Abstract
The hypothalamic–pituitary–adrenal (HPA) axis is the major neuroendocrine axis regulating homeostasis in mammals. Glucocorticoid hormones are rapidly synthesized and secreted from the adrenal gland in response to stress. In addition, under basal conditions glucocorticoids are released rhythmically with both a circadian and an ultradian (pulsatile) pattern. These rhythms are important not only for normal function of glucocorticoid target organs, but also for the HPA axis responses to stress. Several studies have shown that disruption of glucocorticoid rhythms is associated with disease both in humans and in rodents. In this review, we will discuss our knowledge of the negative feedback mechanisms that regulate basal ultradian synthesis and secretion of glucocorticoids, including the role of glucocorticoid and mineralocorticoid receptors and their chaperone protein FKBP51. Moreover, in light of recent findings, we will also discuss the importance of intra-adrenal glucocorticoid receptor signaling in regulating glucocorticoid synthesis.
An overview of the hypothalamic–pituitary–adrenal axis and its rhythms
The hypothalamic–pituitary–adrenal (HPA) axis regulates circulating levels of glucocorticoid hormones (cortisol in humans, corticosterone in the rat and mouse; hereafter referred to as CORT) and is the major neuroendocrine axis regulating homeostasis in mammals. When stress activates the HPA axis the resultant increase in CORT prepares the body to cope with and recover from, the stressor. Glucocorticoids have a wide range of effects; they are involved in the regulation of metabolic processes, immune system, reproduction, behavior and cognitive functions (Cherrington, Citation1999; Chrousos, Citation1995; de Kloet, Citation2000; Macfarlane et al., Citation2008; McEwen, Citation2007; Munck et al., Citation1984). The main activator of the HPA axis is the neuropeptide corticotropin-releasing hormone (CRH), synthetized in the hypothalamic paraventricular nucleus (PVN). Upon activation, CRH is released into portal vessels of the median eminence to reach the anterior pituitary where it stimulates the synthesis and release of adrenocorticotropic hormone (ACTH) from corticotroph cells (Antoni, Citation1986; Vale et al., Citation1981). ACTH in turn is secreted into the blood circulation from where it reaches the adrenal cortex to stimulate the synthesis and secretion of CORT (Dallman et al., Citation1987).
Glucocorticoids exert their effects through activation of the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR) (Reul & de Kloet, Citation1985). These are intracellular receptors belonging to the nuclear receptor family. After binding to CORT, MR and GR translocate to the nucleus where they regulate gene transcription and subsequent protein synthesis (Pratt et al., Citation2006; Robertson et al., Citation1993). Increasing evidence suggests that, in addition to genomic actions, CORT can induce rapid, nongenomic effects occurring within seconds to minutes (Groeneweg et al., Citation2012; Tasker et al., Citation2006). MR and GR are expressed in different tissues and therefore exert different physiological functions. GR expression is ubiquitous throughout the body and is involved in mediating CORT-regulated processes such as energy distribution (e.g. glycogenesis, fat and protein metabolism) and immune function. In the brain, GR levels are particularly high in the hippocampus, amygdala, PVN and prefrontal cortex while MR is mainly expressed in limbic areas, with high levels in the hippocampus, and moderate levels in the amygdala and prefrontal cortex (Reul & de Kloet, Citation1985, Citation1986). Because of their different affinities for CORT, MR is already occupied when CORT concentrations are low, such as during the hormonal circadian nadir, while GR is only activated when CORT levels are high, such as during the circadian peak and in response to stress (Conway-Campbell et al., Citation2007; Kitchener et al., Citation2004; Reul & de Kloet, Citation1985; Reul et al., Citation1990; Spencer et al., Citation1993).
In addition to their effects on metabolism and the immune system, CORT also regulates its own secretion by a negative feedback mechanism that involve activation of GR and MR in various brain regions and in the anterior pituitary.
CORT-mediated negative feedback is fundamental for the termination of the HPA axis response to stress, and, as we will discuss later on, is also important for optimal secretion of CORT in basal (unstressed) conditions. Studies in rodents have identified two temporal distinct negative feedback responses: a fast (sec-min) nongenomic and a delayed (hours-days) genomic feedback (Dallman & Yates, Citation1969; Jones et al., Citation1977, Citation1974; Keller-Wood & Dallman, Citation1984; Osterlund et al., Citation2016). The first involves CORT-mediated rapid inhibition of HPA axis activity though inhibition of CRH and ACTH secretion from the PVN and anterior pituitary, respectively. The second, on the other hand, involves CORT-induced inhibition of CRH expression in the PVN, as well as suppression of transcription of the ACTH precursor pro-opiomelanocortin (POMC) in the pituitary corticotroph cells (de Kloet et al., Citation2005; McEwen, Citation2007). The mechanisms underlying CRH, ACTH and CORT synthesis and secretion, as well as known mechanisms of CORT mediated negative feedback at each node of the HPA axis will be discussed in detail in the next sections.
Rapid changes in CORT secretion are not only observed after stress, but also occur in basal (non-stressed) conditions throughout the 24-h cycle. In basal conditions, the pattern of CORT release is highly dynamic, with a circadian rhythm characterized by high levels of secretion prior to the active phase (night in the rat and day in humans) and low levels during the inactive phase (Carnes et al., Citation1988a; Watts et al., Citation2004). CORT secretion is also characterized by an ultradian rhythm, a high frequency pulsatile secretion, with variable amplitude through the 24-h cycle. Pulses of CORT with higher amplitude occur immediately prior to and during the active phase, so that the circadian changes of CORT are actually due to changes in the amplitude of CORT pulses during the 24-h cycle (Jasper & Engeland, Citation1991; Windle et al., Citation1998). The ultradian rhythm of CORT in the rat was first observed using intra-adrenal microdialysis technique (Jasper & Engeland, Citation1991). Since then, the use of an automated blood sampling systems has allowed researchers to study how the CORT ultradian rhythm changes in physiological and pathological conditions in the rat (Clark et al., Citation1986; Windle et al., Citation1998). Further, ultradian rhythm of CORT has been observed in human and other several mammals (Fulkerson, Citation1978; Henley et al., Citation2009; Holaday et al., Citation1977; Lewis et al., Citation2005). Similarly, pulsatile secretion of ACTH has also been observed in humans (Henley et al., Citation2009), sheep (Apostolakis et al., Citation1992) and in the rat (Carnes et al., Citation1986, Citation1988b). Importantly, both in human and in the rat, dynamic changes in CORT are preceded by similar fluctuations in ACTH concentration in the plasma (Henley et al., Citation2009; Jasper & Engeland, Citation1991). Due to the difficulty of measuring CRH concentration in the median eminence, as a result of the very stressful surgery needed for portal blood sampling in the rat and the low sensitivity of CRH assays, only a few studies have shed light on the pattern of CRH secretion in vivo. Nevertheless, pulsatile CRH secretion in the median eminence has been observed in free-moving rats (Ixart et al., Citation1991, Citation1994). Furthermore, episodic CRH has been shown in sheep (Engler et al., Citation1990).
It is noteworthy that, although CRH in rodents is regarded as the main activator of the HPA axis, in some conditions ACTH and CORT responses are still observed even in the absence of CRH stimulation (Muglia et al., Citation2001). Studies investigating the pituitary and adrenal responses to stress in CRH knock out (KO) mice have shown that CRH is essential for a normal ACTH and CORT response to some stressors, including restraint and fasting (Muglia et al., Citation2000). However, ACTH and CORT responses were observed in CRH KO mice exposed to more and severe stressors, although these responses were lower than in CRH expressing mice (Jacobson et al., Citation2000). The mechanisms regulating the observed stress response in the absence of CRH may result from the hypersecretion of other hypothalamic neuropeptides, including vasopressin and oxytocin (Muglia et al., Citation2001). Indeed, both spontaneous, as well as vasopressin-induced ACTH secretion was observed in vitro in corticotroph cells in which CRH was depleted by selective cytotoxin treatment (Schwartz & Vale, Citation1988). Although the role of vasopressin in regulating ACTH secretion in responses to stress is well known (Volpi et al., Citation2004), current evidence suggests that vasopressin does not regulate basal HPA axis activity. Indeed, basal ACTH secretion was unaltered in vasopressin-deficient Brattleboro rats (Makara et al., Citation2004), as well as in mice lacking the vasopressin receptor V1b (Roper et al., Citation2011). Furthermore, we have shown that blocking vasopressin actions on the anterior pituitary (using a vasopressin V1b receptor antagonist) has no effect on basal circadian and ultradian rhythms of corticosterone secretion (Spiga et al., Citation2009). In addition to vasopressin, HPA axis activity can also be regulated by oxytocin. In vivo experiments have shown that oxytocin administration reduces ACTH and CORT levels in rat exposed to stress (Windle et al., Citation1997, Citation2004). Furthermore, blockade of the oxytocin receptor in the brain results in increased basal and stress-induced ACTH and CORT secretion in the rat (Neumann et al., Citation2000), and increased CORT concentrations were found in oxytocin KO female mice exposed to stress (Amico et al., Citation2004). In contrast to its inhibitory effect at central level, in vitro studies have shown that oxytocin acts at the level of the pituitary to potentiate the CRH-induced ACTH-secretion (Gibbs, Citation1984). Further studies have shown that peripheral oxytocin increases stress-induced ACTH release (Gibbs, Citation1986). Whether physiological levels of oxytocin in unstressed conditions participate in the regulation of CORT ultradian and circadian rhythm is not known.
There is growing evidence for the importance of circadian and ultradian rhythmicity of CORT in maintaining normal responsiveness to CORT in target tissue (Lightman & Conway-Campbell, Citation2010). The regulation of CORT circadian rhythms has been investigated in detail, and several recent reviews have addressed this topic (Dickmeis, Citation2009; Kalsbeek & Fliers, Citation2017; Spiga et al., Citation2014). However, much less is known about the regulation of CORT ultradian rhythms. In this review, we will first give an overview on what is known about the mechanisms underlying glucocorticoid negative feedback within each level of the HPA axis, both describing known nongenomic and genomic mechanisms. We will then discuss evidence for a peripheral CORT pulse generator and discuss how CORT-mediated negative feedback mechanisms may regulate this pulse generator. Finally, we will describe recent findings suggesting a role for FKBP51 in regulating basal CORT secretion, and how the use of FKBP51 antagonists may represent a therapeutic tool in disease where hyperactivity of the HPA axis has been observed.
Regulation of CRH synthesis and secretion
CRH produced in PVN neurons is contained within neurosecretory vesicles localized in axon terminals in the external zone of the median eminence. Activation of neural pathways afferent to CRH neurons in the PVN causes a rapid calcium influx that stimulates the fusion of CRH-containing vesicles to the cell membrane and subsequent release CRH in proximity of the pituitary portal vasculature (Antoni, Citation1986). Concomitant to CRH secretion, activation of CRH neurons also induces CRH gene transcription, followed by de novo synthesis of CRH peptide. CRH transcription is induced by the activation the cAMP/Protein kinase A-regulated pathway, and depends on the downstream binding of phosphorylated cAMP-responsive element binding protein (pCREB) to a cAMP-responsive element (CRE) in the CRH promoter (Liu et al., Citation2010; Seasholtz et al., Citation1988). This is accompanied by binding of CREB to its co-activator CREB-regulated transcriptional co-activator (CRTC; also known as transducer of regulated CREB activity, TORC) (Aguilera, Citation2015). While secretion of CRH allows rapid HPA axis activation and CORT release, de novo synthesis of CRH peptide is necessary to replenish the intravesicular CRH store, in order to maintain responsiveness of the system (Watts, Citation2005).
There is evidence that CORT can negatively regulate both CRH synthesis and secretion (Aguilera et al., Citation2007; Harbuz & Lightman, Citation1989; Kovacs & Makara, Citation1988). Increased CRH expression in the PVN has been shown in adrenalectomized rats both in basal conditions and after exposure to stress. Further, it was shown that in adrenalectomized rats even a minor stressor is sufficient to increase CRH hnRNA, an effect that is not observed in intact animals (Dallman et al., Citation1994; Lightman & Young, Citation1988; Ma & Aguilera, Citation1999). More recent studies have shown overexpression of CRH mRNA, as well as hypersecretion of ACTH and CORT in mice with disrupted GR in the PVN (Laryea et al., Citation2013). Glucocorticoids regulate the activity of PVN CRH neurons by inhibiting neuronal pathways afferent to the PVN (e.g. the, hippocampus, prefrontal cortex and amygdala). In addition, as GR is highly expressed in CRH-secreting neurons of the PVN, CORT can exert negative feedback directly at the level of the PVN (Fenoglio et al., Citation2004; Liposits & Paull, Citation1989).
Nongenomic effects. In addition to the regulation of CRH gene expression (described later in this section), CORT can also exert fast inhibitory effects in the PVN, via nongenomic mechanisms. The mechanism underlying these effects involves CORT-induced endocannabinoid release, presumably via activation of a putative membrane GR (Tasker et al., Citation2006). Using whole-cell patch clamp recordings in the hypothalamus, Tasker and colleagues have shown that CORT can inhibit glutamatergic input to parvocellular neurons of the PVN, and that this is dependent on post-synaptic G- protein couples receptors and pre-synaptic type I cannabinoid receptor (Di et al., Citation2003). The existence of a membrane-associated G protein-coupled GR regulating rapid negative feedback was supported by further studies showing that injection of DEX conjugated to bovine serum albumin (to prevent it from crossing the cell membrane) prevents stress-induced HPA axis activation, and this effect is blocked by the co-administration of a type-I cannabinoid receptor reverse agonist (Evanson et al., Citation2010a, Citation2010b). More recently, the same group has shown that rapid GR-mediated negative feedback is lost in GR KO mice, suggesting that GRs in the membrane may mediate fast feedback, but that a distinct genomic receptor remains a possibility. On the other hand, they also proposed that membrane GR is encoded by a different gene, and that expression of the membrane GR is regulated by the classic GR (Nahar et al., Citation2015). In addition to rapid feedback mechanisms in the PVN, CORT-mediated nongenomic negative feedback regulation occurs in other brain regions, including the hippocampus, amygdala and pre-frontal cortex, which in turn regulate PVN output (Groeneweg et al., Citation2012).
Genomic effects. The mechanisms underlying glucocorticoid induced negative regulation of CRH transcription and mRNA levels are not fully understood. Studies in vitro using AtT-20 cells transfected with the human CRH gene have failed to show the existence of the classical GRE in the CRH promoter. However, the same study demonstrates a glucocorticoid-dependent repression involving direct DNA binding of GR to a negative GRE (nGRE) within the CRH promoter (Malkoski & Dorin, Citation1999; Malkoski et al., Citation1997). In contrast, Evans and colleagues have shown a small decrease in cAMP-induced CRH hnRNA following CORT incubation in primary cultures of fetal rat hypothalamic neurones. However, in the same, study chromatin immuno-precipitation assays showed no difference in GR-binding to the CRH promoter following restraint stress, or in basal conditions in adrenalectomized rats compared to intact animals (Evans et al., Citation2013). Other studies have demonstrated that GR does not bind directly the CRH promoter, and suggest that CORT exerts its regulatory role on CRH transcription by inhibiting CREB binding to the CRE within the CRH promoter, through a mechanisms that involves protein-protein interactions between GR and CREB (Guardiola-Diaz et al., Citation1996; Yamamori et al., Citation2007).
As we have previously discussed, the activity of pCREB at the CRH promoter depends on the binding to its co-regulators CRTC, in particular to CRTC2. Under un-stimulated conditions, phosphorylated CRTC2 is located in cytosol, but upon stimulation, CRTC2 dephosphorylates and translocates to the nucleus to enhance CREB transcriptional activity (Katoh et al., Citation2004; Screaton et al., Citation2004). In a recent study, Jeanneteau and colleagues suggest that phosphorylation and nuclear localization of hypothalamic CRTC2 can be modulated by GR signaling, via a calmodulin-mediated mechanism. Indeed, they have shown that both exposure to stress and administration of the synthetic glucocorticoid dexamethasone (DEX) enhance phosphorylation of CRTC2 in mice (Jeanneteau et al., Citation2012). In addition to its regulation of CRH transcription, there is evidence that CORT can regulate CRH translational activity and CRH mRNA stability (Ma et al., Citation2001).
Regulation of ACTH synthesis and secretion
CRH secreted in the median eminence activates corticotroph cells in the anterior pituitary to stimulate ACTH synthesis and secretion, by both genomic and non-genomic mechanisms. Genomic effects of CRH involve the transcription of the ACTH precursor POMC that is then cleaved to ACTH, and stored in vesicles (Drouin et al., Citation1987; Gagner & Drouin, Citation1985). At the same time, CRH induces the secretion of pre-formed ACTH by vesicular exocytosis, thus allowing rapid ACTH secretion upon stimulation.
Nongenomic mechanisms. CRH binding to CRHR1 leads to activation of the cAMP/PKA pathway. This results in rapid phosphorylation of ion channels and increase in calcium influx, which ultimately causes the fusion of ACTH-containing vesicle to the cell membrane and exocytosis (Ritchie et al., Citation1996). One mechanism through which CORT rapidly inhibits ACTH secretion is by regulating the electropotential properties of the electrically excitable corticotroph cells. Upon CRH stimulation, there is an increase in both bursting and frequency of firing action potentials (Duncan et al., Citation2015). Bursting is an important property of corticotroph cells as it increases intracellular calcium concentrations, and hence the potential to activate the release of ACTH from vesicles (Stojilkovic et al., Citation2005; Tagliavini et al., Citation2016; Van Goor et al., Citation2001). Electrophysiology studies in murine corticotroph cells showed that under un-stimulated conditions cells fire spontaneous spikes, whereas, when stimulated with CRH, the firing frequency increases, ultimately resulting in bursts. Bursting-type firing in corticotroph cells is dependent on activation of large-conductance calcium- and voltage-activated potassium (BK) channels (Duncan et al., Citation2015; CitationTabak et al., 2011; Tsaneva-Atanasova et al., Citation2007), therefore it is thought that activation of BK channels is required for ACTH secretion. One of the mechanisms by which CORT affects ACTH secretion is by reducing bursting. Duncan et al. have shown that CORT decreases both spontaneous firing as well as the CRH -induced bursts (Duncan et al., Citation2016). Further, the same study has shown that CORT-mediated reduction in burst firing, and ACTH release, is BK channel-dependent. The exact mechanism underlying CORT regulation of BK channels is but does seem to involve PKA-mediated BK channel phosphorylation (Chen et al., Citation2005; Tian et al., Citation2001, Citation1998).
The release of ACTH from corticotroph cells is also regulated by the activation of Annexin 1 (ANXA1), a protein produced in pituitary folliculostellate cells and secreted to act on endocrine cells via binding its putative receptor, the formyl peptide receptor. In corticotroph cells ANXA1 inhibits CRH-driven ACTH secretion, presumably by a mechanism downstream of cAMP and Ca2 + influx that involves local reorganization of the actin cytoskeleton (Buckingham et al., Citation2006). This ultimately results in decreased ACTH secretion from vesicles. CORT can inhibit ACTH secretion by promoting ANXA1 translocation from the cytoplasm to the outer surface of the plasma membrane (Chapman et al., Citation2003; John et al., Citation2004). In addition to its activity, CORT also regulate ANXA1 expression. However, while CORT-induced translocation of ANXA1 occurs rapidly and involves post-translational modification of the protein (Buckingham et al., Citation2006), CORT-induced ANXA1 expression is relatively slow. It is, nevertheless, important for replenishing the stored ANXA1 that is depleted after CORT-induced membrane translocation (John et al., Citation2004).
Genomic mechanisms. CRH-induced activation of the cAMP/PKA pathway results in phosphorylation of CREB and subsequent transcription of POMC. In addition, CRH also activates the MAPK pathway (Kovalovsky et al., Citation2002) resulting in activation of the orphan nuclear receptor Nur77 (Philips et al., Citation1997). The binding of Nur77 to the NurRE within the POMC promoter is known to enhance pCREB-mediated gene transcription (Phillips et al., Citation1997). CORT mediates negative feedback by inhibiting ACTH synthesis through a mechanism that requires binding of activated GR to an nGRE within the POMC promoter (Drouin et al., Citation1989a, Citation1989b). In addition, GR can inhibit Nur77-induced POMC transcription through a protein–protein interaction mechanism (Martens et al., Citation2005; Philips et al., Citation1997). A recent study suggests an involvement of NeuroD1 in regulating POMC transcription (Parvin et al., Citation2017). In the absence of CORT, NeuroD1 interacts with the E-box on the POMC promoter and activates transcription (Poulin et al., Citation1997). Increased CORT concentration causes a repression of NeuroD1 expression, and hence less activation of POMC transcription (Parvin et al., Citation2017).
Regulation of corticosterone synthesis
ACTH released into the general circulation induces CORT synthesis by activating its specific receptor melanocortin type-2 receptor (MC2R) within steroidogenic cells of the zona fasciculata of the adrenal cortex. ACTH binding to MC2R increases intracellular levels of cAMP, which in turn activates the PKA pathway. As previously described, CORT is a lipophilic molecule and therefore cannot be stored in vesicles within cells but is rapidly (within minutes) synthetized and released after ACTH stimulation. ACTH regulates nongenomic events such as post-translational modification of steroidogenic proteins. This includes phosphorylation (and hence increased activation) of proteins involved in cholesterol metabolism, namely hormone-sensitive lipase (HSL) and steroidogenic acute regulatory protein (StAR), which regulate the levels of intracellular cholesterol and its transport within the mitochondrial matrix where the steroidogenic process is initiated (Kraemer & Shen, Citation2002; Lin et al., Citation1995; Stocco & Clark, Citation1996). Cholesterol undergoes a series of enzymatic reactions occurring both in the mitochondria and the smooth endoplasmic reticulum, which ultimately lead to the synthesis and secretion of CORT. In parallel to these rapid non-genomic events, ACTH also regulates the transcription of genes encoding for steroidogenic proteins, including StAR and CYP11A (the gene encoding for the cholesterol side-chain cleavage cytochrome protein P450scc, which catalyzes the cleavage of the cholesterol side-chain to produce pregnenolone in the mitochondria). ACTH also regulates the expression of MC2R (Mountjoy et al., Citation1994), and the melanocortin receptor accessory protein (MRAP), which regulates the level and activity of MC2R at the cell surface, and thus the cell's responsiveness to ACTH (Metherell et al., Citation2005).
Our recent studies have shown that the adrenal gland responds rapidly to physiological pulses of ACTH, with pulsatile activation of the steroidogenic pathway. Indeed, we have recently demonstrated that pulsatile ACTH induces pulsatile adrenal responses both at non-genomic and genomic level, including pulsatile phosphorylation of HSL (Spiga et al., Citation2017) as well as pulsatile transcription of steroidogenic genes including StAR, CYP11A and MRAP, and pulsatile phosphorylation of CREB (Spiga et al., Citation2011a, Citation2011b). Interestingly, we have also shown that pulsatile ACTH dynamically regulates the transcription of genes encoding for nuclear receptors involved in the transcription of steroidogenic genes, including steroidogenic factors 1, Nur77 and DAX-1 (Spiga et al., Citation2017). These findings indicate that ultradian secretion of ACTH is important not only for a rapid acute CORT response, but also the long-term maintenance of the steroidogenic activity under basal conditions.
In humans GR is expressed in the adrenal cortex, with functions parallel to that found in other tissues (Briassoulis et al., Citation2011; Spiga et al., Citation2017). This suggests that, in addition to its effect in the pituitary and hypothalamus, CORT could affect its own synthesis by a local feedback mechanism within the adrenal itself. Indeed, a number of in vitro and in vivo studies have shown that prior exposure of the adrenal gland to glucocorticoids results in a decreased response to ACTH. For example, CORT synthesis in response to ACTH is reduced in cultured adrenal cells treated with high levels of CORT or DEX (Carsia & Malamed, Citation1979; Chong et al., Citation2017; Peron, Citation1960). These findings are consistent with studies showing lower responses to ACTH in adrenals collected from hypophysectomized rats treated with CORT, when compared with adrenals from untreated rats (Langecker & Lurie, Citation1957). Other studies have shown that pretreatment with CORT prevents ACTH-induced CORT increase in adrenal vein effluent (Black et al., Citation1961; Richards & Pruitt, Citation1957). Furthermore, pretreatment with ACTH prevents CORT response to further stimuli in the rat (Desouza & Vanloon, Citation1982; Stockham, Citation1964). These data are in keeping with an intra-adrenal CORT negative feedback loop that could constitute an additional GR regulated control mechanism for steroidogenesis.
Genomic effects. A number of studies have shown that CORT can affect steroid synthesis in the adrenal gland both in vitro (Carsia & Malamed, Citation1979; Peron et al., Citation1960) and in vivo (Jones & Stockham, Citation1966; Langecker & Lurie, Citation1957). However, the molecular mechanism underlying CORT-mediated inhibition of steroidogenesis is not clear. There is evidence that, at genomic level, the synthetic glucocorticoid dexamethasone can inhibit the transcription of steroidogenic genes through a mechanism that involves increase in the expression of the steroidogenic co-repressor DAX-1 (Gummow et al., Citation2006). Furthermore, as observed in the pituitary for POMC transcription, CORT can also repress the transcription of StAR by inhibiting both the transcription and the activity of Nur77 via a mechanism that involves active GR (Martin & Tremblay, Citation2008; Song et al., Citation2004). In addition to its role in regulating CRH transcription as previously described for CRH, CRTC2 also regulates CREB-mediate transcription of steroidogenic genes in the adrenal cortex (Takemori et al., Citation2007; Takemori & Okamoto, Citation2008). As previously discussed, nuclear localization of CRTC is essential for its function and a recent study has shown that is inhibited by CORT (Jeanneteau et al., Citation2012). Whether CORT can affect CRTC2 activity in the adrenal cortex is not known, however it is tempting to speculate that this could represent an additional mechanism of CORT-mediated intra-adrenal negative feedback.
Nongenomic effects. Further studies using both mathematical modeling and experimental work in the rat have provided evidence for the existence of a mechanism by which CORT can rapidly inhibit its own synthesis and/or secretion (Spiga et al., Citation2017; Walker et al., Citation2015). This suggests that, in addition to the genomic effects of CORT on StAR transcription, CORT may also regulate its own synthesis by interfering with non-genomic mechanisms within the adrenal steroidogenic pathway. The biological processes underlying this rapid self-inhibition are not known, but we have shown that administration of pulsatile ACTH in the rat does lead to a rapid increase in intra-adrenal CORT concentrations that is followed by a dynamic activation of GR (measured as GR phosphorylation at Serine 211) (Spiga et al., Citation2017). Although to date there is no evidence that CORT can regulate the activity of steroidogenic proteins, CORT can exert nongenomic effects by associating with the catalytic subunit of PKA and regulating its activity (Doucas et al., Citation2000).
Rapid non-genomic effects of CORT in the anterior pituitary involve inhibition of ANXA1 (Taylor et al., Citation1995) and interestingly, ANXA1 has also been identified in the adrenal cortex, where its expression and activity is regulated by CORT (Davies et al., Citation2008). Importantly, the same study has shown a greater CORT response to ACTH stimulation in isolated adrenal gland obtained from ANXA1-null mice, compared to adrenal obtained from WT mice. This suggests that, as well as regulating ACTH secretion, ANXA1 may also be involved in the regulation of CORT synthesis and play a role in CORT-mediated intra adrenal negative feedback (Spiga & Lightman, Citation2015).
It is noteworthy that in addition to ACTH, the steroidogenic activity of the adrenal gland is also regulated by other factors, including neural and humoral factors. Studies in the rat have shown that splanchnic innervation regulates CORT secretion differently depending in the activity state of the adrenal. While adrenal splanchnic innervation increases adrenal sensitivity to ACTH under stress conditions (i.e. when the HPA axis is activated), the opposite effect is observed in basal unstressed conditions (Jasper & Engeland, Citation1994). The splanchnic regulatory effect on adrenal responsiveness not only affects circadian rhythm of CORT (Ulrich-Lai et al., Citation2006), but also affect pulsatile pattern of CORT. Indeed, splanchnic denervation results in increased frequency and amplitude of CORT pulses in the rat (Jasper & Engeland, Citation1994). The mechanisms underlying these effects are not fully understood. Studies have shown no effect of the splanchnic input on the levels of the steroidogenic protein StAR (Ulrich-Lai et al., Citation2006). Splanchnic adrenal innervation has profound effects on catecholamine secretion and on adrenal blood flow that may affect the intra-adrenal levels of ACTH. In addition to splanchnic inputs, other extra-ACTH mechanisms can control adrenal CORT secretion, including pro-inflammatory cytokines and metabolic factors (e.g. leptin, insulin, glucagon) (Bornstein & Chrousos, Citation1999).
Regulation of glucocorticoid pulsatility: the pituitary-adrenal pulse generator
It is well established that the suprachiasmatic nucleus (SCN) of the ventral hypothalamus controls the circadian rhythm of CORT (Kalsbeek et al., Citation2012). Indeed, in rodents, lesion of the SCN causes complete loss of the circadian pattern of corticosterone secretion with increased levels in the morning (Cascio et al., Citation1987; Sage et al., Citation2001; Szafarczyk et al., Citation1979). Studies in SCN-lesioned rats, or in rats kept in constant light for several weeks, showed that, although circadian CORT rhythm was abolished their CORT pulsatile pattern was maintained (Waite et al., Citation2012). These observations led to further investigation on the mechanisms by which ultradian CORT rhythm is generated and maintained, and this review will address this topic in detail.
It has been assumed for many years that the ultradian rhythm of CORT secretion is under hypothalamic regulation, as observed for other hormones such as luteinizing hormone (LH) and growth hormone (GH), whose pulsatile pattern is the result of pulsatile secretion of their respective hypothalamic releasing factors (Belchetz et al., Citation1978; Clarke & Cummins, Citation1982; Plotsky & Vale, Citation1985). CRH is the main neuropeptide regulating basal ACTH secretion in the rat, and, because episodic release of CRH has also been shown in the rat (Ixart et al., Citation1991, Citation1993, Citation1994) (), the existence of a hypothalamic CRH pulse generator was not questioned. However, there is a mismatch between the pulse frequency of CRH (3 pulses/hour) and the pulse frequency of ACTH and corticosterone (∼1 pulse/hour). The existence of the hypothalamic pulse generator was further questioned in light of data showing that, despite a small decrease in mean ACTH levels, blockade of CRH release using a CRF antagonist did not significantly affect the frequency, duration and amplitude of ACTH pulses (Negrovilar et al., Citation1987). Further, a study in the sheep demonstrated maintenance of the ultradian rhythm of CORT even in absence of PVN-mediated regulation of the pituitary (Engler et al., Citation1990). Motivated by this evidence, we addressed this matter using an interdisciplinary approach of mathematical modeling and in vivo experimental work in the rat (). The findings of these studies support the hypothesis that the ultradian CORT rhythm occurs independently of central hypothalamic control and depends on dynamic pituitary-adrenal interactions (Walker et al., Citation2012, Citation2010). This mechanism is based on the ACTH-dependent positive feedforward drive regulating adrenal synthesis and secretion of CORT, and the CORT-mediated negative feedback that regulates ACTH secretion at the level of the anterior pituitary. What makes this feedforward–feedback loop result in pulsatile activity is the existence of time-delays in the secretory response of the adrenal in response to ACTH stimulation, and in the negative feedback mechanism inhibiting ACTH secretion at the level of the pituitary. The pulse of CORT that follows each ACTH pulse has been well characterized both in the rat (Carnes et al., Citation1986; Spiga et al., Citation2017; Walker et al., Citation2015, Citation2012) and in humans (Henley et al., Citation2009). Since CORT cannot be pre-synthesized and stored within adrenal steroidogenic cells due to its lipophilic nature (in contrast to peptides hormones such as CRH and ACTH), the delay in the feedforward drive is due to the time needed by the adrenal for de novo synthesis of CORT in response to ACTH. In contrast, the mechanisms regulating fast CORT negative feedback still remains to be fully characterized, but evidence suggests the involvement of GR (Deng et al., Citation2015; Spiga et al., Citation2017; Walker et al., Citation2015).
Figure 1. Ultradian rhythms of the hypothalamic–pituitary–adrenal (HPA) axis and glucocorticoid rhythms. The hypothalamic paraventricular nucleus (PVN) receives circadian input from the suprachiasmatic nucleus of the hypothalamus and stress inputs from the brainstem and from regions of the limbic system such as the hippocampus and amygdala. The PVN projects to the median eminence where it releases corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) into the hypothalamic-pituitary portal circulation. CRH passes through this vascular route to access corticotroph cells in the anterior pituitary, which respond with the rapid release of adrenocorticotropic hormone (ACTH) from preformed vesicles into the general circulation. In turn, ACTH reaches the adrenal cortex where it activates the synthesis and secretion of glucocorticoid hormones (CORT). CORT regulate the activity of the HPA axis, and thus their own production, through feedback mechanisms acting at the level of the pituitary gland where they inhibit ACTH release, and at the level of the PVN where they inhibit the release of CRH and AVP. Under basal (i.e., unstressed) conditions, an ultradian pattern of secretion underlies all the components of the HPA axis. Note that in the rat, CRH pulse frequency is higher (∼3 pulses/h) than the near-hourly oscillation in ACTH and CORT. Reproduced with permission from (Spiga et al., Citation2014).
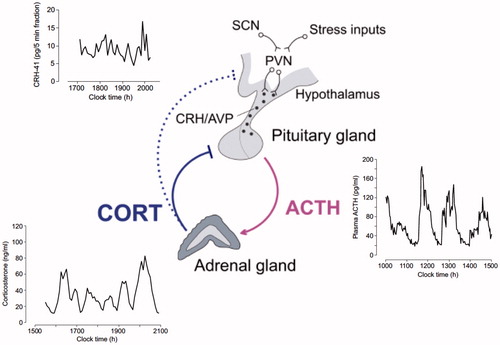
Figure 2. Mathematical modeling predictions and experimental data illustrating the pituitary-adrenal response to different levels of constant CRH drive. (A) Computed two-parameter bifurcation diagram shows that different combinations of constant CRH drive and adrenal delay result in qualitatively different dynamic responses from the pituitary–adrenal system. On one side of the transition curve, the pituitary–adrenal system responds with oscillations in ACTH and glucocorticoids (CORT), despite the fact that the CRH drive is constant (point B). On the other side of the transition curve, the pituitary-adrenal system responds with steady state levels in ACTH and CORT (point C). (B) Model predictions for ACTH (gray) and CORT (black) corresponding to point B (oscillation) and point C (steady state) in panel (A). (C) Constant infusion of low and high doses of CRH induces pulsatile or constant secretion of CORT, respectively. In line with the modeling hypothesis, constant infusion of a low dose of CRH (0.5 μg/h) induces ultradian corticosterone oscillations that persist throughout the infusion period (top graph). In contrast constant infusion of a high-dose CRH (5 μg/h) results in constant secretion of CORT (bottom graph). Samples were collected every 5 min from a freely behaving male rat using an automated blood sampling system. Grey bar indicates the period of infusion. Reproduced with permission from (Spiga et al., Citation2014).
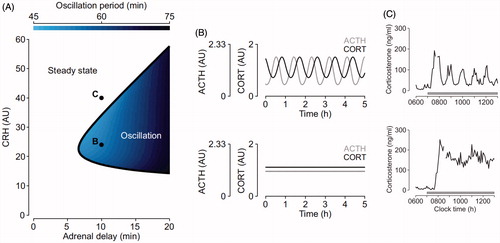
Using mathematical modeling to elucidate the dynamic interaction between the pituitary and the adrenal gland we have been able to demonstrate the importance of the level, rather than the pattern, of CRH seen by the pituitary, for generating pulsatile ACTH and CORT (Walker et al., Citation2010). Consistent with our hypothesis of a sub-hypothalamic pulse generator the mathematical model predicted that the pituitary–adrenal system can indeed support self-sustained ACTH and CORT oscillations, but only when CRH is at ‘physiological’ levels (e.g., during the circadian peak of HPA axis activity), whereas ACTH and CORT oscillations are lost at higher ‘stress-equivalent’ levels of constant CRH as has been observed in vivo following exposure to an acute stressor (Muglia et al., Citation1997; Windle et al., Citation1998). The validity of the prediction of the mathematical model was further confirmed in vivo in the rat by investigating the dynamics of ACTH and CORT in response to constant CRH stimulation. We showed that a constant infusion of a low dose of CRH (0.5 µg/h) results in pulsatile CORT secretion, and importantly the hourly frequency of the CRH-induced pulses is comparable to endogenous physiological pulses measured in the active phase in untreated rats. However, in agreement with the model predictions, the study also showed that pulsatile secretion of CORT is lost with constant infusion CRH at higher doses (1.0 µg and 2.5 µg/h). Our experiments also confirmed the model prediction that in rats infused with low CRH each pulse of ACTH is followed by a pulse of CORT (Walker et al., Citation2012). The prediction of our mathematical model of a pituitary-adrenal pulse generator was also confirmed in vitro. A more recent study using cultured rat anterior pituitary cells perifused with constant levels of CRH has shown that CRH-induced ACTH secretion is indeed subjected to rapid inhibition after incubation with a pulse of CORT, resulting is pulsatile ACTH secretion. Consistent with our hypothesis, the same study also suggests that the CORT effect on ACTH secretion is independent from gene transcription, and relies on a rapid non-genomic mechanism that involves the activation of a cell membrane-associated GR in anterior pituitary corticotroph cells (Deng et al., Citation2015).
Although CRH is the predominant ACTH secretagogue in humans and the rat, its ability to stimulate ACTH secretion can be potentiated by other hypothalamic neuropeptides, including vasopressin and oxytocin. However, our in vivo data on rats infused with constant low CRH alone show consistency between animals in the timing of the initial CORT response to CRH, and subsequent synchrony in CORT oscillation throughout the infusion.
The role of FKBP51 in regulating glucocorticoid negative feedback
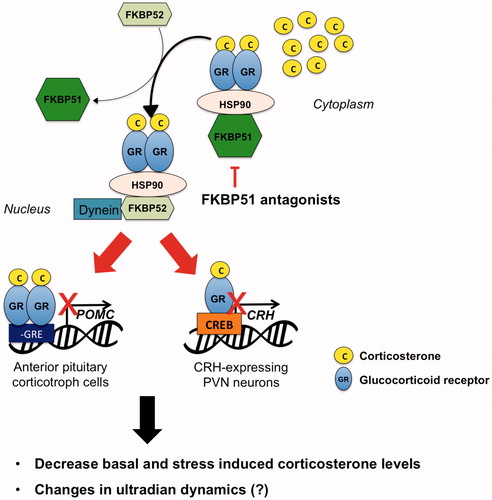
Glucocorticoids are lipophilic and therefore can rapidly cross the cell membrane and bind the GR and MR in the cytosol. This results in conformational changes that lead to nuclear translocation of the receptors and their binding to DNA, with subsequent activation or repression of target genes (Scheschowitsch et al., Citation2017). In their inactive state, GR and MR are predominately found in the cytosol, bound to chaperone proteins including the heat shock proteins hsp90, hsp70 and hsp23, and to immunophilins belonging to the FK506 binding-protein family, including FKBP51 and FKBP52 (encoded by the fkbp5 and fkbp4 genes, respectively; (Echeverria et al., Citation2009; Riggs et al., Citation2003). Heat-shock proteins are involved in GR and MR intracellular trafficking, folding and activation (Pratt et al., Citation2004), whereas FKBP51 and FKBP52 regulate GR and MR affinity for CORT and nuclear translocation (Kirschke et al., Citation2014; Vandevyver et al., Citation2012). The conformational changes induced by the binding of CORT to MR and GR causes FKBP51 to dissociate and FKBP52 to bind (). This allows the receptors to bind the nuclear transporter dynein and to translocate in the nucleus (Echeverria et al., Citation2009; Riggs et al., Citation2003). In addition to its role in GR and MR translocation, FKBP51 is also involved in regulating kinases, including AKT (Fabian et al., Citation2013) and GSK3β (Gassen et al., Citation2016), both involved in regulating GR activity (Rogatsky et al., Citation1998; Stechschulte et al., Citation2014).
Figure 3. Hypothetical mechanisms underlying the effects of a FKBP51 antagonist administration on HPA axis activity. GR is normally localized in the cytosol in a complex with chaperone proteins including FKBP51. CORT increased during stress or at circadian peak induces the detachment of GR from FKBP51 and its binding to FKBP52, leading to GR nuclear translocation and binding to DNA. GR expressed in the anterior pituitary and PVN regulates CORT negative feedback by reducing POMC and CRH synthesis, respectively. Treatment with a FKBP51 will facilitate GR binding to FKBP52 thus GR genomic effect and negative feedback inhibition. This will ultimately result in decreased CORT secretion, and presumably in changes in ultradian rhythmicity.
Many studies have focused on investigating the molecular mechanism underlying dysregulation of the HPA axis, and subsequent imbalances in CORT production, as seen in psychiatric diseases such as major depression, bipolar disorders, post-traumatic stress disorder (PTSD), schizophrenia, and anxiety disorders (de Kloet et al., Citation2005; Holsboer, Citation2000). It is thought that an abnormally enhanced activity of the HPA axis in these disorders results in the failure to adequately terminate the stress response and results in a state of hypersensitivity to stress-related activities. This is mainly thought to be due to a dysfunctional negative feedback response (de Kloet et al., Citation2005) involving a malfunction of GR activity (Holsboer, Citation2000).
The discovery that FKBP51 and FKBP52 have opposite effects on GR activity (Riggs et al., Citation2003; Wochnik et al., Citation2005), together with studies showing GC resistance in non-human primates spontaneously over expressing the FKBP5 gene (Reynolds et al., Citation1999; Scammell et al., Citation2001) led to the intriguing hypothesis that increased expression of FKBP51 may be associated with HPA axis hyperactivity in humans. Binder and colleagues were the first to show an association between certain single nucleotide polymorphisms (SNPs) in the FKBP5 gene and the responsiveness to antidepressant therapy in patients with major depressive disorders (Binder et al., Citation2004). The same group also showed that the polymorphism were correlated with the outcome of the dexamethasone-CRH (DEX-CRH) test, where individuals with SNPs causing higher intracellular protein levels of the FKBP51 showed elevated ACTH and CORT levels (Binder, Citation2009; Binder et al., Citation2004). A number of clinical studies have also showed a correlation between FKBP5 gene variants and the effectiveness of antidepressant treatment (Kirchheiner et al., Citation2008; Laje et al., Citation2009; Lekman et al., Citation2008; Zou et al., Citation2010). The minor T allele of the FKBP5 SNP, FKBP5 rs1360780 (C/T) has been correlated with depression in men. The functional effect of this allele is overexpression of the FKBP5 gene by enhanced binding of the TATA box, which will result in increased FKBP51 protein and enhanced HPA axis activity. (Lavebratt et al., Citation2010; Lekman et al., Citation2008; Velders et al., Citation2011; Willour et al., Citation2009). Importantly, a higher incidence of suicidal events has been reported in patients with the rs1360780TT and with another allele, which causes increased FKBP5 expression, rs3800373GG (Brent et al., Citation2010; Roy et al., Citation2010; Supriyanto et al., Citation2011; Willour et al., Citation2009). Another study also found that patients with the rs1360780TT and rs3800373GG genotype had impaired normalization of the stress-induced cortisol increase from psychosocial stress (Ising et al., Citation2008). These studies suggest that individuals with naturally lower levels of FKBP51 recover faster from depressive states following stress, whereas individuals with naturally higher levels of FKBP51 show a slower recovery subsequent to stress, and consequently a higher risk of developing psychiatric disorders such as depression. Depressed patients with hyperactivity of the HPA axis exhibit larger pulses of CORT throughout the 24-h period (Oster et al., Citation2017). It is therefore possible that the observed changes in pulsatility are linked to overexpression of FKBP51 in these patients, which result in loss of CORT-mediated negative feedback. Interestingly, FKBP5 is a CORT-regulated gene and its expression increases in response to elevated CORT, forming an ultra-short intra-cellular negative feedback loop.
In both mice and rats, FKBP51 is expressed in several brain areas involved in HPA axis regulation, including the PVN, amygdala, hippocampus, dorsal raphe, and prefrontal cortex (Scharf et al., Citation2011; Yang et al., Citation2012), in which GR is also highly expressed. Studies in rodents have shown that FKBP5 mRNA expression is up-regulated in the amygdala and PVN following acute and prolonged stress (O’Leary et al., Citation2013; Scharf et al., Citation2011). In attempts to further elucidate a potential mechanism for FKBP51-mediated alterations of HPA axis activity, animal studies using FKBP51 gene overexpression or knockout mice have been performed. Profound effects on HPA axis activity were observed in FKBP51 KO mice with lower basal and stress induced CORT levels (Hartmann et al., Citation2012; Hoeijmakers et al., Citation2014). Consistent with this, mice treated with a highly specific FKBP51 antagonist show decreased basal CORT secretion (Gaali et al., Citation2015). This further supports the hypothesis of an involvement of FKBP51 in the regulation of the HPA axis activity (Binder, Citation2009). Given the role of FKBP51 in regulating HPA axis activity it is possible that changes in FKBP51 activity and/or expression lead to changes in the pulsatile pattern of CORT. Studies aimed to elucidate this are currently ongoing, and the data obtained will be extremely important to evaluate whether using antagonists of FKBP51 represents a valid pharmaceutical tool to treat disorders in which HPA axis hyperactivity has been observed.
Summary and conclusion
We have discussed the evidence for CORT-mediated negative feedback at the level of CRH and ACTH synthesis, as well as within the adrenal gland steroidogenic pathway. We have provided evidence from both mathematical modeling and experimental in vivo and in vitro studies, that pulsatility of ACTH and CORT is observed even with constant levels of CRH. This has challenged the long-standing hypothesis of a hypothalamic pulse generator-generating pulsatile CRH- to create downstream pulsatile secretion of ACTH and CORT. This suggests there must be a different role for the pulsatile pattern of CRH that has been observed in several species, including the rat. One possibility is that pulsatile CRH release is important for maintaining responsiveness of the pituitary–adrenal system. Indeed, it has been shown that sustained levels of CRH can result in a down regulation and desensitization of CRH receptors (Aguilera et al., Citation2004). CORT negative feed-back can of course still regulate CRH secretion and affect CORT pulsatility, as changes in the levels of CRH, both below and above physiological levels, will result in loss of ACTH and CORT pulsatility (Walker et al., Citation2010). How the pulsatile pattern of CRH modulates and interacts with the endogenously pulsatile pituitary–adrenal system is currently under active investigation.
It has been clear for some time that abnormalities of CORT release occur in several disorders of mental function such as depression and anxiety, and polymorphism in the gene that encodes the GR-regulating protein FKBP51 is associated with increased risk for these disorders. The mechanism remains unclear but it appears that increased levels of FKBP51 inhibit GR-mediated negative feedback and may be involved in increased CORT concentrations. Further research on the role of GR-mediated regulation of ultradian rhythms and CORT negative feedback should contribute to further knowledge of the mechanism and potential treatment options for mental disorders associated with dysregulated HPA axis activity.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aguilera, G. (2015). Molecular regulation of corticotropin-releasing hormone gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Interdisciplinary Information Sciences, 21, 273–282. doi:10.4036/iis.2015.B.13
- Aguilera, G., Kiss, A., Liu, Y., & Kamitakahara, A. (2007). Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress, 10, 153–161. doi:10.1080/10253890701391192
- Aguilera, G., Nikodemova, M., Wynn, P.C., & Catt, K.J. (2004). Corticotropin releasing hormone receptors: Two decades later. Peptides, 25, 319–329. doi:10.1016/j.peptides.2004.02.002
- Amico, J.A., Mantella, R.C., Vollmer, R.R., & Li, X. (2004). Anxiety and stress responses in female oxytocin deficient mice. Journal of Neuroendocrinology, 16, 319–324. doi:10.1111/j.0953-8194.2004.01161.x
- Antoni, F.A. (1986). Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocrine Reviews, 7, 351–378. doi:10.1210/edrv-7-4-351
- Apostolakis, E.M., Longo, L.D., Veldhuis, J.D., & Yellon, S.M. (1992). Dissociation of pulsatile cortisol and adrenocorticotropin secretion in fetal sheep during late gestation. Endocrinology, 130, 2571–2578. doi:10.1210/endo.130.5.1315248
- Belchetz, P.E., Plant, T.M., Nakai, Y., Keogh, E.J., & Knobil, E. (1978). Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science, 202, 631–633.
- Binder, E.B. (2009). The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology, 34(Suppl 1), S186–S195. doi:10.1016/j.psyneuen.2009.05.021
- Binder, E.B., Salyakina, D., Lichtner, P., Wochnik, G.M., Ising, M., Putz, B., … Muller-Myhsok, B. (2004). Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature Genetics, 36, 1319–1325. doi:10.1038/ng1479
- Black, W.C., Crampton, R.S., Hilton, J.G., Verdesca, A.S., & Nedeljkovic, R.I. (1961). Inhibitory effect of hydrocortisone and analogues on Adrenocortical Secretion in dogs. American Journal of Physiology, 201, 1057–1060. doi:10.1152/ajplegacy.1961.201.6.1057
- Bornstein, S.R., & Chrousos, G.P. (1999). Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: Neural and immune inputs. Journal of Clinical Endocrinology and Metabolism, 84, 1729–1736.
- Brent, D., Melhem, N., Ferrell, R., Emslie, G., Wagner, K.D., Ryan, N., … Keller, M. (2010). Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. American Journal of Psychiatry, 167, 190–197. doi:10.1176/appi.ajp.2009.09040576
- Briassoulis, G., Damjanovic, S., Xekouki, P., Lefebvre, H., & Stratakis, C.A. (2011). The Glucocorticoid receptor and its expression in the anterior pituitary and the adrenal cortex: a source of variation in hypothalamic-pituitary-adrenal axis function; implications for pituitary and adrenal tumors. Endocrine Practice, 17, 941–948. doi:10.4158/EP11061.RA
- Buckingham, J.C., John, C.D., Solito, E., Tierney, T., Flower, R.J., Christian, H., & Morris, J. (2006). Annexin 1, glucocorticoids, and the neuroendocrine-immune interface. Annals of New York Academy of Sciences, 1088, 396–409. doi:10.1196/annals.1366.002
- Carnes, M., Brownfield, M.S., Kalin, N.H., Lent, S., & Barksdale, C.M. (1986). Episodic secretion of ACTH in rats. Peptides, 7, 219–223. doi:10.1016/0196-9781(86)90216-0
- Carnes, M., Kalin, N.H., Lent, S.J., Barksdale, C.M., & Brownfield, M.S. (1988a). Pulsatile ACTH secretion: variation with time of day and relationship to cortisol. Peptides, 9, 325–331.
- Carnes, M., Lent, S.J., Erisman, S., & Feyzi, J. (1988b). Changes in mean plasma ACTH reflect changes in amplitude and frequency of secretory pulses. Life Sciences, 43, 1785–1790.
- Carsia, R.V., & Malamed, S. (1979). Acute Self-Suppression of Corticosteroidogenesis in isolated adrenocortical cells. Endocrinology, 105, 911–914. doi:10.1210/endo-105-4-911
- Cascio, C.S., Shinsako, J., & Dallman, M.F. (1987). The suprachiasmatic nuclei stimulate evening ACTH secretion in the rat. Brain Research, 423, 173–178.
- Chapman, L.P., Epton, M.J., Buckingham, J.C., Morris, J.F., & Christian, H.C. (2003). Evidence for a role of the adenosine 5′-triphosphate-binding cassette transporter A1 in the externalization of annexin I from pituitary folliculo-stellate cells. Endocrinology, 144, 1062–1073. doi:10.1210/en.2002-220650
- Chen, L., Tian, L., MacDonald, S.H., McClafferty, H., Hammond, M.S., Huibant, J.M., … Shipston, M.J. (2005). Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. Journal of Biological Chemistry, 280, 33599–33609. doi:10.1074/jbc.M505383200
- Cherrington, A.D. (1999). Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes, 48, 1198–1214.
- Chong, C., Hamid, A., Yao, T., Garza, A.E., Pojoga, L.H., Adler, G.K., Romero, J.R., & Williams, G.H. (2017). Regulation of aldosterone secretion by mineralocorticoid receptor-mediated signaling. Journal of Endocrinology, 232, 525–534. doi:10.1530/JOE-16-0452
- Chrousos, G.P. (1995). The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. New England Journal of Medicine, 332, 1351–1362. doi:10.1056/NEJM199505183322008
- Clark, R.G., Chambers, G., Lewin, J., & Robinson, I.C.A.F. (1986). Automated repetitive microsampling of blood: Growth hormone profiles in conscious male rats. Endocrinology, 111, 27–35.
- Clarke, I.J., & Cummins, J.T. (1982). The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology, 111, 1737–1739. doi:10.1210/endo-111-5-1737
- Conway-Campbell, B.L., McKenna, M.A., Wiles, C.C., Atkinson, H.C., de Kloet, E.R., & Lightman, S.L. (2007). Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology, 148, 5470–5477. doi:10.1210/en.2007-0585
- Dallman, M.F., Akana, S.F., Jacobson, L., Levin, N., Cascio, C.S., & Shinsako, J. (1987). Characterization of corticosterone feedback regulation of ACTH secretion. Annals of the New York Academy of Sciences, 512, 402–414.
- Dallman, M.F., Akana, S.F., Levin, N., Walker, C.D., Bradbury, M.J., Suemaru, S., & Scribner, K.S. (1994). Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Annals of the New York Academy of Sciences, 746, 22–31. discussion 31-22, 64-27.
- Dallman, M.F., & Yates, F.E. (1969). Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Annals of the New York Academy of Sciences, 156, 696–721.
- Davies, L., Karthikeyan, N., Lynch, J.T., Sial, E.A., Gkourtsa, A., Demonacos, C., & Krstic-Demonacos, M. (2008). Cross talk of signaling pathways in the regulation of the glucocorticoid receptor function. Molecular Endocrinology, 22, 1331–1344. doi:10.1210/me.2007-0360
- de Kloet, E.R. (2000). Stress in the brain. European Journal of Pharmacology, 405, 187–198.
- de Kloet, E.R., Joels, M., & Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nature Reviews. Neuroscience, 6, 463–475. doi:10.1038/nrn1683
- Deng, Q., Riquelme, D., Trinh, L., Low, M.J., Tomic, M., Stojilkovic, S., & Aguilera, G. (2015). Rapid glucocorticoid feedback inhibition of ACTH secretion involves ligand-dependent membrane association of glucocorticoid receptors. Endocrinology, 156, 3215–3227. doi:10.1210/EN.2015-1265
- Desouza, E.B., & Vanloon, G.R. (1982). Stress-induced inhibition of the Plasma-Corticosterone response to a subsequent stress in rats - a Nonadrenocorticotropin-mediated mechanism. Endocrinology, 110, 23–33. doi:10.1210/endo-110-1-23
- Di, S., Malcher-Lopes, R., Halmos, K.C., & Tasker, J.G. (2003). Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. The Journal of Neuroscience, 23, 4850–4857.
- Dickmeis, T. (2009). Glucocorticoids and the circadian clock. The Journal of Endocrinology, 200, 3–22. doi:10.1677/JOE-08-0415
- Doucas, V., Shi, Y., Miyamoto, S., West, A., Verma, I., & Evans, R.M. (2000). Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America, 97, 11893–11898. doi:10.1073/pnas.220413297
- Drouin, J., Charron, J., Gagner, J.P., Jeannotte, L., Nemer, M., Plante, R.K., & Wrange, O. (1987). Pro-opiomelanocortin gene: a model for negative regulation of transcription by glucocorticoids. Journal of Cellular Biochemistry, 35, 293–304. doi:10.1002/jcb.240350404
- Drouin, J., Sun, Y.L., & Nemer, M. (1989a). Glucocorticoid repression of pro-opiomelanocortin gene transcription. Journal of Steroid Biochemistry, 34, 63–69.
- Drouin, J., Trifiro, M.A., Plante, R.K., Nemer, M., Eriksson, P., & Wrange, O. (1989b). Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Molecular and Cellular Biology, 9, 5305–5314.
- Duncan, P.J., Sengul, S., Tabak, J., Ruth, P., Bertram, R., & Shipston, M.J. (2015). Large conductance Ca(2)(+)-activated K(+) (BK) channels promote secretagogue-induced transition from spiking to bursting in murine anterior pituitary corticotrophs. The Journal of Physiology, 593, 1197–1211. doi:10.1113/jphysiol.2015.284471
- Duncan, P.J., Tabak, J., Ruth, P., Bertram, R., & Shipston, M.J. (2016). Glucocorticoids inhibit CRH/AVP-Evoked bursting activity of male murine anterior pituitary corticotrophs. Endocrinology, 157, 3108–3121. doi:10.1210/en.2016-1115
- Echeverria, P.C., Mazaira, G., Erlejman, A., Gomez-Sanchez, C., Piwien Pilipuk, G., & Galigniana, M.D. (2009). Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Molecular and Cellular Biology, 29, 4788–4797. doi:10.1128/MCB.00649-09
- Engler, D., Pham, T., Liu, J.P., Fullerton, M.J., Clarke, I.J., & Funder, J.W. (1990). Studies of the regulation of the hypothalamic-pituitary-adrenal axis in sheep with hypothalamic-pituitary disconnection. II. Evidence for in vivo ultradian hypersecretion of proopiomelanocortin peptides by the isolated anterior and intermediate pituitary. Endocrinology, 127, 1956–1966. doi:10.1210/endo-127-4-1956
- Evans, A.N., Liu, Y., Macgregor, R., Huang, V., & Aguilera, G. (2013). Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids. Molecular Endocrinology, 27, 1796–1807. doi:10.1210/me.2013-1095
- Evanson, N.K., Herman, J.P., Sakai, R.R., & Krause, E.G. (2010a). Nongenomic actions of adrenal steroids in the central nervous system. Journal of Neuroendocrinology, 22, 846–861. doi:10.1111/j.1365-2826.2010.02000.x
- Evanson, N.K., Tasker, J.G., Hill, M.N., Hillard, C.J., & Herman, J.P. (2010b). Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology, 151, 4811–4819. doi:10.1210/en.2010-0285
- Fabian, A.K., Marz, A., Neimanis, S., Biondi, R.M., Kozany, C., & Hausch, F. (2013). InterAKTions with FKBPs - mutational and pharmacological exploration. PLoS One, 8, e57508. doi:10.1371/journal.pone.0057508
- Fenoglio, K.A., Brunson, K.L., Avishai-Eliner, S., Chen, Y., & Baram, T.Z. (2004). Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology, 145, 2702–2706. doi:10.1210/en.2004-0111
- Fulkerson, W.J. (1978). Synchronous episodic release of cortisol in the sheep. The Journal of Endocrinology, 79, 131–132.
- Gaali, S., Kirschner, A., Cuboni, S., Hartmann, J., Kozany, C., Balsevich, G., … Hausch, F. (2015). Selective inhibitors of the FK506-binding protein 51 by induced fit. Nature Chemical Biology, 11, 33–37. doi:10.1038/nchembio.1699
- Gagner, J.P., & Drouin, J. (1985). Opposite regulation of pro-opiomelanocortin gene transcription by glucocorticoids and CRH. Molecular and Cellular Endocrinology, 40, 25–32.
- Gassen, N.C., Hartmann, J., Zannas, A.S., Kretzschmar, A., Zschocke, J., Maccarrone, G., … Rein, T. (2016). FKBP51 inhibits GSK3beta and augments the effects of distinct psychotropic medications. Molecular Psychiatry, 21, 277–289. doi:10.1038/mp.2015.38
- Gibbs, D.M. (1984). Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sciences, 35, 487–491.
- Gibbs, D.M. (1986). Stress-specific modulation of acth-secretion by oxytocin. Neuroendocrinology, 42, 456–458. doi:10.1159/000124487
- Groeneweg, F.L., Karst, H., de Kloet, E.R., & Joels, M. (2012). Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and Cellular Endocrinology, 350, 299–309. doi:10.1016/j.mce.2011.06.020
- Guardiola-Diaz, H.M., Kolinske, J.S., Gates, L.H., & Seasholtz, A.F. (1996). Negative glucorticoid regulation of cyclic adenosine 3′, 5′-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Molecular Endocrinology, 10, 317–329. doi:10.1210/mend.10.3.8833660
- Gummow, B.M., Scheys, J.O., Cancelli, V.R., & Hammer, G.D. (2006). Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Molecular Endocrinology, 20, 2711–2723. doi:10.1210/me.2005-0461
- Harbuz, M.S., & Lightman, S.L. (1989). Glucocorticoid inhibition of stress-induced changes in hypothalamic corticotrophin-releasing factor messenger RNA and proenkephalin A messenger RNA. Neuropeptides, 14, 17–20.
- Hartmann, J., Wagner, K.V., Liebl, C., Scharf, S.H., Wang, X.D., Wolf, M., … Schmidt, M.V. (2012). The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology, 62, 332–339. doi:10.1016/j.neuropharm.2011.07.041
- Henley, D.E., Leendertz, J.A., Russell, G.M., Wood, S.A., Taheri, S., Woltersdorf, W.W., & Lightman, S.L. (2009). Development of an automated blood sampling system for use in humans. Journal of Medical Engineering and Technology, 33, 199–208. doi:10.1080/03091900802185970
- Hoeijmakers, L., Harbich, D., Schmid, B., Lucassen, P.J., Wagner, K.V., Schmidt, M.V., & Hartmann, J. (2014). Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS One, 9, e95796. doi:10.1371/journal.pone.0095796
- Holaday, J.W., Martinez, H.M., & Natelson, B.H. (1977). Synchronized ultradian cortisol rhythms in monkeys - persistence during corticotropin infusion. Science, 198, 56–58. doi:10.1126/science.197603
- Holsboer, F. (2000). The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology, 23, 477–501. doi:10.1016/S0893-133X(00)00159-7
- Ising, M., Depping, A.M., Siebertz, A., Lucae, S., Unschuld, P.G., Kloiber, S., … Holsboer, F. (2008). Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. European Journal of Neuroscience, 28, 389–398. doi:10.1111/j.1460-9568.2008.06332.x
- Ixart, G., Barbanel, G., Nouguier-Soule, J., & Assenmacher, I. (1991). A quantitative study of the pulsatile parameters of CRH-41 secretion in unanesthetized free-moving rats. Experimental Brain Research, 87, 153–158.
- Ixart, G., Siaud, P., Barbanel, G., Mekaouche, M., Givalois, L., & Assenmacher, I. (1993). Circadian variations in the amplitude of corticotropin-releasing hormone 41 (CRH41) episodic release measured in vivo in male rats: Correlations with diurnal fluctuations in hypothalamic and median eminence CRH41 contents. Journal of Biological Rhythms, 8, 297–309. doi:10.1177/074873049300800403
- Ixart, G., Siaud, P., Mekaouche, M., Barbanel, G., Givalois, L., & Assenmacher, I. (1994). Short-term but not long-term adrenalectomy modulates amplitude and frequency of the CRH41 episodic release in push-pull cannulated median eminence of free-moving rats. Brain Research, 658, 185–191.
- Jacobson, L., Muglia, L.J., Weninger, S.C., Pacak, K., & Majzoub, J.A. (2000). CRH deficiency impairs but does not block pituitary-adrenal responses to diverse stressors. Neuroendocrinology, 71, 79–87. doi:10.1159/000054524
- Jasper, M.S., & Engeland, W.C. (1991). Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. American Journal of Physiology, 261, R1257–R1268. doi:10.1152/ajpregu.1991.261.5.R1257
- Jasper, M.S., & Engeland, W.C. (1994). Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology, 59, 97–109. doi:10.1159/000126645
- Jeanneteau, F.D., Lambert, W.M., Ismaili, N., Bath, K.G., Lee, F.S., Garabedian, M.J., & Chao, M.V. (2012). BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proceedings of the National Academy of Sciences of United States of America, 109, 1305–1310. doi:10.1073/pnas.1114122109
- John, C.D., Christian, H.C., Morris, J.F., Flower, R.J., Solito, E., & Buckingham, J.C. (2004). Annexin 1 and the regulation of endocrine function. Trends in Endocrinology and Metabolism, 15, 103–109. doi:10.1016/j.tem.2004.02.001
- Jones, M.T., Hillhouse, E.W., & Burden, J.L. (1977). Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. Journal of Endocrinology, 73, 405–417.
- Jones, M.T., & Stockham, M.A. (1966). Effect of previous stimulation of Adrenal Cortex by Adrenocorticotrophin on Function of Pituitary-Adreonocortical axis in response to stress. The Journal of Physiology, 184, 741–750.
- Jones, M.T., Tiptaft, E.M., Brush, F.R., Fergusson, D.A., & Neame, R.L. (1974). Evidence for dual corticosteroid-receptor mechanisms in the feedback control of adrenocorticotrophin secretion. Journal of Endocrinology, 60, 223–233.
- Kalsbeek, A., & Fliers, E. (2017). Circadian and endocrine rhythms. Best Practice and Research: Clinical Endocrinology and Metabolism, 31, 443.
- Kalsbeek, A., van der Spek, R., Lei, J., Endert, E., Buijs, R.M., & Fliers, E. (2012). Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Molecular and Cellular Endocrinology, 349, 20–29. doi:10.1016/j.mce.2011.06.042
- Katoh, Y., Takemori, H., Min, L., Muraoka, M., Doi, J., Horike, N., & Okamoto, M. (2004). Salt-inducible kinase-1 represses cAMP response element-binding protein activity both in the nucleus and in the cytoplasm. European Journal of Biochemistry, 271, 4307–4319. doi:10.1111/j.1432-1033.2004.04372.x
- Keller-Wood, M.E., & Dallman, M.F. (1984). Corticosteroid inhibition of ACTH secretion. Endocrine Reviews, 5, 1–24. doi:10.1210/edrv-5-1-1
- Kirchheiner, J., Lorch, R., Lebedeva, E., Seeringer, A., Roots, I., Sasse, J., & Brockmoller, J. (2008). Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics, 9, 841–846. doi:10.2217/14622416.9.7.841
- Kirschke, E., Goswami, D., Southworth, D., Griffin, P.R., & Agard, D.A. (2014). Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell, 157, 1685–1697. doi:10.1016/j.cell.2014.04.038
- Kitchener, P., Di Blasi, F., Borrelli, E., & Piazza, P.V. (2004). Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. European Journal of Neuroscience, 19, 1837–1846. doi:10.1111/j.1460-9568.2004.03267.x
- Kovacs, K.J., & Makara, G.B. (1988). Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Research, 474, 205–210.
- Kovalovsky, D., Refojo, D., Liberman, A.C., Hochbaum, D., Pereda, M.P., Coso, O.A., Stalla, G.K., Holsboer, F., & Arzt, E. (2002). Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: Involvement of calcium, protein kinase A, and MAPK pathways. Molecular Endocrinology, 16, 1638–1651. doi:10.1210/mend.16.7.0863
- Kraemer, F.B., & Shen, W.J. (2002). Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. Journal of Lipid Research, 43, 1585–1594.
- Laje, G., Perlis, R.H., Rush, A.J., & McMahon, F.J. (2009). Pharmacogenetics studies in STAR*D: Strengths, limitations, and results. Psychiatric Services, 60, 1446–1457. doi:10.1176/appi.ps.60.11.1446
- Langecker, H., & Lurie, R. (1957). Die hemmung der corticotropin-sekretion durch steroide. Acta Endocrinologica-Cop, 25, 54–58.
- Laryea, G., Schutz, G., & Muglia, L.J. (2013). Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Molecular Endocrinology, 27, 1655–1665. doi:10.1210/me.2013-1187
- Lavebratt, C., Aberg, E., Sjoholm, L.K., & Forsell, Y. (2010). Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. Journal of Affective Disorders, 125, 249–255. doi:10.1016/j.jad.2010.02.113
- Lekman, M., Laje, G., Charney, D., Rush, A.J., Wilson, A.F., Sorant, A.J., … Paddock, S. (2008). The FKBP5-gene in depression and treatment response–an association study in the sequenced treatment alternatives to relieve depression (STAR*D) Cohort. Biolological Psychiatry, 63, 1103–1110. doi:10.1016/j.biopsych.2007.10.026
- Lewis, J.G., Bagley, C.J., Elder, P.A., Bachmann, A.W., & Torpy, D.J. (2005). Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chimica Acta, 359, 189–194. doi:10.1016/j.cccn.2005.03.044
- Lightman, S.L., & Conway-Campbell, B.L. (2010). The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nature Reviews Neuroscience, 11, 710–718. doi:10.1038/nrn2914
- Lightman, S.L., & Young, W.S. 3rd. (1988). Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. The Journal of Physiology, 403, 511–523.
- Lin, D., Sugawara, T., Strauss, J.F., 3rd, Clark, B.J., Stocco, D.M., Saenger, P., Rogol, A., & Miller, W.L. (1995). Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science, 267, 1828–1831.
- Liposits, Z., & Paull, W.K. (1989). Association of dopaminergic fibers with corticotropin releasing hormone (CRH)-synthesizing neurons in the paraventricular nucleus of the rat hypothalamus. Histochemistry, 93, 119–127.
- Liu, Y., Coello, A.G., Grinevich, V., & Aguilera, G. (2010). Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription. Endocrinology, 151, 1109–1118. doi:10.1210/en.2009-0963
- Ma, X.M., & Aguilera, G. (1999). Transcriptional responses of the vasopressin and corticotropin-releasing hormone genes to acute and repeated intraperitoneal hypertonic saline injection in rats. Brain Research: Molecualr Brain Research, 68, 129–140.
- Ma, X.M., Camacho, C., & Aguilera, G. (2001). Regulation of corticotropin-releasing hormone (CRH) transcription and CRH mRNA stability by glucocorticoids. Cellular and Molecular Neurobiology, 21, 465–475.
- Macfarlane, D.P., Forbes, S., & Walker, B.R. (2008). Glucocorticoids and fatty acid metabolism in humans: Fuelling fat redistribution in the metabolic syndrome. The Journal of Endocrinology, 197, 189–204. doi:10.1677/JOE-08-0054
- Makara, G.B., Mergl, Z., & Zelena, D. (2004). The role of vasopressin in hypothalamo-pituitary-adrenal axis activation during stress: An assessment of the evidence. Annals of the New York Academy of Sciences, 1018, 151–161.
- Malkoski, S.P., & Dorin, R.I. (1999). Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Molecular Endocrinologu, 13, 1629–1644. doi:10.1210/mend.13.10.0351
- Malkoski, S.P., Handanos, C.M., & Dorin, R.I. (1997). Localization of a negative glucocorticoid response element of the human corticotropin releasing hormone gene. Molecular and Cellular Endocrinology, 127, 189–199. doi:10.1016/S0303-7207(96)04004-X
- Martens, C., Bilodeau, S., Maira, M., Gauthier, Y., & Drouin, J. (2005). Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Molecular Endocrinology, 19, 885–897. doi:10.1210/me.2004-0333
- Martin, L.J., & Tremblay, J.J. (2008). Glucocorticoids antagonize cAMP-induced Star transcription in Leydig cells through the orphan nuclear receptor NR4A1. Journal of Molecular Endocrinology, 41, 165–175. doi:10.1677/JME-07-0145
- McEwen, B.S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87, 873–904. doi:10.1152/physrev.00041.2006
- Metherell, L.A., Chapple, J.P., Cooray, S., David, A., Becker, C., Ruschendorf, F., … Clark, A.J. (2005). Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nature Genetics, 37, 166–170. doi:10.1038/ng1501
- Mountjoy, K.G., Mortrud, M.T., Low, M.J., Simerly, R.B., & Cone, R.D. (1994). Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology, 8, 1298–1308. doi:10.1210/mend.8.10.7854347
- Muglia, L.J., Bethin, K.E., Jacobson, L., Vogt, S.K., & Majzoub, J.A. (2000). Pituitary-adrenal axis regulation in CRH-deficient mice. Endocrine Research, 26, 1057–1066.
- Muglia, L.J., Jacobson, L., Weninger, S.C., Karalis, K.P., Jeong, K.H., & Majzoub, J.A. (2001). The physiology of corticotropin-releasing hormone deficiency in mice. Peptides, 22, 725–731.
- Muglia, L.J., Jacobson, L., Weninger, S.C., Luedke, C.E., Bae, D.S., Jeong, K.H., & Majzoub, J.A. (1997). Impaired diurnal adrenal rhythmicity restored by constant infusion of corticotropin-releasing hormone in corticotropin-releasing hormone-deficient mice. Journal of Clinical Investigation, 99, 2923–2929. doi:10.1172/JCI119487
- Munck, A., Guyre, P.M., & Holbrook, N.J. (1984). Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews, 5, 25–44. doi:10.1210/edrv-5-1-25
- Nahar, J., Haam, J., Chen, C., Jiang, Z., Glatzer, N.R., Muglia, L.J., Dohanich, G.P., Herman, J.P., & Tasker, J.G. (2015). Rapid nongenomic glucocorticoid actions in male mouse hypothalamic neuroendocrine cells are dependent on the nuclear glucocorticoid receptor. Endocrinology, 156, 2831–2842. doi:10.1210/en.2015-1273
- Negrovilar, A., Culler, M.D., Valenca, M.M., Flack, T.B., & Wisniewski, G. (1987). Pulsatile peptide secretion – Encoding of brain messages regulating endocrine and reproductive functions. Environmental Health Perspectives, 75, 37–43.
- Neumann, I.D., Wigger, A., Torner, L., Holsboer, F., & Landgraf, R. (2000). Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. Journal of Neuroendocrinology, 12, 235–243.
- O’Leary, J.C. III, Zhang, B., Koren, J. III, Blair, L., & Dickey, C.A. (2013). The role of FKBP5 in mood disorders: Action of FKBP5 on steroid hormone receptors leads to questions about its evolutionary importance. CNS & Neurological Disorders – Drug Targets, 12, 1157–1162.
- Oster, H., Challet, E., Ott, V., Arvat, E., de Kloet, E.R., Dijk, D.J., … Van Cauter, E. (2017). The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocrine Reviews, 38, 3–45. doi:10.1210/er.2015-1080
- Osterlund, C.D., Rodriguez-Santiago, M., Woodruff, E.R., Newsom, R.J., Chadayammuri, A.P., & Spencer, R.L. (2016). Glucocorticoid fast feedback inhibition of stress-induced ACTH secretion in the male rat: rate independence and stress-state resistance. Endocrinology, 157, 2785–2798. doi:10.1210/en.2016-1123
- Parvin, R., Saito-Hakoda, A., Shimada, H., Shimizu, K., Noro, E., Iwasaki, Y., Fujiwara, K., Yokoyama, A., & Sugawara, A. (2017). Role of NeuroD1 on the negative regulation of Pomc expression by glucocorticoid. PLoS One, 12, e0175435. doi:10.1371/journal.pone.0175435
- Peron, F.G. (1960). The isolation and identification of some adrenocorticosteroids released by rat adrenal tissue incubated in vitro. Endocrinology, 66, 458–469. doi:10.1210/endo-66-3-458
- Peron, F.G., Moncloa, F., & Dorfman, R.I. (1960). Studies on the possible inhibitory effect of corticosterone on corticosteroidogenesis at the adrenal level in the rat. Endocrinology, 67, 379–388. doi:10.1210/endo-67-3-379
- Phillips, A., Lesage, S., Gingras, R., Maira, M.H., Gauthier, Y., Hugo, P., & Drouin, J. (1997). Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Molecular and Cellular Biology, 17, 5946–5951.
- Philips, A., Maira, M., Mullick, A., Chamberland, M., Lesage, S., Hugo, P., & Drouin, J. (1997). Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Molecular and Cellular Biology, 17, 5952–5959.
- Plotsky, P.M., & Vale, W. (1985). Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science, 230, 461–463.
- Poulin, G., Turgeon, B., & Drouin, J. (1997). NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Molecular and Cellular Biology, 17, 6673–6682.
- Pratt, W.B., Galigniana, M.D., Harrell, J.M., & DeFranco, D.B. (2004). Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal, 16, 857–872. doi:10.1016/j.cellsig.2004.02.004
- Pratt, W.B., Morishima, Murphy, Y.M., & Harrell, M. (2006). Chaperoning of glucocorticoid receptors. Handbook of Experimental Pharmacology,172, 111–138.
- Reul, J.M., & de Kloet, E.R. (1985). Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology, 117, 2505–2511. doi:10.1210/endo-117-6-2505
- Reul, J.M., & de Kloet, E.R. (1986). Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. Journal of Steroid Biochemistry, 24, 269–272.
- Reul, J.M., de Kloet, E.R., van Sluijs, F.J., Rijnberk, A., & Rothuizen, J. (1990). Binding characteristics of mineralocorticoid and glucocorticoid receptors in dog brain and pituitary. Endocrinology, 127, 907–915. doi:10.1210/endo-127-2-907
- Reynolds, P.D., Ruan, Y., Smith, D.F., & Scammell, J.G. (1999). Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. Journal of Clinical Endocrinology and Metabolism, 84, 663–669. doi:10.1210/jcem.84.2.5429
- Richards, J.B., & Pruitt, R.L. (1957). Hydrocortisone suppression of stress-induced adrenal 17-Hydroxycorticosteroid secretion in dogs. Endocrinology, 60, 99–104.
- Riggs, D.L., Roberts, P.J., Chirillo, S.C., Cheung-Flynn, J., Prapapanich, V., Ratajczak, T., … Smith, D.F. (2003). The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. The Embo Journal, 22, 1158–1167. doi:10.1093/emboj/cdg108
- Ritchie, A.K., Kuryshev, Y.A., & Childs, G.V. (1996). Corticotropin-releasing hormone and calcium signaling in corticotropes. Trends in Endocrinology and Metabolism, 7, 365–369.
- Robertson, N.M., Schulman, G., Karnik, S., Alnemri, E., & Litwack, G. (1993). Demonstration of nuclear translocation of the mineralocorticoid receptor (MR) using an anti-MR antibody and confocal laser scanning microscopy. Molecular Endocrinology, 7, 1226–1239. doi:10.1210/mend.7.9.8247024
- Rogatsky, I., Waase, C.L.M., & Garabedian, M.J. (1998). Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3) – Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. Journal of Biological Chemistry, 273, 14315–14321.
- Roper, J.A., O’Carroll, A.M., Young, W.S., & Lolait, S.J. (2011). The vasopressin Avpr1b receptor: Molecular and pharmacological studies. Stress-the International Journal on the Biology of Stress, 14, 98–115. doi:10.3109/10253890.2010.512376
- Roy, A., Gorodetsky, E., Yuan, Q., Goldman, D., & Enoch, M.A. (2010). Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology, 35, 1674–1683. doi:10.1038/npp.2009.236
- Sage, D., Maurel, D., & Bosler, O. (2001). Involvement of the suprachiasmatic nucleus in diurnal ACTH and corticosterone responsiveness to stress. American Journal of Physiology-Endocrinology and Metabolism, 280, E260–E269. doi:10.1152/ajpendo.2001.280.2.E260
- Scammell, J.G., Denny, W.B., Valentine, D.L., & Smith, D.F. (2001). Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. General and Comparative Endocrinology, 124, 152–165. doi:10.1006/gcen.2001.7696
- Scharf, S.H., Liebl, C., Binder, E.B., Schmidt, M.V., & Muller, M.B. (2011). Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS One, 6, e16883. doi:10.1371/journal.pone.0016883
- Scheschowitsch, K., Leite, J.A., & Assreuy, J. (2017). New insights in Glucocorticoid receptor signaling-more than just a ligand-binding receptor. Frontiers in Endocrinology), 8, 16. doi:10.3389/fendo.2017.00016
- Schwartz, J., & Vale, W. (1988). Dissociation of the adrenocorticotropin secretory responses to corticotropin-releasing factor (Crf) and vasopressin or oxytocin by using a specific Cyto-Toxic analog of Crf. Endocrinology, 122, 1695–1700. doi:10.1210/endo-122-4-1695
- Screaton, R.A., Conkright, M.D., Katoh, Y., Best, J.L., Canettieri, G., Jeffries, S., … Montminy, M. (2004). The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell, 119, 61–74.
- Seasholtz, A.F., Thompson, R.C., & Douglass, J.O. (1988). Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Molecular Endocrinology, 2, 1311–1319.
- Song, K.H., Park, Y.Y., Park, K.C., Hong, C.Y., Park, J.H., Shong, M., … Choi, H.S. (2004). The atypical orphan nuclear receptor DAX-1 interacts with orphan nuclear receptor Nur77 and represses its transactivation. Molecular Endocrinology, 18, 1929–1940.
- Spencer, R.L., Miller, A.H., Moday, H., Stein, M., & McEwen, B.S. (1993). Diurnal differences in basal and acute stress levels of type I and type II adrenal steroid receptor activation in neural and immune tissues. Endocrinology, 133, 1941–1950.
- Spiga, F., Harrison, L.R., Wood, S., Knight, D.M., MacSweeney, C.P., Thomson, F., Craighead, M., & Lightman, S.L. (2009). Blockade of the V(1b) receptor reduces ACTH, but not corticosterone secretion induced by stress without affecting basal hypothalamic-pituitary-adrenal axis activity. The Journal of Endocrinology, 200, 273–283. doi:10.1677/JOE-08-0421
- Spiga, F., & Lightman, S.L. (2015). Dynamics of adrenal glucocorticoid steroidogenesis in health and disease. Molecular and Cellular Endocrinology, 408, 227–234. doi:10.1016/j.mce.2015.02.005
- Spiga, F., Liu, Y., Aguilera, G., & Lightman, S.L. (2011a). Temporal effect of adrenocorticotrophic hormone on adrenal glucocorticoid steroidogenesis: Involvement of the transducer of regulated cyclic AMP-response element-binding protein activity. Journal of Neuroendocrinology, 23, 136–142. doi:10.1111/j.1365-2826.2010.02096.x
- Spiga, F., Waite, E.J., Liu, Y., Kershaw, Y.M., Aguilera, G., & Lightman, S.L. (2011b). ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology, 152, 1448–1457. doi:10.1210/en.2010-1209
- Spiga, F., Walker, J.J., Terry, J.R., & Lightman, S.L. (2014). HPA axis-rhythms. Comprehensive Physiology, 4, 1273–1298. doi:10.1002/cphy.c140003
- Spiga, F., Zavala, E., Walker, J.J., Zhao, Z., Terry, J.R., & Lightman, S.L. (2017). Dynamic responses of the adrenal steroidogenic regulatory network. Proceedings of the National Academy of Sciences of Sciences, 114, E6466–E6474. doi:10.1073/pnas.1703779114
- Stechschulte, L.A., Hinds, T.D., Jr., Ghanem, S.S., Shou, W., Najjar, S.M., & Sanchez, E.R. (2014). FKBP51 reciprocally regulates GRα and PPARγ activation via the Akt-p38 pathway. Molecular Endocrinology (Baltimore, Md.), 28, 1254–1264. doi:10.1210/me.2014-1023
- Stocco, D.M., & Clark, B.J. (1996). Regulation of the acute production of steroids in steroidogenic cells. Endocrine Reviews, 17, 221–244. doi:10.1210/edrv-17-3-221
- Stockham, M.A. (1964). Responses to acth – comparison of peripheral plasma + adrenal corticosterone concentrations. Journal of Physiology-London, 170, P63.
- Stojilkovic, S.S., Zemkova, H., & Van Goor, F. (2005). Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends in Endocrinology and Metabolism, 16, 152–159. doi:10.1016/j.tem.2005.03.003
- Supriyanto, I., Sasada, T., Fukutake, M., Asano, M., Ueno, Y., Nagasaki, Y., … Hishimoto, A. (2011). Association of FKBP5 gene haplotypes with completed suicide in the Japanese population. Progress in Neuropsychopharmacology and Biological Psychiatry, 35, 252–256. doi:10.1016/j.pnpbp.2010.11.019
- Szafarczyk, A., Ixart, G., Malaval, F., Nouguier-Soule, J., & Assenmacher, I. (1979). Effects of lesions of the suprachiasmatic nuclei and of p-chlorophenylalanine on the circadian rhythms of adrenocorticotrophic hormone and corticosterone in the plasma, and on locomotor activity of rats. Journal of Endocrinology, 83, 1–16.
- Tabak, J., Tomaiuolo, M., Gonzalez-Iglesias, A.E., Milescu, L.S., & Bertram, R. (2011). Fast-activating voltage- and calcium-dependent potassium (BK) conductance promotes bursting in pituitary cells: A dynamic clamp study. Journal of Neuroscience, 31, 16855–16863. doi:10.1523/JNEUROSCI.3235-11.2011
- Tagliavini, A., Tabak, J., Bertram, R., & Pedersen, M.G. (2016). Is bursting more effective than spiking in evoking pituitary hormone secretion? A spatiotemporal simulation study of calcium and granule dynamics. American Journal of Physiology–Endocrinology and Metabolism, 310, E515–E525. doi:10.1152/ajpendo.00500.2015
- Takemori, H., Kanematsu, M., Kajimura, J., Hatano, O., Katoh, Y., Lin, X.Z., … Okamoto, M. (2007). Dephosphorylation of TORC initiates expression of the StAR gene. Molecular and Cellular Endocrinology, 265, 196–204. doi:10.1016/j.mce.2006.12.020
- Takemori, H., & Okamoto, M. (2008). Regulation of CREB-mediated gene expression by salt inducible kinase. Journal of Steroid Biochemistry and Molecular Biology, 108, 287–291. doi:10.1016/j.jsbmb.2007.09.006
- Tasker, J.G., Di, S., & Malcher-Lopes, R. (2006). Minireview: Rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology, 147, 5549–5556. doi:10.1210/en.2006-0981
- Taylor, A.D., Flower, R.J., & Buckingham, J.C. (1995). Dexamethasone inhibits the release of Tsh from the rat anterior-pituitary gland in-vitro by mechanisms dependent on de-novo protein-synthesis and lipocortin-1. Journal of Endocrinology, 147, 533–544.
- Tian, L., Hammond, M.S., Florance, H., Antoni, F.A., & Shipston, M.J. (2001). Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. The Journal of Physiology, 537, 57–68.
- Tian, L., Knaus, H.G., & Shipston, M.J. (1998). Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. Journal of Biological Chemistry, 273, 13531–13536.
- Tsaneva-Atanasova, K., Sherman, A., van Goor, F., & Stojilkovic, S.S. (2007). Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. Journal of Neurophysiology, 98, 131–144. doi:10.1152/jn.00872.2006
- Ulrich-Lai, Y.M., Arnhold, M.M., & Engeland, W.C. (2006). Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. American Journal of Physiology, Regulatory Integrative and Comparative Physiology, 290, R1128–R1135. doi:10.1152/ajpregu.00042.2003
- Vale, W., Spiess, J., Rivier, C., & Rivier, J. (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science, 213, 1394–1397.
- Van Goor, F., Zivadinovic, D., Martinez-Fuentes, A.J., & Stojilkovic, S.S. (2001). Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. Cell type-specific action potential secretion coupling. Journal of Biological Chemistry, 276, 33840–33846. doi:10.1074/jbc.M105386200
- Vandevyver, S., Dejager, L., & Libert, C. (2012). On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic (Copenhagen, Denmark), 13, 364–374. doi:10.1111/j.1600-0854.2011.01288.x
- Velders, F.P., Kuningas, M., Kumari, M., Dekker, M.J., Uitterlinden, A.G., Kirschbaum, C., … Tiemeier, H. (2011). Genetics of cortisol secretion and depressive symptoms: a candidate gene and genome wide association approach. Psychoneuroendocrinology, 36, 1053–1061. doi:10.1016/j.psyneuen.2011.01.003
- Volpi, S., Rabadan-Diehl, C., & Aguilera, G. (2004). Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress-the International Journal on the Biology of Stress, 7, 75–83.
- Waite, E.J., McKenna, M., Kershaw, Y., Walker, J.J., Cho, K., Piggins, H.D., & Lightman, S.L. (2012). Ultradian corticosterone secretion is maintained in the absence of circadian cues. European Journal of Neuroscience, 36, 3142–3150. doi:10.1111/j.1460-9568.2012.08213.x
- Walker, J.J., Spiga, F., Gupta, R., Zhao, Z., Lightman, S.L., & Terry, J.R. (2015). Rapid intra-adrenal feedback regulation of glucocorticoid synthesis. Journal of the Royal Society, Interface/the Royal Society, 12, 20140875. doi:10.1098/rsif.2014.0875
- Walker, J.J., Spiga, F., Waite, E., Zhao, Z., Kershaw, Y., Terry, J.R., & Lightman, S.L. (2012). The origin of glucocorticoid hormone oscillations. PLoS Biology, 10, e1001341. doi:10.1371/journal.pbio.1001341
- Walker, J.J., Terry, J.R., & Lightman, S.L. (2010). Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proceedings of Biological Science, 277, 1627–1633. doi:10.1098/rspb.2009.2148
- Watts, A.G. (2005). Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Frontiers in Neuroendocrinol, 26, 109–130. doi:10.1016/j.yfrne.2005.09.001
- Watts, A.G., Tanimura, S., & Sanchez-Watts, G. (2004). Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: Daily rhythms and their interactions with corticosterone. Endocrinology, 145, 529–540. doi:10.1210/en.2003-0394
- Willour, V.L., Chen, H., Toolan, J., Belmonte, P., Cutler, D.J., Goes, F.S., … Schweizer, B., G. Bipolar Disorder Phenome, N.G.I.B.D. Consortium (2009). Family-based association of FKBP5 in bipolar disorder. Molecular Psychiatry, 14, 261–268. doi:10.1038/sj.mp.4002141
- Windle, R.J., Kershaw, Y.M., Shanks, N., Wood, S.A., Lightman, S.L., & Ingram, C.D. (2004). Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. Journal of Neuroscience, 24, 2974–2982. doi:10.1523/JNEUROSCI.3432-03.2004
- Windle, R.J., Shanks, N., Lightman, S.L., & Ingram, C.D. (1997). Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology, 138, 2829–2834. doi:10.1210/endo.138.7.5255
- Windle, R.J., Wood, S.A., Shanks, N., Lightman, S.L., & Ingram, C.D. (1998). Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology, 139, 443–450. doi:10.1210/endo.139.2.5721
- Wochnik, G.M., Ruegg, J., Abel, G.A., Schmidt, U., Holsboer, F., & Rein, T. (2005). FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. Journal of Biological Chemistry, 280, 4609–4616. doi:10.1074/jbc.M407498200
- Yamamori, E., Iwasaki, Y., Taguchi, T., Nishiyama, M., Yoshida, M., Asai, M., Oiso, Y., Itoi, K., Kambayashi, M., & Hashimoto, K. (2007). Molecular mechanisms for corticotropin-releasing hormone gene repression by glucocorticoid in BE(2)C neuronal cell line. Molecular and Cellular Endocrinology, 264, 142–148. doi:10.1016/j.mce.2006.11.001
- Yang, X., Ewald, E.R., Huo, Y., Tamashiro, K.L., Salvatori, R., Sawa, A., … Lee, R.S. (2012). Glucocorticoid-induced loss of DNA methylation in non-neuronal cells and potential involvement of DNMT1 in epigenetic regulation of Fkbp5. Biochemical and Biophysics Research Communication, 420, 570–575. doi:10.1016/j.bbrc.2012.03.035
- Zou, Y.F., Wang, F., Feng, X.L., Li, W.F., Tao, J.H., Pan, F.M., … Su, H. (2010). Meta-analysis of FKBP5 gene polymorphisms association with treatment response in patients with mood disorders. Neuroscience Letters, 484, 56–61. doi:10.1016/j.neulet.2010.08.019
