of pasireotide as well as its suppressive effects on corticosterone secretion were over-ridden by the effects of acute stress, thus shedding new light into improving our understanding of several aspects that underlie pasireotide treatment on glucose and corticosterone homeostasis.
Summary
This study shows that pasireotide, a new somatostatin analog approved for treatment of both Cushing's disease and acromegaly in humans, does not significantly change the baseline blood glucose and corticosterone levels in rats treated with this compound for two weeks. Furthermore, pasireotide preserves the acute regulatory stress responses in a stress model typically related to social behavior, in spite of its well-known actions on both the central and peripheral neurotransmission.
Acknowledgements
We thank Norvatis SA for kindly donating pasireotide acetate for the experiments.
Disclosure statement
There is no conflict of interest declared for this study.
Additional information
Funding
References
- Breitschaft, A., Hu, K., Hermosillo Reséndiz, K., Darstein, C., & Golor, G. (2014). Management of hyperglycemia associated with pasireotide (SOM230): Healthy volunteer study. Diabetes Research and Clinical Practice, 103, 458–465. doi:10.1016/j.diabres.2013.12.011
- Busnardo, C., Tavares, R.F., Resstel, L.B., Elias, L.L., & Correa, F.M. (2010). Paraventricular nucleus modulates autonomic and neuroendocrine responses to acute restraint stress in rats. Autonomic Neuroscience, 158, 51–57. doi:10.1016/j.autneu.2010.06.003
- Carvalho Miranda, P.A., Simões, E., Silva, A.C., de Oliveira Longo, J.R., Magalhães Madureira, M., Bastos Fóscolo, R., … Ribeiro-Oliveira, A. (2011). Angiotensin-converting enzyme inhibition changes the metabolic response to neuroglucopenic stress. Journal of the Renin Angiotensin Aldosterone System, 12, 153–160. doi:10.1177/1470320310390726
- Castillo, V., Theodoropoulou, M., Stalla, J., Gallelli, M.F., Cabrera-Blatter, M.F., Haedo, M.R., … Arzt, E. (2011). Effect of SOM230 (pasireotide) on corticotropic cells: action in dogs with Cushing's disease. Neuroendocrinology, 94, 124–136. doi:10.1159/000327429
- Cescato, R., Loesch, K.A., Waser, B., Mäcke, H.R., Rivier, J.E., Reubi, J.C., & Schonbrunn, A. (2010). Agonist-biased signaling at the sst2A receptor: the multi-somatostatin analogs KE108 and SOM230 activate and antagonize distinct signaling pathways. Molecular Endocrinology, 24, 240–249. doi:10.1210/me.2009-0321
- Csaba, Z., Peineau, S., & Dournaud, P. (2012). Molecular mechanisms of somatostatin receptor trafficking. Journal of Molecular Endocrinology, 48, R1–12. doi:10.1530/JME-11-0121
- Cuevas-Ramos, D., & Fleseriu, M. (2016). Pasireotide: a novel treatment for patients with acromegaly. Drug Design Development and Therapy, 10, 227–239. doi:10.2147/DDDT.S77999
- Golor, G., Hu, K., Ruffin, M., Buchelt, A., Bouillaud, E., Wang, Y., & Maldonado, M. (2012). A first-in-man study to evaluate the safety, tolerability, and pharmacokinetics of pasireotide (SOM230), a multireceptor-targeted somatostatin analog, in healthy volunteers. Drug Design Development and Therapy, 6, 71–79. doi:10.2147/DDDT.S29125
- Harms, P.G., & Ojeda, S.R. (1974). A rapid and simple procedure for chronic cannulation of the rat jugular vein. Journal of Applied Physiology, 36, 391–392. doi:10.1152/jappl.1974.36.3.391
- Henry, R.R., Ciaraldi, T.P., Armstrong, D., Burke, P., Ligueros-Saylan, M., & Mudaliar, S. (2013). Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. Journal of Clinical Endocrinology & Metabolism, 98, 3446–3453. doi:10.1210/jc.2013-1771
- Kao, Y.J., Ghosh, M., & Schonbrunn, A. (2011). Ligand-dependent mechanisms of sst2A receptor trafficking: role of site-specific phosphorylation and receptor activation in the actions of biased somatostatin agonists. Molecular Endocrinology, 25, 1040–1054. doi:10.1210/me.2010-0398
- Kvetnansky, R., Sun, C.L., Lake, C.R., Thoa, N., Torda, T., & Kopin, I.J. (1978). Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology, 103, 1868–1874. doi:10.1210/endo-103-5-1868
- Lesche, S., Lehmann, D., Nagel, F., Schmid, H.A., & Schulz, S. (2009). Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. Journal of Clinical Endocrinology & Metabolism, 94, 654–661. doi:10.1210/jc.2008-1919
- Pacak, K., Palkovits, M., Yadid, G., Kvetnansky, R., Kopin, I.J., & Goldstein, D.S. (1998). Heterogeneous neurochemical responses to different stressors: a test of Selye's doctrine of nonspecificity. American Journal of Physiology, 275(4 Pt 2), R1247–R1255. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9756557
- Patel, Y.C. (1999). Somatostatin and its receptor family. Frontiers in Neuroendocrinology, 20, 157–198. doi:10.1006/frne.1999.0183
- Pecori Giraldi, F., Pagliardini, L., Cassarino, M.F., Martucci, F., Sesta, A., Castelli, L., … Cavagnini, F. (2012). Stimulatory effect of SOM230 on human and rat adrenal corticosteroid secretion in vitro. General and Comparative Endocrinology, 178, 436–439. doi:10.1016/j.ygcen.2012.05.003
- Pöll, F., Lehmann, D., Illing, S., Ginj, M., Jacobs, S., Lupp, A., … Schulz, S. (2010). Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Molecular Endocrinology, 24, 436–446. doi:10.1210/me.2009-0315
- Reis, F.M., Ribeiro-de-Oliveira, A., Guerra, R.M., Reis, A.M., & Coimbra, C.C. (1996). Blood glucose and prolactin in hyperprolactinemic rats exposed to restraint and surgical stress. Life Sciences, 58, 155–161. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8606624
- Ribeiro-de-Oliveira, A., Guerra, R.M., Fóscolo, R.B., Marubayashi, U., Reis, A.M., & Coimbra, C.C. (1999). Effects of chronic bromocriptine (CB-154) treatment on the plasma glucose and insulin secretion response to neurocytoglucopenia in rats. Journal of Endocrinology, 162, 237–242. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/?term=ribeiro+de+oliveira+1999
- Schmid, H.A., & Brueggen, J. (2012). Effects of somatostatin analogs on glucose homeostasis in rats. Journal of Endocrinology, 212, 49–60. doi:10.1530/JOE-11-0224
- Schmid, H.A., Brue, T., Colao, A., Gadelha, M.R., Shimon, I., Kapur, K., … Fleseriu, M. (2016). Effect of pasireotide on glucose- and growth hormone-related biomarkers in patients with inadequately controlled acromegaly. Endocrine, 53, 210–219. doi:10.1007/s12020-016-0895-8
- Silva, A.P., Schoeffter, P., Weckbecker, G., Bruns, C., & Schmid, H.A. (2005). Regulation of CRH-induced secretion of ACTH and corticosterone by SOM230 in rats. European Journal of Endocrinology, 153, R7–R10. doi:10.1530/eje.1.01998
- Silverstein, J.M. (2016). Hyperglycemia induced by pasireotide in patients with Cushing's disease or acromegaly. Pituitary, 19, 536–543. doi:10.1007/s11102-016-0734-1
- Stewart, L.Q., Roper, J.A., Young, W.S., O'Carroll, A.M., & Lolait, S.J. (2008). Pituitary-adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. Journal of Neuroendocrinology, 20, 597–605. doi:10.1111/j.1365-2826.2008.01704.x
- Sun, L., & Coy, D.H. (2016). Somatostatin and its Analogs. Current Drug Targets, 17, 529–537. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/ 26951062
- Vachon, P., & Moreau, J.P. (2001). Serum corticosterone and blood glucose in rats after two jugular vein blood sampling methods: comparison of the stress response. Contemporary Topics in Laboratory Animal Science, 40, 22–24. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11560401
- Vergès, B. (2017). Effects of anti-somatostatin agents on glucose metabolism. Diabetes & Metabolism, 43, 411–415. doi:10.1016/j.diabet.2017.05.003
- Weckbecker, G., Lewis, I., Albert, R., Schmid, H.A., Hoyer, D., & Bruns, C. (2003). Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nature Reviews Drug Discovery, 2, 999–1017. doi:10.1038/nrd1255
