Abstract
Chronic mild stress can lead to negative health outcomes. Frequency, duration, and intensity of acute stressors can affect health-related processes. We tested whether the temporal pattern of daily acute stressors (clustered or dispersed across the day) affects depression-related physiology. We used a rodent model to keep stressor frequency, duration, and intensity constant, and experimentally manipulated the temporal pattern of acute stressors delivered during the active phase of the day. Adult male Sprague-Dawley rats were exposed to one of three chronic mild stress groups: Clustered: stressors that occurred within 1 hour of each other (n = 21), Dispersed: stressors that were spread out across the active phase (n = 21), and Control: no stressors presented (n = 21). Acute mild stressors included noise, strobe lights, novel cage, cage tilt, wet bedding, and water immersion. Depression-related outcomes included: sucrose preference, body weight, circulating glucocorticoid (corticosterone) concentration after a novel acute stressor and during basal morning and evening times, and endotoxin-induced circulating interleukin-6 concentrations. Compared to control rats, those in the Clustered group gained less weight, consumed less sucrose, had a blunted acute corticosterone response, and an accentuated acute interleukin-6 response. Rats in the Dispersed group had an attenuated corticosterone decline during the active period and after an acute stressor compared to the Control group. During a chronic mild stress experience, the temporal distribution of daily acute stressors affected health-related physiologic processes. Regular exposure to daily stressors in rapid succession may predict more depression-related symptoms, whereas exposure to stressors dispersed throughout the day may predict diminished glucocorticoid negative feedback.
Introduction
Accumulation of acute daily stressors can amount to chronic stress with clear health implications. Research with both humans and animals indicates that chronic stress is related to the development and progression of several negative health outcomes (Lupien, McEwen, Gunnar, & Heim, Citation2009; Steptoe & Kivimaki, Citation2012; Tamashiro, Sakai, Shively, Karatsoreos, & Reagan, Citation2011). As a general construct, chronic stress has been characterized as ongoing or frequent intermittent challenging life events that are associated with prolonged changes in physiology which may increase vulnerability to pathology (de Kloet, Joëls, & Holsboer, Citation2005; Joëls, Karst, Krugers, & Lucassen, Citation2007; McEwen, Citation2004; Smyth, Zawadzki, & Gerin, Citation2013). In rodents, these effects have been well-documented in depression-related biologic processes: exposure to several weeks of repeated, acute, physical or social challenges leads to anhedonic behavior, altered glucocorticoid circulation, and accentuated pro-inflammatory responses (Avitsur, Stark, & Sheridan, Citation2001; Bielajew, Konkle, & Merali, Citation2002; Dal-Zotto, Martí, & Armario, Citation2000; Stark et al., Citation2001). In humans, similar associations have been documented between chronic stress and depressive symptoms, glucocorticoid regulation, and pro-inflammatory cytokines (Hammen, Citation2005; Miller, Chen, & Zhou, Citation2007; Robles, Glaser, & Kiecolt-Glaser, Citation2005).
Studies on the characteristics of discrete stressors that make up chronic stress have focused on stressor intensity, duration, and/or frequency. Many of these studies tracked glucocorticoid hormone responses to these different characteristics because of clear associated health implications. In rats, acute stressor intensity, frequency, and duration affect the degree and duration of circulating adrenocorticotropic & glucocorticoid hormone elevations (Dhabhar & McEwen, Citation1997; García et al., Citation2000; García, Martí, Vallès, Dal-Zotto, & Armario, Citation2000; Grissom & Bhatnagar, Citation2009; Marquez, Belda, & Armario, Citation2002; Martí et al., Citation2001). Similar effects have been documented in humans for stressor intensity and frequency (Dickerson & Kemeny, Citation2004; Kudielka, Hellhammer, & Wust, Citation2009; Stawski, Cichy, Piazza, & Almeida, Citation2013). Recent conceptual models have discussed the potential importance of the temporal patterning of acute stressors within chronic stress (Smyth et al., Citation2013), but there is little empirical evidence about whether or how the temporal distribution of chronic daily acute stressors affects physiology over time. Thus, in the current study, we tested whether the daily temporal pattern of repeat exposure to acute stressors affects health-related physiology. We used a rodent model to determine if daily acute stressors clustered close together in time or dispersed throughout the day differentially influenced depression-related outcomes (altered weight gain, hedonic behavior, glucocorticoid regulation, and pro-inflammatory responses). With a rodent model, we were able to manipulate acute stressor temporal patterns while keeping stressor intensity, frequency, and duration constant.
We tested three hypotheses: (i) the temporal summation hypothesis, (ii) the anticipatory hypothesis, and (iii) the null hypothesis. The temporal summation hypothesis proposes that health consequences are more extensive when daily acute stressors occur in rapid succession as opposed to spread out over the day. This is based on the assumption that biologic responses to temporally clustered acute stressors summate over time if there is not enough time between stressors to allow for return to basal activity. If supported, we expected that rats exposed to daily stressors in rapid succession (“Clustered”) would show the greatest evidence of slowed growth, dampened hedonic behavior, glucocorticoid dysregulation (slower daily decline, dampened acute response, and/or slower recovery following acute stressor), and increased inflammatory responses compared to Control rats. The anticipatory hypothesis proposes that health consequences are worse when daily stressors are dispersed across the day because the organism must maintain elevated physiologic preparedness throughout the day. If this hypothesis is accurate, we expected that rats exposed to daily acute stressors throughout the day (“Dispersed”) would show the greatest evidence of slowed growth, decreased hedonic behavior, greater glucocorticoid dysregulation, and increased peripheral inflammatory responses. Under the null hypothesis, the temporal pattern of acute stressors within chronic stress is of no consequence, and both forms of chronic stress (“Clustered” versus “Dispersed”) will lead to similar outcomes.
Methods
Animals
As an initial study on stressor temporal dynamics, we studied male rats because their circulating glucocorticoid concentration and behavioral activity are less variable from day-to-day than females. Sixty-three adult male Sprague-Dawley rats (60 days of age) were purchased from Charles River Laboratories (Wilmington, MA). To minimize additional complex and uncontrollable stressors associated with male–male social interactions within a cage, rats were single-housed in standard (20 × 26 × 46 cm) cages containing sawdust bedding and changed once a week. Prior to any testing, all rats were allowed to acclimate to the laboratory housing for 1 week, and experimental stress manipulations did not begin for 16 days. Conditions within the colony room were controlled at 30–70% humidity and 21 ± 1 °C. Food and water were available ad libitum except during periods of stress exposure. A wooden chewing block and a clear red hard plastic cylinder were also provided in the home cage for enrichment. Rats were housed in a reverse 12 L:12 D lighting schedule (lights off at 09:00 h and on at 21:00 h EST). All manipulations and measures were conducted during, just prior to, or after the onset of the dark phase of the lighting schedule—i.e. during the nocturnal rat active phase. This timing was selected to best mimic conditions when organisms normally encounter naturalistic stressors. Thus, in the current study, “morning” samples refer to those collected at the beginning of the dark (active) phase, and “evening” samples refer to those collected at the end of the dark phase. Rats were weighed weekly to monitor weight gain. Procedures complied with National Institutes for Health Guide for the Care and Use of Laboratory Animals, and were approved by the Pennsylvania State University Institutional Animal Care and Use Committee (IACUC No. 45196).
Experimental design
The study was divided into three stages relative to a chronic mild stress (CMS) manipulation: pre-CMS, CMS, and post-CMS (). During pre-CMS, basal hedonic behavior was measured using the sucrose preference test (68–69 days of age). Rats were then randomly divided into three groups for the CMS phase: Control (n = 21), Clustered (n = 21), and Dispersed (n = 21). During CMS (76–102 days of age), rats in the Clustered and Dispersed groups received three different stressors every day, each lasting 15 min; Control rats were not exposed to these stressors but handled every day. For the Clustered group, rats were exposed to the three daily acute stressors all within 60 min of each other every day. For the Dispersed group, rats were exposed to the same three daily acute stressors, with at least 135 min between each stressor (). Across the two experimental groups (Clustered and Dispersed), daily acute stressor type, intensity, duration, and frequency were kept constant. In the Dispersed group, rats experienced one stressor during each third of the active period (one in the morning, 09:30–13:00 h, one in the afternoon, 13:15–16:45 h, and one in the evening, 17:00–20:30 h). In the Clustered group, to control for time of day effects (Romeo, Karatsoreos, & McEwen, Citation2006), on each day, one third of rats were exposed to stressors within each of the above times during the active phase (morning, afternoon, evening), and each rat was equally rotated across all time periods for the duration of CMS. Basal circulating corticosterone concentration was measured in the morning and evening on the last day of CMS (102 days of age). During the post-CMS phase, physiologic and behavioral responses were measured in the following order: sucrose preference test, innate immune response to endotoxin (lipopolysaccharide injection), and acute corticosterone response to restraint (102–103, 104 and 107 days of age, respectively; ). We made group sizes relatively large (i.e. 21 rats/condition) because of two factors: (i) we expected our experimental manipulations to lead to subtle effects, if any, because (for logistical reasons) the two stress groups only received 45 min of stressors/day which is less than CMS protocols used by others, and (ii) we were using outbred rats which necessarily introduces additional variance into the data. The sample size was based on power analyses using group mean and variance values based on adult corticosterone levels in male Sprague-Dawley rats previously exposed to stress- versus control conditions. Sample size was calculated using two-tailed significance of 0.05, and power of 0.80. All procedures were carried out in red light conditions unless otherwise stated. All rats were processed simultaneously, but to minimize time required to collect blood samples on any given day, the 63 rats were equally divided across three sub-groups, and each sub-group started the full protocol on one of three consecutive days. Each sub-group contained equal numbers of rats from each experimental group—i.e. 7 rats/condition/sub-group.
Figure 1. Study timeline. Order of events during the three phases of the study, relative to the chronic mild stress (CMS) protocol: Pre-CMS, CMS, post-CMS. During pre-CMS, all rats were handled and tested for sucrose preference (SPT; 68–69 days of age). During CMS, rats were exposed to one of three conditions (Control, Dispersed, Clustered), with the latter two groups exposed to three daily 15 min stressors (76–102 days of age). To measure alterations in circadian glucocorticoid regulation, basal serum corticosterone concentration was measured in the morning and evening on the last day of CMS (102 days of age). Post-CMS physiology and behavior were measured in the following order: sucrose preference test (102–103 days of age), innate immune response to lipopolysaccharide (104 days of age), and acute corticosterone response to restraint (107 days of age).
Table 1. A representative day in the modified chronic mild stress protocol.
CMS protocol
We used a modified version of the unpredictable CMS protocol (Willner, Citation1997). CMS is a widely utilized rodent protocol that leads to behavioral and physiologic signs often seen in human depression—e.g. reduced body weight gain, reduced preference for palatable food (sucrose solution), increased depressive-like behavior (immobility in a forced swim test), heightened adrenocorticotrophic hormone (ACTH) and corticosterone responses to stress, flattened corticosterone circadian rhythm, and elevated interleukin-6 (IL-6) production after lipopolysaccharide challenge compared to non-stressed rats (Bielajew et al., Citation2002; Herman, Citation2013; Isgor, Kabbaj, Akil, & Watson, Citation2004; Jayatissa, Henningsen, West, & Wiborg, Citation2009; Manikowska, Mikołajczyk, Mikołajczak, & Bobkiewicz-Kozłowska, Citation2014; Varga et al., Citation2011; Willner, Citation2005). We exposed rats to three of six different 15-min stressors on a daily basis. Exposure was conducted during the dark phase, in dim red light, in a room that was separate from the colony room but on the same light schedule as the colony room (). The three stressors used each day were pseudo-randomized such that no rat experienced the same stressor on consecutive days and all rats were exposed to each stressor the same number of times across the 4 weeks of CMS ( and ). Stressors included: (i) novel cage: new clean cage identical to home cage but with no bedding; (ii) water immersion: new cage with 2 cm of room temperature tap water; (iii) wet bedding: new cage with 2 cm of wet bedding made of equal parts corncob bedding and room temperature water; (iv) tilted cage: home cage tilted at 45° angle; (v) noise: exposed to 80 dB white noise; and (vi) strobe light: exposed to stroboscopic illumination (300 flashes/min). All rats were returned to the colony room immediately after stressor exposure.
Body weight
Rats were weighed once a week starting at 76 days of age (Day 1 of CMS) until 102 days of age (Day 27 of CMS). To minimize handling, body weight was measured in the evening at the same time as other procedures.
Sucrose preference
Sucrose preference testing was conducted at 68–69 days of age. The sucrose preference test was administered in the colony room to measure hedonic behavior. To measure basal sucrose preference, we did not water- or food-deprive rats prior to this test. Lack of deprivation was important here because we expected differential weight gain among experimental groups, in which case a standard water or food deprivation method across all groups could have led to different deprivation rates relative to body weight across groups. Two bottles were placed on the home cage and rats allowed to drink freely for 24 h. One bottle contained regular tap water and the other contained 1% sucrose solution. The position of the water and sucrose bottles was switched 12 h after the start of testing to control for potential side preferences. Percent sucrose solution consumed was defined as: [amount of sucrose solution consumed (grams)/amount of sucrose consumed (grams)+amount of water consumed (grams)] × 100. To control for loss of fluids from passive dripping and handling, we recorded the amount of sucrose solution and water (grams) lost from bottles on three empty cages on the same rack as study rats and handled in the same manner. Average sucrose and water lost to passive dripping and bottle handling was subtracted from each rat’s total sucrose and water consumption prior to calculating percent sucrose consumption.
Morning and evening basal circulating concentration and acute corticosterone response
On the last day of CMS (102 days of age), morning and evening circulating corticosterone concentrations were measured from blood samples rapidly collected at the beginning and at the end of the active period. Blood was collected in the morning (08:00–09:00 h) and evening (21:00–22:00 h) in a room separate from the colony and CMS-administration rooms, under white lights. Evening blood draws occurred >90 min after the last stress session so that circulating corticosterone had time to return to basal concentrations. Rats were transported to the blood collection room in their home cage, removed from their cage, and briefly restrained to collect a small blood sample (50–100 μl) from the left lateral tail vein with a 25 gauge needle (no anesthetic used). On average, it took <2 min from cage disturbance to collect a complete blood sample, with only 8% of samples taking longer than 3 min (mean, median, and range: 109, 99, and 57–272 s). Blood was kept on ice in an untreated tube until all samples were collected, then centrifuged at 3000g for 10 min at 4 °C. Serum was collected and stored at −80 °C until assayed.
Five days after completion of CMS (107 days of age), an acute corticosterone response to restraint was measured from blood samples collected during the latter half of the active period (17:30–20:30 h), in a room separate from the colony and CMS-administration rooms. This entire procedure was conducted under white lights. Rats were transported to the procedure room individually and a blood sample collected using the same procedure as described above. Immediately after the baseline sample was collected, rats were restrained in tapered plastic film tubes (DecapiCone; Braintree Scientific, Hagerstown, MA, USA) and returned to their home cage. Rats were secured in the film tube with their nose in the narrow end and tail coming out the wider open end. The open end was cinched around the tail and taped to itself to inhibit movement or escape. After 15 min, rats were removed from the restrainers and kept in the home cage until the second and third blood samples (50–100 μl each) were collected 30 and 105 min after the baseline sample. Blood was kept on ice until all samples were collected, then centrifuged at 3000 g for 10 min at 4 °C. Serum was collected and stored at −80 °C until assayed. Serum corticosterone concentration was measured using a commercial [125I] radioimmunoassay for rat and mouse serum/plasma with minimum sensitivity of 25 ng/ml (MP Biomedicals, Solon, OH, USA). Samples were run in duplicate across six assays, with CMS conditions balanced within each assay. Mean intra-assay coefficient of variation (CV) for low and high controls was 14.8 and 8.9%; inter-assay coefficient of variation for these controls was 7.9 and 8.7%.
Innate immune response
Two days after CMS was complete (104 days of age), we quantified the innate pro-inflammatory response. Rats were injected with lipopolysaccharide (cat. no. L3012; Sigma-Aldrich, St. Louis, MO, USA) and as a basic estimate of the acute pro-inflammatory response, circulating IL-6 concentration was measured at the time of injection and 2 h later. We used a lipopolysaccharide dose of 25 μg/kg diluted with sterile endotoxin-free saline (USP; cat. no. 2F7124; Baxter, Deerfield, IL) to arrive at an injection volume of 0.5 ml. Lipopolysaccharide was administered intraperitoneally (IP) during the beginning of the active period (09:30–10:30 h). At each time point, blood (200 μl) was collected by transecting the tip of the tail (∼2 mm) with a scalpel blade (no anesthetic) and gently massaging the tail from base to tip. Blood was collected into an untreated tube, stored on ice for ∼1 h, centrifuged, and serum stored at −80 °C until assayed. The entire procedure was conducted under white lights, in a procedure room separate from the colony room. Rats were sequentially processed; each rat was individually transported to the procedure room, immediately bled and received the lipopolysaccharide injection, and returned to their home cage on a rack in the procedure room until their second blood sample was collected 2 h later. Serum IL-6 concentration was measured with a commercial ELISA kit (Rat IL-6 Quantikine, cat. no. R6000B; R&D Systems, Minneapolis, MN) with a standard range of 62.5–4000 pg/ml and a sensitivity of 36 pg/ml. Samples were processed according to manufacturer’s instructions; briefly, 75 μl of frozen rat serum was thawed and diluted 1:1 with Calibrator Diluent RD5-16 (R&D Systems, Minneapolis, MN) and samples analyzed in duplicate. Rats from the three different experimental conditions were evenly distributed across nine plates. Mean intra-assay coefficient of variation for mid-range control samples and for all duplicate samples was 6.9 and 6.3%; inter-assay coefficient of variation for the control sample was 13.0%.
Data analyses
Distributions for each outcome measure (weight, behavior, glucocorticoid concentration, innate immune response) were examined for normality. Variables with the expected positively skewed distributions (corticosterone concentration, IL-6 concentration) were transformed using the natural logarithm of raw values, and sucrose preference percentages were logit transformed to arrive at normal distributions. Once data were normalized, data points that were above or below 2.5 standard deviations from the group mean were excluded as outliers. The number and distribution of outliers across experimental groups for each analysis are given in the results. To aid interpretation, figures display raw data means. Results in the text are given as mean ± standard error of mean (SEM).
To estimate change in adrenal activity across the active period, we calculated the difference in basal corticosterone concentrations from the beginning to end of the dark phase; “corticosterone active period decline.” To estimate acute adrenal feedforward (“corticosterone reactivity”), we used the difference in corticosterone concentrations from 0 to 30 min after restraint initiation, with greater positive values indicating stronger feedforward regulation. We also analyzed corticosterone concentrations at 0 and 30 min separately. To estimate acute negative feedback (“corticosterone recovery”), we used corticosterone concentrations at 105 min post-restraint onset. Estimates of corticosterone reactivity and recovery were not correlated (r61 = −0.210, p > .10), hence these two data points were considered independent. We used corticosterone reactivity and recovery measures, instead of area-under-the-curve, in order to estimate adrenal feedforward and feedback processes independently. To determine innate immune response to lipopolysaccharide, we analyzed circulating IL-6 concentrations at 0 and 2 h post-injection.
We first tested for pre-CMS differences in body weight and behavior among CMS groups with univariate analysis of variances (ANOVAs). Repeated measures ANOVA was used to analyze the effect of CMS condition on body weight, with a follow-up univariate ANOVA with planned pair-wise comparisons of body weights on the last day of CMS across groups. We also used repeated measures ANOVA to analyze the change in corticosterone production from morning to evening on the final day of CMS and from 0 to 30 to 105 min from the beginning of acute restraint stress several days after CMS was complete. Univariate ANOVAs were used to compare hedonic behavior (SPT), circulating glucocorticoid concentrations (basal corticosterone active period decline, baseline corticosterone before restraint, acute corticosterone reactivity, and acute corticosterone recovery following restraint), and innate immune function (rapid IL-6 response to lipopolysaccharide) across experimental groups. In each ANOVA, CMS treatment was used as a fixed factor. When pre-CMS measures were available (i.e. SPT, and body weight), these values were used as covariates, and if there were testing day effects on an outcome measure (i.e. IL-6 concentration, SPT sucrose preference), then test day means were used as a covariate. Planned pair-wise comparisons were used to determine which experimental group(s) differed from the Control group. The criterion for statistical significance was set at p ≤ .05. When an experimental group was found to differ significantly from the control group, we report an estimate of the effect size (η2) to help interpret results.
Results
Body weight
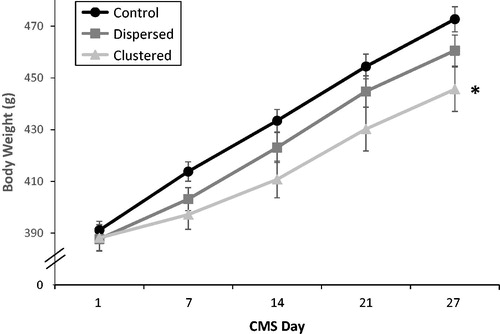
Body weights at the beginning of CMS did not differ among groups (F2,60 = 0.179, p = .84), but by the end of CMS, body weight differed significantly among groups (time × stress condition interaction: F2,59 = 7.41, p < .01, ). By the end of CMS, Clustered rats weighed significantly less than Control rats (planned comparisons of weight on Day 27 of CMS, p < .05, η2=0.196) and this was not the case for Dispersed rats (p = .20). Clustered and Dispersed rats gained 70 and 89% as much weight as the Control rats, respectively. There were no outliers.
Figure 2. Body weight gain during chronic mild stress (CMS). Mean body weights (g) from Day 1 to Day 27 in the three stress conditions. Estimated marginal mean and SEM are presented. The number of rats in each group at each time point was 21. *On Day 27, Clustered rat body weights were significantly lower than Control rats (planned pair-wise comparisons, p < .05).
Sucrose preference
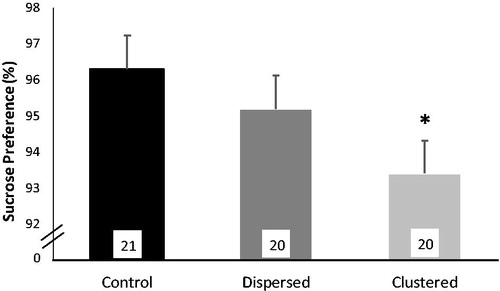
Prior to CMS, sucrose preference did not differ among experimental groups (mean ± SEM: Control, n = 21 versus Dispersed, n = 20 versus Clustered, n = 20: 91.0 ± 1.5 versus 92.1 ± 1.4 versus 93.3 ± 1.3% sucrose solution consumed; F2,57 = 1.18, p = .31), but at the end of CMS there was a small but statistically significant difference in sucrose consumption across groups (F2,56 = 3.13, p < .05; ). Rats in the Clustered condition consumed significantly less sucrose than those in the Control group (p < .05, η2 = 0.089), although the actual difference was relatively small (; clustered rats consumed 3% less sucrose solution than the Control rats, indicating that they were still at high sucrose consumption; i.e. 93 versus 96% preference). Group differences in relative sucrose preference were not a result of group differences in water consumption (F2,56 = 0.88, p = .42). Two outliers were removed from these analyses, one in the Dispersed group with sucrose preference <50% and one in the Clustered group where the bottle leaked during testing.
Figure 3. Sucrose preference at end of chronic mild stress (CMS). Percent sucrose solution consumed over a 24 h period during the sucrose preference test administered on the final day of CMS. Estimated marginal means, which control for pre-CMS sucrose consumption, are shown. Error bars represent SEM and the number of rats per group are in the bars. *Clustered rats consumed significantly less sucrose than Control rats (planned pair-wise comparisons, p < .05).
Decline in circulating corticosterone concentration during active period
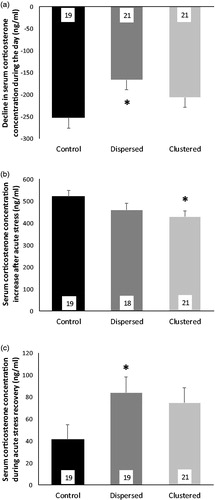
On the last day of CMS, circulating corticosterone concentrations were significantly greater in the morning versus the evening, and there was a trend for this daily decline to differ among experimental groups (repeated measures ANOVA main effect of time: F1,58 = 221.29, p <.001; interaction of time × stress group: F2,58 = 2.0, p = .053). Experimental groups differed on the total daily decline in circulating corticosterone concentration during the active phase (F2,58 = 3.43, p < .05; ). Rats in the Dispersed group had less decline from morning to evening than rats in the Control groups (p < .05, η2 = 0.106). Statistically, group differences were driven by the change from morning to evening concentrations, rather than by absolute concentrations at the morning or evening. Although, with more power, it would be possible to evaluate if the attenuated daily decline in the Dispersed versus Clustered group was a result of elevated evening corticosterone concentration in the Dispersed rats in combination with a dampened morning levels in both Dispersed and Clustered groups (morning corticosterone concentration mean ± SEM for Control versus Dispersed versus Clustered: 277.5 ± 21.2 versus 237.2 ± 20.2 versus 236.6 ± 20.2 ng/ml, F2,58 = 1.26, p = 0.29; evening corticosterone concentration mean ± SEM for Control versus Dispersed versus Clustered: 24.6 ± 16.8 versus 70.1 ± 16.0 versus 28.0 ± 16.0 ng/ml, F2,58 = 1.87, p = .16). It is not necessarily the case that the dampened decline in daily corticosterone in Dispersed rats resulted from the fact that all Dispersed rats experienced one stressor in the evening whereas only one-third of Clustered rats received their stressors in the evening, on the day of blood collection. In a comparison of the corticosterone decline among rats in the three Clustered sub-groups, there was no difference in daily decline among sub-groups that received stressors during the morning, noon, or evening windows on the day of blood collection, although there is indication that this decline may have been less in the evening stressed Cluster sub-group (mean ± SEM for morning, n = 7 versus noon, n = 8 versus evening, n = 6 subgroups: −219 ± 31 versus −219 ± 29 versus −175 ± 34 ng/ml; F2,18 = 0.59, p = .57). Two outliers were removed from these analyses, both in the Control group.
Figure 4. Circulating glucocorticoid concentration at end and after chronic mild stress (CMS). (a) Mean decline in basal circulating corticosterone concentration during the active period of the last day of CMS for all three groups. *Dispersed rats had an attenuated decrease in corticosterone during the active period of the day compared to Control rats (planned pair-wise comparisons, p < .05). (b) Corticosterone reactivity to 15 min restraint: Mean change in circulating corticosterone concentration from baseline to 30 min after the onset of 15 min restraint for all three groups. *Clustered rat corticosterone reactivity was significantly attenuated compared to Control rats (planned pair-wise comparisons, p < .05). (c) Corticosterone concentration during recovery phase: Mean circulating corticosterone concentration 105 min after onset of 15-min restraint for all three groups. *Dispersed rats had significantly higher corticosterone concentrations during recovery than Control rats (planned pair-wise comparisons, p < .05). Across all figures, error bars indicate SEM and the number of rats per group are in the bars.
Acute-circulating corticosterone response to restraint
Five days after the end of CMS, restraint stress during the latter half of the active period produced a significant change in circulating corticosterone concentration across the three sampling time points (repeated measures ANOVA main effect of time: F1.8,97 = 1145.57, p < .001; interaction of time × stress group: F3.6,97 = 2.02, p = .105). There were no group differences in baseline corticosterone concentrations (mean ± SEM for Control versus Dispersed versus Clustered: 78.3 ± 14.4 versus 98.7 ± 14.8 versus 89.9 ± 14.1 ng/ml; F2,55 = 0.49, p = .61). But there were trends toward groups differences in restraint stress-induced corticosterone reactivity and recovery (F2,55 = 2.86, p = .066; F2,56 = 2.87, p = .065; ). Raw corticosterone concentration at 30 min did not differ among groups (mean ± SEM for Control versus Dispersed versus Clustered: 599.3 ± 23.1 versus 559.8 ± 23.7 versus 542.7 ± 22.5 ng/ml; F2,55 = 1.61, p = .21). Given marginally significant omnibus test results for corticosterone reactivity, we proceeded with planned pair-wise comparisons (although we note that these should be interpreted with caution). Clustered rats had dampened corticosterone reactivity compared to Control rats (mean ± SEM: Clustered = 430 ± 26 ng/ml versus Control = 521 ± 28 ng/ml; p < .05, η2 = 0.092; ), and Dispersed rats had dampened corticosterone recovery compared to Control rats (mean ± SEM: Dispersed = 84 ± 14 ng/ml versus Control = 41 ± 14 ng/ml; p < .05, η2 = 0.087; ). Two samples could not be collected, and three outliers were removed from these analyses, two from the Control group and three from the Dispersed group.
Acute-circulating IL-6 response to endotoxin
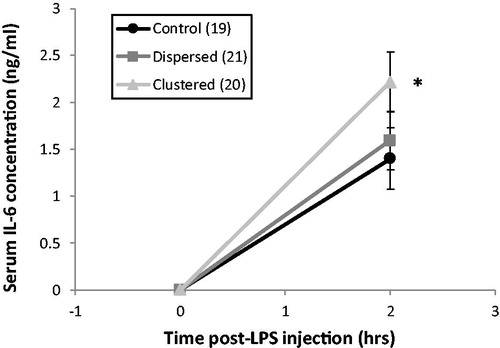
Two days after CMS was complete, there was a trend toward group differences in the acute-circulating IL-6 response to lipopolysaccharide stimulation (F2,56 = 2.84, p = .067; ). In planned pair-wise comparisons, rats in the Clustered group had significantly greater circulating IL-6 concentrations 2 h after lipopolysaccharide injection compared to Control rats (p < .05, η2 = 0.091). There were no group differences in baseline circulating IL-6 concentration at the time of lipopolysaccharide injection (F2,56 = 0.014, p = 0.99). Three outliers were removed from analyses, two from the Control group, and one from the Clustered group.
Figure 5. Circulating interleukin-6 (IL-6) response to lipopolysaccharide (LPS) 2 days after chronic mild stress (CMS). Mean IL-6 concentrations for each group at 0 and 2 h after LPS injection. Error bars indicate SEM. Sample size for each group is in the key. *Clustered rats had a significant greater acute IL-6 response to LPS than Control rats (planned pair-wise comparisons, p < .05).
Discussion
In the current study, the temporal patterning of acute stressors within a chronic stress experience had mild effects on several health-related processes. When rats experienced repeated exposure to acute stressors during the active phase that were clustered together, we saw evidence of decreased body weight gain, decreased preference for a palatable substance (sucrose solution), decreased glucocorticoid reactivity to an acute stressor, and an elevated IL-6 response to endotoxin compared to unstressed rats. Broadly, these findings suggest that timing matters—i.e. rats are sensitive to the temporal patterning of daily stressors and different temporal stressor patterns lead to different regulation of physiologic processes over time. The current results are somewhat more consistent with the temporal summation hypothesis which proposes that repeat exposure to a rapid succession of acute stressors may lead to more negative health consequences over time than exposure to the same stressors but dispersed throughout the day. However, it should be noted that at least one regulatory process was more affected by exposure to acute stressors dispersed across the day (instead of clustered): glucocorticoid return to baseline was attenuated in individuals exposed to daily stressors that were spread out over the day. These results emerged when stressor exposure and outcome measures were conducted during the rats’ active period (dark phase), which is different from many studies that normally involve stressors and outcome measures during the rodent inactive period.
The magnitude of differences between experimental and control groups reported in the current study were small—i.e. effect sizes of ∼0.10—which may reflect the finding that temporal patterning of stressors has mild effects on physiologic regulation. Alternatively, the mild effects reported here may reflect the fact that, by necessity, our stressor protocol was relatively modest—i.e. 45 min of stressor/day—rather than the normal 1–2 + h of daily stressors used in most rodent CMS protocols. Either way, the results test a novel idea about the relative significance of daily stressor temporal distribution. Replication of the current results are warranted, and given the reported effect sizes, future study designs are informed about the level of power required, particularly if the goal is to compare outcome differences among experimental groups that are exposed to different temporal patterns of daily stressors.
Body weight and sucrose preference
Rats in the Clustered condition gained the least weight and consumed the least amount of sucrose solution by the end of CMS. These results indicate that daily stressors experienced close together can lead to decreased food palatability or hedonic responses. Decreased body weight and sucrose preference in the current study were moderate compared to previously documented decreases after more dramatic stressors and/or following food/water deprivation (Willner, Citation2005). Most CMS protocols involve exposing rodents to more than 2 h of stressors/day, with some stressors lasting more than half the day (Matthews, Forbes, & Reid, Citation1995). Prior studies that did not use food/water deprivation have found reduced sucrose preference after CMS, although the difference is typically much smaller (∼10% reduction: Castro et al., Citation2011; Larsen, Mikkelsen, Hay-Schmidt, & Sandi, Citation2010). The magnitude of results in the current study are likely modulated by type, intensity, duration, and frequency of daily stressors.
Glucocorticoid regulation
Previous research has shown that CMS tends to affect basal corticosterone concentrations more than acute stress reactivity (Bielajew et al., Citation2002; Grippo et al., Citation2004). In the current study, circulating glucocorticoid concentrations evoked by a novel acute stressor were 20% lower in rats that had experienced chronic stress in a Clustered temporal pattern compared to Control rats. This attenuated acute corticosterone response to a novel stressor is the opposite of what has been seen after several days of repeat exposure to homotypic social or physical stressors or classic CMS protocols (Bhatnagar & Dallman, Citation1998; Bielajew et al., Citation2002, Herman Citation2013). The attenuated response to a novel physical stressor after several weeks of repeat exposure to tightly clustered acute physical stressors may minimize the temporal summation of circulating glucocorticoids during these daily events. In contrast, in the current study, glucocorticoid decline and recovery after a peak was slowed when daily stressors were spaced far apart (Dispersed). Individuals that experience stress made up of frequent acute stressors throughout the day may be well-served by a slower decrease in glucocorticoid concentrations following challenges in order to maintain elevated circulating glucocorticoid concentrations for future challenges. This may lead to risk over time due to the cumulative impacts of persistent activation.
The divergent patterns of CMS-induced hypothalamus–pituitary–adrenal (HPA) axis dysregulation displayed by rats in the Clustered versus Dispersed conditions suggest that each temporal pattern could target different underlying neural circuitry. In the current study, we used multiple different (heterotypic) stressors, which can have a greater influence on physiologic indices of stress than repeated exposure to the same (homotypic) stressor (Herman, Citation2013). Chronic heterotypic stress regimens impair the stress habituation process wherein the magnitude of physiologic responses is diminished with repeat exposure (Grissom & Bhatnagar, Citation2009; Herman, Citation2013). Studies suggest that habituation following homotypic stress is mediated by neuroplastic changes in the ventral prefrontal cortex, basolateral amygdala, and paraventricular thalamus (Bhatnagar, Huber, Nowak, & Trotter, Citation2002). Notably, habituating homotypic stress regimens augment subsequent HPA axis responses to a novel stressor (Bhatnagar & Dallman, Citation1998) similar to our observations from rats in the Dispersed condition. This HPA axis facilitation effect is associated with enhanced drive from the paraventricular thalamus and locus coeruleus (Herman, Citation2013). Non-habituating heterotypic stress regimens cause a long-lasting diminution of future HPA axis responses (Ostrander, Ulrich-Lai, Choi, Richtand, & Herman, Citation2006) reminiscent of the CMS-induced alterations displayed by rats in the Clustered condition. Interestingly, sustained neuronal activation has been observed in the medial prefrontal cortex, nucleus of the solitary tract, and posterior hypothalamic nucleus following chronic heterotypic, but not homotypic, stress (Flak, Solomon, Jankord, Krause, & Herman, Citation2012). Given that selective recruitment of this circuit by heterotypic stress has been associated with stress controllability of a stressor (Herman, Citation2013), it would be interesting to assess whether stress predictability influences the divergent responses to the Clustered and Distributed conditions.
In human depression and chronic stress (for which CMS was meant to model), morning glucocorticoid concentrations are often suppressed and evening concentrations elevated, leading to a flatter decline in circulating glucocorticoid concentration across the day (e.g. Burke, Davis, Otte, & Mohr, Citation2005). In the current study, we saw a similar attenuated daily decline in rats that experienced Dispersed stressors. This suggests that different aspects of glucocorticoid regulation may be affected by the temporal pattern of acute stressors within chronic stress. Acute glucocorticoid responses (i.e. feedforward mechanisms) may be more affected by repeat exposure to temporally clustered acute stressors, whereas glucocorticoid recovery (i.e. negative feedback mechanisms) may be more affected by repeat exposure to temporally dispersed stressors across the day. Future research may do well to examine the temporal spacing of stressors in human experiences that are characterized as chronic stress. There may be important health implications if humans living under different daily temporal patterns of chronic stress display divergent glucocorticoid regulation phenotypes (Herman, Citation2013; Kiecolt-Glaser, Marucha, Malarkey, Mercado, & Glaser, Citation1995).
Innate inflammatory response
In the current study, rats that experienced Clustered stressors had an elevated rapid IL-6 response to endotoxin compared to Control rats. This result is consistent with previous research in which inescapable tail shock led to increased pro-inflammatory cytokine concentrations after lipopolysaccharide challenge (Johnson et al., Citation2002). Glucocorticoids are potent anti-inflammatory agents, thus it follows that Clustered rats, with dampened acute glucocorticoid responses to a challenge, have heightened acute IL-6 responses to lipopolysaccharide. This may also explain why rats in the Dispersed condition, with an attenuated corticosterone recovery, did not show comparable elevations in IL-6 responses to lipopolysaccharide. Acute inflammation is a necessary defense against infection, but chronically high concentrations of IL-6 have been noted as a likely contributor to the development of coronary artery disease (Hartman & Frishman, Citation2014). In humans, IL-6 responses do not appear to habituate to repeated acute stress (von Kanel, Kudielka, Preckel, Hanebuth, & Fischer, Citation2006), and environments characterized by chronic stress are associated with greater stimulated IL-6 responses (Schreier, Roy, Frimer, & Chen, Citation2014). Future research may benefit from examining the effect of the temporal spacing of repeated stressors on inflammatory responses as this may have prevention and treatment implications for individuals in stressful environments.
Study limitations
In the current study, we individually housed and exposed rats to stressors of shorter duration than previous CMS studies. A reasonable concern is that rats would not experience significant stress. However, we observed weight gain impairment and decreased sucrose preference in the stress versus control groups, indicating that the milder form of CMS had similar, albeit smaller, effects as seen in prior studies (Willner, Citation2005). In addition, we did not use food and water deprivation prior to sucrose preference testing—a common procedure in CMS studies. However, we still documented small decreases in post-CMS sucrose preferences compared to control rats. It is possible that a more severe CMS protocol may produce more pronounced stress effects, with either accentuated or muted differences between stressor temporal pattern conditions (clustered versus dispersed). Future work should replicate and extend this work to include females and socially housed rodents. In addition, the dampened daily corticosterone decline observed in Dispersed stressor rats should be replicated given the possibility that this shallower decline may have resulted from residual acute corticosterone responses to evening stressors. The acute corticosterone response results should also be replicated in groups that are not previously exposed to lipopolysaccharide. Several of the outcome variables in the current study involved outliers which may have minimized our power to identify group differences. However, we had relatively large initial group sizes because we expected our modified CMS protocol to have modest effects, thus the overall truncation of power was minimized.
Another limitation of the current study is that we did not measure acute physiologic responses to individual stressors delivered during CMS. We do not know what glucocorticoid release, immune signaling, or brain activity looked like following three tightly clustered versus temporally dispersed stressors, and thus cannot conclude that stressors that arrive in tight succession lead to a greater cumulative physiologic response compared to stressors spread out over time (i.e. the assumption underlying the temporal summation hypothesis). Prior work indicates that circulating glucocorticoid concentrations and hypothalamic paraventricular nucleus cell activity are similar after a complex multimodal stressor (noise, restraint, bright lights, crowding, shaking) versus a simple unimodal stressor (restraint or noise alone; Maras et al., Citation2014). However, multi- versus uni-modal stressors may differentially activate hypothalamic and dorsal hippocampal function and memory responses (Deak et al., Citation2005; Maras et al., Citation2014). Thus, future studies need to document acute physiologic responses to different stressor temporal patterns. Ideally this should involve multiple physiologic responses (e.g. adrenocorticotropic and glucocorticoid hormones, norepinephrine, pro-inflammatory cytokines, acute brain responses) to identify which (and how) systems are activated by differentially distributed stressors.
Measures such as those suggested above may provide insight into how rats perceive multi-modal acute stressors that are clustered versus dispersed in time. Exposure to the same array of stressors presented in different temporal patterns may lead to qualitatively different experiences. Particularly in the kind of paradigm used in the current study, where three different stressors are presented each day, a rapid succession of different stressors may be perceived as a complex multi-modal stressor invoking different processing systems than a unimodal stressor (Maras et al., Citation2014). Multiple mild stressors that always occur in rapid succession may also increase the predictability of these stressors and/or stimulate an anticipatory response. Prior studies on stressor predictability and anticipation suggest that these processes lead to accentuated corticosterone reactivity rather than the attenuated responses seen in the current study (Juster, Perna, Marin, Sindi, & Lupien, Citation2012; Lovelock & Deak, Citation2017; Pitman et al., Citation1995). However, an important difference is that prior studies used the same stressors in the same order to induce predictability/anticipation, whereas the current study involved rapid succession of different stressors in different orders on each day. Qualitatively different cognitive processing of multi-modal stressors, presented sequentially versus disparately, is an area of research that may be particularly important to address in human studies, with a particular focus on predictability and anticipation.
Conclusions
The results from this study indicate that repeated (chronic) daily exposure to temporally clustered mild acute stressors may lead to different chronic stress effects than daily exposure to temporally dispersed stressors. These results support speculation of a short-term stress response threshold that exacerbates the detrimental effects of chronic stress (i.e. the temporal summation hypothesis). Chronic stress is often viewed as being comprised of some combination of repeated acute stressors (typically of varying duration and intensity). It has also been speculated that the distribution of stressors over time, even assuming equal “total” overall exposure, may influence the impact of these stressors on health-related behavior and physiology (Smyth et al., Citation2013). This study provides an initial experimental test of this idea, and the results indicate that acute stressors that occur further apart (i.e. temporally dispersed) may have less negative physiologic impact than stressors that occur more closely together in time (i.e. temporally clustered). This may be specific to conditions in which stressors are relatively mild or last for a relatively short time during each day. Moderate stressors that are spread out over time may allow sufficient time for specific effects of each stressor to dissipate before additional stressors occur; this may attenuate the accumulation of physiologic consequences over time. Potential temporal mechanisms will need to be tested in future studies. If these observed patterns are replicable and generalize, this may have important implications for stress research in a variety of contexts. At the broadest level, this study provides preliminary experimental evidence that temporal patterning of repeated exposure to acute stressors can influence physiologic regulation.
Acknowledgements
Many students in the Behavioral Neuroendocrinology Laboratory, at the Pennsylvania State University, provided assistance at all hours of the day: J. Baffoe-Bonnie, J. T. Carp, T. L. Coppage, N. R. Chirichella, I. M. Kaplan, A. M. Kech, S. M. Koo, and A. C. Motchenbacher. In addition, we appreciate critical manuscript feedback from Drs. Hannah Schreier and Idan Shalev.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Avitsur, R., Stark, J.L., & Sheridan, J.F. (2001). Social stress induces glucocorticoid resistance in subordinate animals. Hormones and Behavior, 39, 247–257. doi:10.1006/hbeh.2001.1653
- Bhatnagar, S., & Dallman, M.F. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience, 84, 1025–1039. doi:10.1016/S0306-4522(97)00577-0
- Bhatnagar, S., Huber, R., Nowak, N., & Trotter, P. (2002). Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary- adrenal responses to repeated restraint. Journal of Neuroendocrinology, 14, 403–410. doi:10.1046/j.0007-1331.2002.00792.x
- Bielajew, C., Konkle, A.T.M., & Merali, Z. (2002). The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats: I. Biochemical and physiological analyses. Behavioural Brain Research, 136, 583–592. doi:10.1016/S0166-4328(02)00222-X
- Burke, H.M., Davis, M.C., Otte, C., & Mohr, D.C. (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30, 846–856. doi:10.1016/j.psyneuen.2005.02.010
- Castro, J.E., Diessler, S., Varea, E., Marquez, C., Larsen, M.H., Cordero, M.I., & Sandi, C. (2011). Personality traits in rats predict vulnerability and resilience to developing stress- induced depression-like behaviors, HPA axis hyper-reactivity and brain changes in pERK ½ activity. Psychoneuroendocrinology, 37, 1209–1223. doi:10.1016/j.psyneuen.2011.12.014
- Dal-Zotto, S., Martí, O., & Armario, A. (2000). Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behavioural Brain Research, 114, 175–181. doi:10.1016/S0166-4328(00)00220-5
- Deak, T., Bordner, K.A., McElderry, N.K., Barnum, C.J., Blandino, P., Deak, M.M., & Tammariello, S.P. (2005). Stress-induced increases in hypothalamic IL-1: A systematic analysis of multiple stressor paradigms. Brain Research Bulletin, 64, 541–556. doi:10.1016/j.brainresbull.2004.11.003
- de Kloet, E.R., Joëls, M., & Holsboer, F. (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6, 463–475. doi:10.1038/nrn1683
- Dhabhar, F.S., & McEwen, B.S. (1997). Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain, Behavior, and Immunity, 11, 285–306. doi:10.1006/brbi.1997.0508
- Dickerson, S.S., & Kemeny, M.E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–391. doi:10.1037/0033-2909.130.3.355
- Flak, J.N., Solomon, M.B., Jankord, R., Krause, E.G., & Herman, J.P. (2012). Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. European Journal of Neuroscience, 36, 2547–2555. doi:10.1111/j.1460-9568.2012.08161.x
- García, A., Martí, O., Vallès, A., Dal-Zotto, S., & Armario, A. (2000). Recovery of the hypothalamic-pituitary-adrenal response to stress: Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology, 72, 114–125. doi:10.1159/000054578
- Grippo, A.J., Santos, C.M., Johnson, R.F., Beltz, T.G., Martins, J.B., Felder, R.B., & Johnson, A.K. (2004). Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. American Journal of Physiology-Heart and Circulatory Physiology, 286, H619–H626. doi:10.1152/ajpheart.00450.2003
- Grissom, N., & Bhatnagar, S. (2009). Habituation to repeated stress: Get used to it. Neurobiology of Learning and Memory, 92, 215–224. doi:10.1016/j.nlm.2008.07.001
- Hammen, C. (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293–319. doi:10.1146/annurev.clinpsy.1.102803.143938
- Hartman, J., & Frishman, W.H. (2014). Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiology in Review, 22, 147–151. doi:10.1097/CRD.0000000000000021
- Herman, J.P. (2013). Neural control of chronic stress adaptation. Frontiers in Behavioral Neuroscience, 7, 61. doi:10.3389/fnbeh.2013.00061
- Isgor, C., Kabbaj, M., Akil, H., & Watson, S.J. (2004). Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus, 14, 636–648. doi:10.1002/hipo.10207
- Jayatissa, M.N., Henningsen, K., West, M.J., & Wiborg, O. (2009). Decreased cell proliferation in the dentate gyrus does not associate with development of anhedonic-like symptoms in rats. Brain Research, 1290, 133–141. doi:10.1016/j.brainres.2009.07.001
- Joëls, M., Karst, H., Krugers, H.J., & Lucassen, P.J. (2007). Chronic stress: Implications for neuronal morphology, function and neurogenesis. Frontiers in Neuroendocrinology, 28, 72–96. doi:10.1016/j.yfrne.2007.04.001
- Johnson, J.D., O’connor, K.A., Deak, T., Stark, M., Watkins, L.R., & Maier, S.F. (2002). Prior stressor exposure sensitizes LPS-induced cytokine production. Brain, Behavior, and Immunity, 16, 461–476. doi:10.1006/brbi.2001.0638
- Juster, R.-P., Perna, A., Marin, M.-F., Sindi, S., & Lupien, S.J. (2012). Timing is everything: Anticipatory stress dynamics among cortisol and blood pressure reactivity and recovery in healthy adult. Stress, 15, 569–577. doi:10.3109/10253890.2012.661494
- Kiecolt-Glaser, J.K., Marucha, P.T., Malarkey, W.B., Mercado, A.M., & Glaser, R. (1995). Slowing of wound healing by psychological stress. The Lancet, 346, 1194–1196. doi:10.1016/S0140-6736(95)92899-5
- Kudielka, B.M., Hellhammer, D.H., & Wust, S. (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34, 2–18. doi:10.1016/j.psyneuen.2008.10.004
- Larsen, M.H., Mikkelsen, J.D., Hay-Schmidt, A., & Sandi, C. (2010). Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. Journal of Psychiatric Research, 44, 808–816. doi:10.1016/j.jpsychires.2010.01.005
- Lovelock, D.F., & Deak, T. (2017). Repeated exposure to two stressors in sequence demonstrates that corticosterone and paraventricular nucleus of the hypothalamus interleukin-1β responses habituate independently. Journal of Neuroendocrinology, 29, e12514. doi:10.1111/jne.12514
- Lupien, S.J., McEwen, B.S., Gunnar, M.R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. doi:10.1038/nrn2639
- Manikowska, K., Mikołajczyk, K., Mikołajczak, P.L., & Bobkiewicz-Kozłowska, T. (2014). The influence of mianserin on TNF-α, IL-6 and IL-10 serum levels in rats under chronic mild stress. Pharmacological Reports, 66, 22–27. doi:10.1016/j.pharep.2013.06.003
- Maras, P.M., Molet, J., Chen, Y., Rice, C., Ji, S.G., Solodkin, A., & Baram, T.Z. (2014). Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Molecular Psychiatry, 19, 811–822. doi:10.1038/mp.2014.12
- Marquez, C., Belda, X., & Armario, A. (2002). Post-stress recovery of pituitary–adrenal hormones and glucose, but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Research, 926, 181–185. doi:10.1016/S0006-8993(01)03112-2
- Martí, O., Garcia, A., Velles, A., Harbuz, M.S., & Armario, A. (2001). Evidence that a single exposure to aversive stimuli triggers long-lasting effects in the hypothalamus-pituitary-adrenal axis that consolidate with time. European Journal of Neuroscience, 13, 129–136. doi:10.1046/j.1460-9568.2001.01355.x
- Matthews, K., Forbes, N., & Reid, I.C. (1995). Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiological Behavior, 57, 241–248. doi:10.1016/0031-9384(94)00286-E
- McEwen, B.S. (2004). Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032, 1–7. doi:10.1196/annals.1314.001
- Miller, G.E., Chen, E., & Zhou, E.S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi:10.1037/0033-2909.133.1.25
- Ostrander, M.M., Ulrich-Lai, Y.M., Choi, D.C., Richtand, N.M., & Herman, J.P. (2006). Hypoactivity of the hypothalamo-pituitary- adrenocortical axis during recovery from chronic variable stress. Endocrinology, 147, 2008–2017. doi:10.1210/en.2005-1041
- Pitman, D.L., Natelson, B., Ottenweller, J., McCarty, R., Pritzel, T., & Tapp, W. (1995). Effects of exposure to stressors of varying predictability on adrenal function in rats. Behavioral Neuroscience, 109, 767–776. doi:10.1037/0735-7044.109.4.767
- Robles, T.F., Glaser, R., & Kiecolt-Glaser, J.K. (2005). Out of balance: A new look at chronic stress, depression, and immunity. Current Directions in Psychological Science, 14, 111–115. doi:10.1111/j.0963-7214.2005.00345.x
- Romeo, R.D., Karatsoreos, I.N., & McEwen, B.S. (2006). Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Hormones and Behavior, 50, 463–468. doi:10.1016/j.yhbeh.2006.06.002
- Schreier, H.M., Roy, L.B., Frimer, L.T., & Chen, E. (2014). Family chaos and adolescent inflammatory profiles: The moderating role of socioeconomic status. Psychosomatic Medicine, 76, 460–467. doi:10.1097/PSY.0000000000000078
- Smyth, J., Zawadzki, M., & Gerin, W. (2013). Stress and disease: A structural and functional analysis. Social and Personality Psychology Compass, 7, 217–227. doi:10.1111/spc3.12020
- Stark, J.L., Avitsur, R., Padgett, D.A., Campbell, K.A., Beck, F.M., & Sheridan, J.F. (2001). Social stress induces glucocorticoid resistance in macrophages. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 280, R1799–R1805. doi:10.1152/ajpregu.2001.280.6.R1799
- Stawski, R.S., Cichy, K.E., Piazza, J.R., & Almeida, D.M. (2013). Associations among daily stressors and salivary cortisol: Findings from the National Study of Daily Experiences. Psychoneuroendocrinology, 38, 2654–2665. doi:10.1016/j.psyneuen.2013.06.023
- Steptoe, A., & Kivimaki, M. (2012). Stress and cardiovascular disease. Nature Reviews Cardiology, 9, 360–370. doi:10.1038/nrcardio.2012.45
- Tamashiro, K.L., Sakai, R.R., Shively, C.A., Karatsoreos, I.N., & Reagan, L.P. (2011). Chronic stress, metabolism, and metabolic syndrome. Stress, 14, 468–474. doi:10.3109/10253890.2011.606341
- Varga, J., Domokos, Á., Barna, I., Jankord, R., Bagdy, G., & Zelena, G. (2011). Lack of vasopressin does not prevent the behavioural and endocrine changes induced by chronic unpredictable stress. Brain Research Bulletin, 84, 45–52. doi:10.1016/j.brainresbull.2010.09.014
- von Kanel, R., Kudielka, B.M., Preckel, D., Hanebuth, D., & Fischer, J.E. (2006). Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain, Behavior, and Immunity, 20, 40–48. doi:10.1016/j.bbi.2005.03.013
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology, 134, 319–329. doi:10.1007/s002130050456
- Willner, P. (2005). Chronic mild stress (CMS) revisited: Consistency and behavioral-neurobiological concordance in the effects of CMS. Neuropsychobiology, 52, 90–110. doi:10.1159/000087097
