Abstract
Stress is an important and modifiable determinant of health, and its association with hair cortisol concentrations (HCC) during pregnancy remains unclear. We selected a random sample of 97 participants from a cohort of pregnant participants attending prenatal clinics in Lima, Peru. Each provided a hair sample at enrollment (mean gestational age = 13.1 weeks) and again at full-term delivery. Hair samples were segmented to reflect HCC in preconception and each trimester. At enrollment, measures of stress included: difficulty accessing basic goods, educational attainment, exposure to violence, fair or poor general health, perceived stress, and symptoms of depression, general anxiety, and post-traumatic stress disorder. Linear mixed models evaluated the association between each stress measure and absolute and relative changes in HCC. Pearson correlation coefficients (r) assessed correlations between HCC and continuous stress scores. Educational attainment of ≤12 years was associated with higher HCC in preconception and the 1st trimester, and general anxiety with lower preconception HCC. When modeling HCC patterns across the 4 hair segments, an educational attainment of ≤12 years was associated with higher HCC, high perceived stress with lower HCC, and general anxiety with steeper increases in HCC (group by time p value = .02). Only preconception HCC and GAD scores correlated (r = −0.22, p = .04). We observed few associations between stress and HCC. However, those that were seen were generally restricted to the preconception and 1st trimester. Further investigations into the association between stress and changes in HCC across pregnancy are warranted, and should include the preconception where possible.
Lay Summary
Few associations between stress and hair cortisol concentrations (HCC) were observed from preconception to the 3rd trimester. Observed differences in HCC based on educational attainment and anxiety were generally restricted to the preconception and 1st trimester when circulating cortisol concentrations are at their lowest compared to later in pregnancy. Further investigations into the association between stress and HCC across pregnancy are warranted, and should consider the evaluation of overall increases in HCC and the addition of the preconception period where possible.
1. Introduction
Stress is an important and modifiable determinant of health. Given its potential impacts during pregnancy (Coussons-Read et al., Citation2012; Davis & Sandmann, Citation2010, Citation2012; Diego et al., Citation2006; Glover, Citation2015; Glynn, Davis, & Sandman, Citation2013; Manuck et al., Citation2015; Wadhwa, Entringer, Buss, & Lu, Citation2011), the American College of Obstetricians and Gynecologists (Citation2006) advocates for screenings of stress-related risk factors at least once per trimester followed by the appropriate referrals (2006). In addition, an understanding of mediating neuroendocrine stress-response mechanisms remains a priority (Shannon, King, & Kennedy, Citation2007). One such mechanism is the hypothalamic pituitary adrenal (HPA) axis, and cortisol is a key biomarker of HPA axis activity. Cortisol concentrations in hair reflect long-term cortisol secretion during pregnancy and are positively correlated with salivary cortisol (D'Anna-Hernandez, Ross, Natvig, & Laudenslager, Citation2011; Kirschbaum, Tietze, Skoluda, & Dettenborn, Citation2009; Stalder & Kirschbaum, Citation2012). Findings for the association between hair cortisol concentrations (HCC) and stress during pregnancy remain unclear. Furthermore, the association between stress and HCC in the preconception has not been reported.
On balance, no consistent relationship has been observed between HCC and measures of stress. For example, a recent correlation-based meta-analysis found no consistent relationship between HCC and self-reported perceived stress or depression but found a 17% reduction in HCC with anxiety disorders (Stalder et al., Citation2017). When restricted to studies of pregnant participants or those who have recently delivered, reported findings are also mixed. For example, some studies report positive associations between HCC, perceived stress scores, and depression (Caparros-Gonzalez et al., Citation2017; Hoffman, Mazzoni, Wagner, Laudenslager, & Ross, Citation2016; Kalra, Einarson, Karaskov, Van Uum, & Koren, Citation2007). In contrast, a similar amount of studies report no such associations (Braig et al., Citation2016; Wikenius et al., Citation2016; Scharlau et al., Citation2018). To help provide clarity on the topic, we performed a comprehensive evaluation of the relationship between HCC patterns from preconception to the third trimester and multiple measures of stress during pregnancy, and used hair samples collected from a cohort of pregnant women in Lima, Peru (once at enrollment and again at full-term delivery). In doing so, we sought to determine whether changes in cortisol secretion (as reflected in maternal scalp hair samples) differed based on measures of stress assessed in early pregnancy.
2. Methods and materials
2.1 Study participants and procedures
Data for this study was drawn from participants of the Pregnancy Outcomes, Maternal, and Infant Study (PrOMIS), a prospective cohort study consisting of pregnant women attending prenatal clinics and previously described elsewhere (Barrios et al., Citation2015). Briefly, the PrOMIS cohort was designed to examine maternal social and behavioral risk factors on the development of preterm birth and other adverse pregnancy outcomes among Peruvian women. The cohort was comprised of women attending prenatal care clinics at the Instituto Nacional Materno Perinatal (INMP) in Lima, Peru, the primary reference establishment for maternal and perinatal care operated by the Ministry of Health of the Peruvian government. Written informed consent was obtained from all participants, and the institutional review board of the INMP and the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health approved all study procedures. Recruitment began in February 2012, and scalp hair samples were collected from participants during the period of October 2014 to November 2015. Participants who were 18 years of age or older, were able to speak and read in Spanish, and initiated prenatal care in early pregnancy were invited to participate (mean gestational age (GA) = 13.1 weeks, standard deviation (SD) = 3.9). Participants were then followed until delivery, and analyses were restricted to participants with full-term delivery to help ensure that segmented hair segments at delivery reflected the 2nd and 3rd trimesters. At enrollment in the 1st trimester, structured interviewer-administered questionnaires were used to collect information on stress, hair and sociodemographic characteristics, and medical and reproductive history. Among enrolled participants (N = 2,068), 96% provided a first hair sample at enrollment (N = 1,991), and 32% contributed a second hair sample after full-term delivery while women were still in the hospital (N = 653) (mean GA 39.0 weeks, SD = 1.0). Participants who contributed two hair samples were comparable to the full cohort (e.g. mean age: 27.5 vs. 27.9 and mean GA at enrollment: 12.0 weeks vs. 11.1 weeks, respectively). Since it was not financially feasible to conduct biochemical analyses using hair samples from all participants, we randomly selected 100 individuals from the pool of eligible individuals with two hair samples. Our final sample size of 97 participants excluded three participants whose cortisol was either undetectable or exceeded 100 pg/mg (>4 SD above the mean).
2.2 Hair collection and laboratory analysis
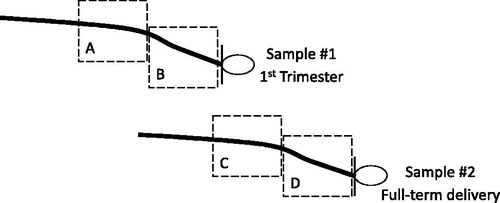
Trained research staff collected hair samples from the posterior vertex region as close to the scalp as possible at enrollment and again at full-term delivery. Prior to lab analysis, individual’s hair samples were randomly ordered and segmented for a total of four 3 cm hair segments per individual. Given that the average hair growth in humans is approximately one centimeter per month (Astore, Pecoraro, & Pecoraro, Citation1979; Barman, Astore, & Pecoraro, Citation1965; Barth, Citation1986; Loussouarn, El Rawadi, & Genain, Citation2005; Pecoraro, Astore, & Barman, Citation1969), the four hair segments reflected the following time periods: preconception (3–6 cm from the scalp at enrollment), 1st trimester (3 cm from the scalp at enrollment), 2nd trimester (3–6 cm from the scalp at delivery), and 3rd trimester (3 cm from the scalp at delivery) (). Segments further from the scalp were excluded due to concerns of measurement error from cortisol degradation and washout (Hamel et al., Citation2011; Li et al., Citation2012; Russell, Koren, Rieder, & Van Uum, Citation2012; Orta et al., Citation2018). To remove variability due to batch from within-subject contrasts, hair segments from the same woman were extracted and assayed in the same batch. The immunoassay used was the Cortisol Saliva Luminescence Immunoassay, IBL International®, for which the lower limit of detection was 0.1 pg/mg. Six blinded quality control (QC) samples were randomly dispersed per batch to assess variability (Tworoger & Hankinsonn, Citation2006). Briefly, segmented hair samples were washed in 2.5 milliliters (ml) of isopropanol twice for three minutes each time, and then dried for 12 hours in an extractor hood. After drying, 7.5 mg of whole non-pulverized hair was weighed out per segment and then minced into small pieces. Hair samples were then incubated for 18 hours in 1.8 ml high-grade methanol at room temperature, after which 1.6 ml of clear supernatant was transferred to a vial and the methanol evaporated off at 55 °C using a steady stream of nitrogen for 30 minutes. The residue from each segment was then resuspended in 225 microliters (µl) of distilled water, and 20 µl of internal standard (cortisol-d4) was added. Calculated inter-assay and intra-assay coefficients of variation were 19.4% and 11.9%, respectively, and within the acceptable range (Tworoger & Hankinsonn, Citation2006). Since cortisol concentrations in hair may be influenced by ultraviolet light (UV) exposure (Hansen, Garde, Skovgaard, & Christensen, Citation2001; Li et al., Citation2012), UV categories of low, intermediate, and high were established using dates of enrollment and meteorological data.
Figure 1 : Diagram showing the four hair segments used in analyses. Dashed boxes indicate segments on each hair sample used in analyses: (A) 3–6 cm from the scalp on hair sample 1 reflecting the preconception period, (B) 3 cm from the scalp on hair sample 1 reflecting the 1st trimester, (C) 3–6 cm from the scalp on hair sample 2 reflecting the 2nd trimester, and (D) 3 cm from the scalp on hair sample 2 reflecting the 3rd trimester.
2.3 Stress measures
Stress measures were identified using criteria from Miller, Chen, & Zhou, Citation2007 and Stalder et al., Citation2017, which required exposure to conditions that would reasonably be appraised as threatening and exceeding coping resources, or psychological in nature. Based on these criteria, at enrollment we assessed economic stressors, exposure to violence, perceived health and stress, and psychological distress. Economic stressors included: difficulty accessing basic goods (yes/no), unemployment (yes/no), and educational attainment (≤12 vs. >12 years). We assessed a participant’s exposure to violence by asking about their history of physical or sexual abuse prior to age 18 (yes/no) and their history of intimate partner violence across their lifetime (yes/no). We assessed a participant’s perceived health by asking their current general health status (fair/poor vs. good/excellent). Measures of psychological distress were assessed at enrollment using previously translated scales validated for use in Spanish-speaking populations, and included: perceived stress in the past month using the 14-item Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, Citation1983), general anxiety symptoms in the past two weeks using the Generalized Anxiety Disorder-7 scale and a cutoff score of 7 (Zhong et al., Citation2015), depressive symptoms in the past week using the Patient Health Questionnaire-9 scale and a cutoff score of 10 (Zhong et al., Citation2014), and post-traumatic stress disorder (PTSD) symptoms in the past month using the Civilian version of the Posttraumatic Stress Disorders Checklist (PCL-C) and a cutoff score of 26 (Gelaye et al., Citation2017).
2.4 Statistical analysis
The Shapiro–Wilk test statistic was used to evaluate skewness of HCC (pg/mg). Based on findings of right-skewness, values were transformed on the natural logarithm scale to approximate normality (logHCC), and mean logHCC and standard deviations (SD) are reported. Pair-wise differences in mean logHCC across hair segments were evaluated using paired t-tests. Pearson correlation coefficients (r) were used to assess correlation of logHCC across hair segments and with continuous scores from psychological distress scales (perceived stress, general anxiety, and depression). Differences in mean logHCC across categorical measures of maternal stress were evaluated using independent t-tests and linear mixed models. For categorical analyses of perceived stress, the median score of 29.0 was used as the cutoff for distinguishing high vs. low perceived stress. For depression, general anxiety, and PTSD the aforementioned cutoffs were used to create categories. Likelihood ratio tests (LRT) were used to determine best fitting models for both the logHCC means and within-subject covariance. Based on LRT results, the final model contained a linear time trend across the 4 hair segments (0, 1, 2, 3) and an unstructured covariance matrix. Each categorical index of stress was included as a main group effect with time. In separate models each stress measure (or group) was evaluated for its interaction with time (group × time) which characterizes the extent to which the slope of HCC across the time points (preconception to the 3rd trimester) differ according to categories of that stress measure. Multivariable models adjusted for factors that could influence cortisol concentrations in hair, such as: early-pregnancy body mass index (BMI), alcohol consumption, hair structure, chemical hair treatment and UV exposure (Stalder et al., Citation2017). In sensitivity analyses we further adjusted for gestational age (Wikenius et al., Citation2016). All statistical analyses used SAS® version 9.4 software (SAS Institute, Inc., Cary, North Carolina) and p values are two-sided at the alpha 0.05 level.
3. Results
Sociodemographic and hair characteristics of study participants are described in , and stress was common. For example, 41.2% of participants had difficulty paying for basic goods, 39.2% a low educational attainment (≤12 years), 53.6% were unemployed during pregnancy, 68.0% reported fair or poor general health during the current pregnancy, 29.9% had a history of physical or sexual abuse as a child, and 16.5% had a history of intimate partner violence. Further, the mean perceived stress score was 29.0 (SD = 4.9), 23.7% presented with depression (PHQ-9 score ≥10), 34.0% presented with generalized anxiety disorder (GAD-7 score ≥7), and 19.6% presented with PTSD (PCL-C score ≥26). LogHCC values on the same hair sample were highly correlated (r = 0.83 on hair sample 1, and r = 0.75 on hair sample 2), while LogHCC values across hair samples were not (r’s ranged from 0.08 to 0.23, p values >.11) (). Mean logHCC values significantly increased from preconception (1.28, standard deviation (SD) = 1.0) to the 1st trimester (1.64, SD = 0.96) and with each subsequent trimester (2nd trimester = 1.75, SD = 0.89; 3rd trimester = 2.22, SD = 0.88) (). With the exception of the 1st and 2nd trimester difference, all pairwise differences in logHCC were statistically significant.
Figure 2 : Mean log-transformed hair cortisol concentrations at the four hair segments (preconception, 1st trimester, 2nd trimester, 3rd trimester), by hair sample (1 or 2) (N = 97 participants). Gray boxes indicate 95% error bars. The difference in mean logHCC comparing preconception to the first trimester was 1.28 (standard deviation (SD) = 1.00) vs. 1.64 (SD = 0.96), p-value <.0001. The difference in mean logHCC comparing the first and second trimesters was 1.64 (SD = 0.96) vs. 1.75 (SD = 0.89), p-value >.05. The difference in mean logHCC comparing the second and third trimesters was 1.75 (SD = 0.89) vs. 2.22 (SD = 0.88), p-value <.0001.
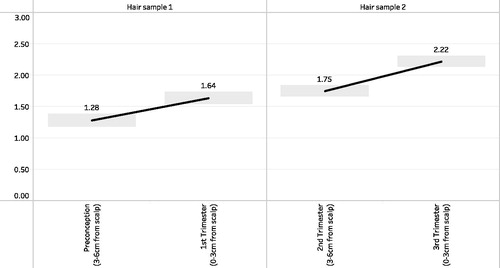
Table 1: Distribution of participant characteristics at enrollment (N = 97 participants).
Table 2: Pearson correlation coefficients (r), and mean differences (∂) and standard deviations (SD) of log-transformed hair cortisol concentrations from preconception to the 3rd trimester (N = 97 participants).
Table 3. Mean log-transformed hair cortisol concentrations at the four hair segments (preconception, 1st trimester, 2nd trimester, 3rd trimester) by each stress measure (N = 97 participants).
On average, mean logHCC values and overall patterns from preconception to the 3rd trimester did not differ according to categories of stress, and differences that were seen were generally restricted to segments reflecting the preconception and the 1st trimester ( and ). For example, an educational attainment of ≤12 years was associated with higher mean logHCC values in both preconception (1.58 vs. 1.09, p value = .02) and the 1st trimester (2.01 vs. 1.39, p value = .002), while general anxiety (GAD-7 score ≥7) was associated with lower mean logHCC in preconception (0.88 vs. 1.49, p value = .004) (). In adjusted models, increases in mean logHCC by categories of time were evident across all categories of stress (p values <.0001), and although slopes were similar, mean logHCC values were generally higher with low education (Beta = 0.32, p = .01) and lower with high perceived stress (Beta = −0.28, p value = .02) (). The overall rate of increase in logHCC from preconception to the 3rd trimester was steeper among individuals with general anxiety at enrollment (group × time p value = .02) (). With the exception of preconception HCC and general anxiety scores (r = −0.22, p value = .04), correlations of HCC with continuous scores of psychological distress were not observed (). General anxiety scores and depression scores were highly correlated (r = 0.75, p value < .0001). Models adjusted for gestational age did not appreciably differ (data not shown).
Figure 3. Mean log-transformed hair cortisol concentrations for the four hair segments (preconception, 1st trimester, 2nd trimester, 3rd trimester), by hair sample (1 or 2) and measure of stress assessed (gray = no, black = yes). CA = child abuse; IPV = intimate partner violence; PSS = perceived stress score; PTSD = Post-traumatic Stress Disorder. *An asterisk indicates that the difference in mean logHCC values comparing the two groups at that segment was statistically significant at the alpha 0.05 level. For depression at enrollment (N = 96).
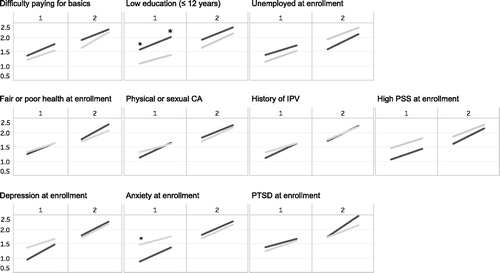
Table 4: Linear model results for the effect of stress (group), time (preconception, 1st trimester, 2nd trimester, 3rd trimester), and group by time on mean logHCC patterns from preconception to the 3rd trimester.
Table 5. Pearson correlation coefficients (r) of log-transformed hair cortisol concentrations (logHCC) from preconception to the 3rd trimester with continuous scores of psychological distress at enrollment (N = 97 participants).
4. Discussion
In this sample of participants with a high prevalence of stress in early pregnancy, we observed expected increases in HCC with pregnancy progression and few associations between measures of stress and absolute and relative changes in HCC. With the exception of general anxiety, overall patterns of HCC from preconception to the 3rd trimester were similar across the selected measures of stress. Additionally, observed differences in HCC by education and general anxiety were generally restricted to the preconception and 1st trimester when cortisol concentrations are lower relative to later in pregnancy, and closer to when measures of stress were assessed. Unexpectedly, participants with high perceived stress had generally lower HCC from preconception to the 3rd trimester.
On average, our HCC values were lower than other studies that collected hair samples at comparable times in pregnancy, although observed increases were generally similar. For example, one study of 103 participants who provided hair samples at delivery observed mean HCC values of approximately 13, 20, and 40 pg/mg in 1st, 2nd, and 3rd trimester hair segments, respectively (Kirschbaum et al., Citation2009). Another study of 21 participants who provided hair samples at each trimester observed mean HCC values of approximately 25, 33, and 40 pg/mg in 1st, 2nd, and 3rd trimester hair segments, respectively (D’Anna Hernandez, Ross, Natvig, & Laudenslager, Citation2011) . For comparison, the geometric mean HCC values observed in our study of 97 participants were approximately 5, 6, and 9 pg/mg in 1st, 2nd, and 3rd trimester hair segments, respectively. In contrast, our values were higher than a study of 180 participants who observed geometric mean HCC values of 3–4 pg/mg across the trimesters (Schreier et al., Citation2016). Despite the fact that our study observed lower absolute values of HCC, the approximately 2-fold increase in HCC from the 1st trimester to the 3rd trimester in our study is within range of the 1–3-fold increases observed in the aforementioned studies. We found no studies reporting on preconception HCC levels.
A recent correlation-based meta-analysis showed no consistent association of HCC with perceived stress or depression, and 17% lower HCC with anxiety disorders (Stalder et al., Citation2017). When restricted to studies that evaluated the association between stress and HCC during pregnancy or the postpartum, similar inconsistencies are observed (Braig et al., Citation2016; Caparros-Gonzalez et al., Citation2017; Hoffman et al., Citation2016; Kalra, Einarson, Kalra et al., Citation2007; Scharlau et al., Citation2018; Wikenius et al., Citation2016) (Supplemental Table). For example, in a study of 44 participants recruited during prenatal visits in Spain, Caparros-Gonzalez and colleagues report higher HCC using 3 cm hair segments reflecting each trimester when comparing participants with and without depression in the postpartum period (Caparros-Gonzalez et al., Citation2017). In contrast, a larger study (N = 181) using a similar recruitment strategy in Norway and a similar screening instrument found no association between HCC from 1 cm hair segments reflecting the 2nd trimester and depression scores assessed in the 2nd trimester (Pearson correlation coefficient = 0.1; multivariable beta coefficient p value = .38) (Wikenius et al., Citation2016). A US study recruited 90 participants at prenatal clinics and found significant correlations between HCC from 3 cm hair segments and scores of perceived stress, depression, and anxiety in all trimesters (Hoffman et al., Citation2016). They found no association between HCC and education or socioeconomic status. In the largest study to date, 768 participants were recruited during hospital stays after delivery in Germany and 3 cm hair segments reflecting the 3rd trimester were collected (Braig et al., Citation2016). No association was observed between HCC and scores of anxiety, depression, and chronic stress (Spearman correlation coefficients ranged from 0.0–0.1, p values >.06). The chronic stress scale in that study included questions regarding employment and social burdens. Lastly, in a study using a sample of 25 callers requesting medication safety information in Canada, modest correlations between HCC from 1–1.5 cm hair segments reflecting the late 1st to early 2nd trimester and perceived stress scale scores were observed (Spearman correlation coefficient = 0.47, p < .05) (Kalra et al., 2007). Recently, although no association was observed between HCC from 1 cm hair segments reflecting the 2nd and 3rd trimester and depression scores, a negative correlation with cortisone was observed (Scharlau et al., Citation2018). We found no studies reporting on stress and HCC in the preconception. On balance, our study’s findings of few associations between stress and HCC during pregnancy are consistent with the literature. Potential reasons for discrepancies across studies include differences in the trimesters reflected by cortisol and stress, country, and screening instruments.
Lower HCC levels among individuals with anxiety have been previously reported (Steudte et al., Citation2011; Stalder et al., Citation2017) and are consistent with hypotheses of wear-and-tear due to chronic stress (McEwen, Citation1998; Pruessner, Hellhammer, & Kirschbaum, Citation1999), specifically cortisol hyposecretion subsequent to a period of hypersecretion (Trickett, Noll, Susman, Shenk, & Putnam, Citation2010). In our study, lower HCC values among participants with general anxiety were restricted to the preconception and 1st trimester, while 2nd and 3rd trimester HCC levels did not differ. Therefore, it is likely that lower HCC in the preconception and 1st trimester explain our observation of a steeper slope of increase in HCC values from preconception to the 3rd trimester. We know of no comparable study reporting on such changes in HCC across pregnancy by anxiety. However, similar findings of steeper cortisol increases across pregnancy among those with anxiety have been previously reported using salivary cortisol (Kane, Dunkel Schetter, Glynn, Hobel, & Sandman, Citation2014). Overall, the results from our study are in conjunction with prior research suggesting that measures of stress may not play a strong role in determining absolute levels of HCC in during pregnancy, and that future researchers should more closely examine their association with their changes in HCC across pregnancy (including the preconception) and subsequent health outcomes. Similar recommendations were reported in a study of non-pregnant women using salivary cortisol (Vedhara et al., Citation2003). Further, the association between cortisol changes during pregnancy and depression status is an important gap in the literature (Orta, Gelaye, Bain, & Williams, Citation2018).
Our findings of differences in HCC restricted to preconception and 1st trimester hair segments may be explained by several reasons. First, higher placental-derived corticotropin releasing hormone during the 2nd and 3rd trimesters of pregnancy lead to substantial increases in maternal circulating cortisol concentrations upwards of twice that of pre-pregnancy levels (Goland, Wardlaw, Stark, Brown, & Frantz, Citation1986). This, in conjunction with research suggesting that physiologic responses to psychosocial stressors attenuate with advancing gestation (Entringer et al., Citation2010), suggests that the impact of stress may no longer be physiologically detected using hair cortisol in late pregnancy. This implies a potential “ceiling effect”, a commonly observed phenomenon in which discrimination is only detectable at low to moderate levels (Austin & Brunnerr, Citation2003). Further, in our study, instruments used to assess distress asked participants to reflect on symptoms in the weeks prior to enrollment. Despite the trait stability of distress symptoms such as anxiety during pregnancy (Schubert et al., Citation2017), the preconception and 1st trimester period may have been the most biologically relevant time window for such comparisons. Furthermore, since stress levels were assessed only once at enrollment, we do not know if symptoms persisted throughout pregnancy.
In an effort to evaluate whether measures of stress were associated with absolute HCC and its patterns, our study assessed multiple aspects of the stress experience that encompassed socioeconomic status, history of violence, and psychological distress using validated instruments. This comprehensive evaluation addresses some of the issues of previous studies that focused on only some potential stressors, or one, and helps lay the foundation for the role of multiple stressors on cortisol secretion patterns across pregnancy. Our study also used multiple hair collections using standardized collection techniques and trained personnel. Our inclusion of hair segments reflecting the preconception period is novel and helps fill an important gap in the literature. An additional strength to our study was our statistical approach, which evaluated differences in absolute HCC and HCC patterns while accounting for covariance across hair segments and potential covariates.
Despite our strengths, our study has some limitations. First, multiple assessments of stress at each trimester of pregnancy would have provided opportunities to examine HCC patterns in relation to changes in stress during pregnancy. Second, our study may not have been sufficiently powered to detect small group differences and group by time interactions. Further, we did not correct for multiple tests. Third, poor correlations across hair samples in our study may have influenced why observed differences on hair sample 1 did not extend to hair sample 2. Fourth, in some cases psychological distress scales assessed time windows shorter than the 3 cm hair segments reflecting the past 3-months. However, observed differences based on general anxiety, a generally stable trait (Schubert et al., Citation2017), may not have been as impacted as some of the other psychological distress scales. Furthermore, although our CV’s were on the higher end of the acceptable range, high batch to batch variation may have impacted our findings. However, our study design ensured that segments from all women were assayed in the same batch, and that random ordering ensured that individuals with high and low stress were randomly distributed across batches. Therefore, potential measurement error is likely non-differential with respect to stress. Furthermore, our findings may be impacted by residual confounding from factors associated with both stress and cortisol secretion during pregnancy. Lastly, although HCC in segments up to 6 cm from the scalp are thought to be least impacted by cortisol “washout” over time, HCC in preconception and second trimester hair segments (3–6 cm from the scalp) are likely somewhat attenuated, compared to if they were collected as proximal segments (3 cm from the scalp). Therefore, confirmation of such findings in larger studies designed to assess stress measured at multiple time points in pregnancy using proximal hair segments (3 cm from the scalp) in all trimesters and the preconception is warranted.
5. Conclusion
To our knowledge, we are the first to evaluate the association between measures of stress with both absolute HCC levels and their changes from preconception to the 3rd trimester. With the exception of educational attainment, perceived stress, and general anxiety, few indicators of stress were observed to be associated with hair cortisol and its patterns. Further, observed differences were restricted to the preconception and first trimester when cortisol levels are low relative to later in pregnancy. Future studies seeking to assess the impacts of stress on biomarker secretion patterns in hair during pregnancy should consider the addition of the preconception time window where possible.
Supplemental_Table.docx
Download MS Word (14.4 KB)Acknowledgments
The authors are indebted to the participants of the PrOMIS study for their cooperation. They are also grateful to Juliane Grasz, Elena Sanchez, and the dedicated staff members of Asociacion Civil Proyectos en Salud (PROESA), Peru and Instituto Nacional Materno Perinatal, Peru for their expert technical assistance with this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- American College of Obstetricians and Gynecologists. (2006). “ACOG committee opinion No. 43: Psychosocial risk factors: Perinatal screening and intervention.” Obstetrics and Gynecology, 108.2, 8.
- Astore, I.P., Pecoraro, V., & Pecoraro, E.G. (1979). The normal trichogram of pubic hair. The British Journal of Dermatology, 101, 441–445. doi:10.1111/j.1365-2133.1979.tb00023.x
- Austin, P.C., & Brunner, L.J. (2003). Type I error inflation in the presence of a ceiling effect. The American Statistician, 57, 97. doi:10.1198/0003130031450
- Barman, J.M., Astore, I., & Pecoraro, V. (1965). The normal trichogram of the adult. The Journal of Investigative Dermatology, 44, 233–236. doi:10.1038/jid.1965.42
- Barrios, Y.V., Gelaye, B., Zhong, Q., Nicolaidis, C., Rondon, M.B., Garcia, P.J., … Williams, M.A. (2015). Association of childhood physical and sexual abuse with intimate partner violence, poor general health and depressive symptoms among pregnant women. PloS One, 10, e0116609. doi:10.1371/journal.pone.0116609
- Barth, J.H. (1986). Measurement of hair growth. Clinical and Experimental Dermatology, 11, 127–138. doi:10.1111/j.1365-2230.1986.tb00437.x
- Braig, S., Grabher, F., Ntomchukwu, C., Reister, F., Stalder, T., Kirschbaum, C., … Genuneit, J. (2016). The association of hair cortisol with self-reported chronic psychosocial stress and symptoms of anxiety and depression in women shortly after delivery. Paediatric and Perinatal Epidemiology, 30, 97–104. doi:10.1111/ppe.12255
- Caparros-Gonzalez, R.A., Romero-Gonzalez, B., Strivens-Vilchez, H., Gonzalez-Perez, R., Martinez-Augustin, O., & Peralta-Ramirez, M.I. (2017). Hair cortisol levels, psychological stress and psychopathological symptoms as predictors of postpartum depression. PloS One, 12, e0182817. doi:10.1371/journal.pone.0182817
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. doi:10.2307/2136404
- Coussons-Read, M.E., Lobel, M., Carey, J.C., Kreither, M.O., D'Anna, K., Argys, L., … Cole, S. (2012). The occurrence of preterm delivery is linked to pregnancy-specific distress. And Elevated Inflammatory Markers across Gestation. Brain, Behavior, and Immunity, 26, 650–659. doi:10.1016/j.bbi.2012.02.009
- D'Anna-Hernandez, K.L., Ross, R.G., Natvig, C.L., & Laudenslager, M.L. (2011). Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology & behavior 104, 348–353. doi:10.1016/j.physbeh.2011.02.041
- Davis, E.P., & Sandman, C.A. (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81, 131–148. doi:10.1111/j.1467-8624.2009.01385.x
- Davis, E.P., & Sandman, C.A. (2012). Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology, 37, 1224–1233. doi:10.1016/j.psyneuen.2011.12.016
- Diego, M.A., Jones, N.A., Field, T., Hernandez-Reif, M., Schanberg, S., Kuhn, C., & Gonzalez-Garcia, A. (2006). Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosomatic Medicine, 68, 747–753. doi:10.1097/01.psy.0000238212.21598.7b
- Entringer, S., Buss, C., Shirtcliff, E.A., Cammack, A.L., Yim, I.S., Chicz-DeMet, A., … Wadhwa, P.D. (2010). Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress, 13, 258–268. doi:10.3109/10253890903349501
- Gelaye, B., Zheng, Y., Medina-Mora, M.E., Rondon, M.B., Sanchez, S.E., & Williams, M.A. (2017). Validity of the posttraumatic stress disorders (PTSD) checklist in pregnant women. BMC Psychiatry, 17, 179. doi:10.1186/s12888-017-1304-4
- Glover, V. (2015). Prenatal stress and its effects on the fetus and the child: Possible underlying biological mechanisms. Advances in neurobiology 10.] In Perinatal programming of neurodevelopment (pp. 269–283). New York, NY: Springer. doi:10.1007/978-1-4939-1372-5_13
- Glynn, L.M., Davis, E.P., & Sandman, C.A. (2013). New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides, 47, 363–370. doi:10.1016/j.npep.2013.10.007
- Goland, R.S., Wardlaw, S.L., Stark, R.I., Brown, L.S., Jr., & Frantz, A.G. (1986). High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. The Journal of Clinical Endocrinology and Metabolism, 63, 1199–1203. doi:10.1210/jcem-63-5-1199
- Hamel, A.F., Meyer, J.S., Henchey, E., Dettmer, A.M., Suomi, S.J., & Novak, M.A. (2011). Effects of shampoo and water washing on hair cortisol concentrations. Clinica Chimica Acta; International Journal of Clinical Chemistry, 412, 382–385. doi:10.1016/j.cca.2010.10.019
- Hansen, A.M., Garde, A.H., Skovgaard, L.T., & Christensen, J.M. (2001). Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clinica Chimica Acta; International Journal of Clinical Chemistry, 309, 25–35. doi:10.1016/S0009-8981(01)00493-4
- Hoffman, M.C., Mazzoni, S.E., Wagner, B.D., Laudenslager, M.L., & Ross, R.G. (2016). Measures of maternal stress and mood in relation to preterm birth. Obstetrics and Gynecology, 127, 545–552. doi:10.1097/AOG.0000000000001287
- Kalra, S., Einarson, A., Karaskov, T., Van Uum, S., & Koren, G. (2007). The relationship between stress and hair cortisol in healthy pregnant women. Clinical & Investigative Medicine, 30, 103–107. doi:10.25011/cim.v30i2.986
- Kane, H.S., Dunkel Schetter, C., Glynn, L.M., Hobel, C.J., & Sandman, C.A. (2014). Pregnancy anxiety and prenatal cortisol trajectories. Biological Psychology, 100, 13–19. doi:10.1016/j.biopsycho.2014.04.003
- Kirschbaum, C., Tietze, A., Skoluda, N., & Dettenborn, L. (2009). Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34, 32–37. doi:10.1016/j.psyneuen.2008.08.024
- Li, J., Xie, Q., Gao, W., Xu, Y., Wang, S., Deng, H., & Lu, Z. (2012). Time course of cortisol loss in hair segments under immersion in hot water. Clinica Chimica Acta; International Journal of Clinical Chemistry, 413, 434–440. doi:10.1016/j.cca.2011.10.024
- Loussouarn, G., El Rawadi, C., & Genain, G. (2005). Diversity of hair growth profiles. International Journal of Dermatology, 44 Suppl 1, 6–9. doi:10.1111/j.1365-4632.2005.02800.x
- Manuck, T.A., Esplin, M.S., Biggio, J., Bukowski, R., Parry, S., Zhang, H., … Ilekis, J. (2015). The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. American Journal of Obstetrics and Gynecology, 212, S11–487 e411. doi:10.1016/j.ajog.2015.02.010
- McEwen, B.S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. doi:10.1111/j.1749-6632.1998.tb09546.x
- Miller, G.E., Chen, E., & Zhou, E.S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi:10.1037/0033-2909.133.1.25
- Orta, O.R., Gelaye, B., Bain, P.A., & Williams, M.A. (2018). The association between maternal cortisol and depression during pregnancy, a systematic review. Archives of Women's Mental Health, 21, 43–53. doi:10.1007/s00737-017-0777-y
- Orta, O.R., Tworoger, S.S., Terry, K.L., Coull, B.A., Gelaye, B., Kirschbaum, C., … Williams, M.A. (2018). An evaluation of distal hair cortisol concentrations collected at delivery. Stress, 21, 355–365. doi:10.1080/10253890.2018.1458088
- Pecoraro, V., Astore, I.P.L., & Barman, J.M. (1969). The normal trichogram of pregnant women. Advances in biology of skin, 9, 203–210.
- Pruessner, J.C., Hellhammer, D.H., & Kirschbaum, C. (1999). Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine, 61, 197–204. doi:10.1097/00006842-199903000-00012
- Russell, E., Koren, G., Rieder, M., & Van Uum, S. (2012). Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology, 37, 589–601. doi:10.1016/j.psyneuen.2011.09.009
- Scharlau, F., Pietzner, D., Vogel, M., Gaudl, A., Ceglarek, U., Thiery, J., … Kiess, W. (2018). Evaluation of hair cortisol and cortisone change during pregnancy and the association with self-reported depression, somatization, and stress symptoms. Stress, 21, 43–50. doi:10.1080/10253890.2017.1392507.
- Schreier, H.M., Bosquet Enlow, M., Ritz, T., Coull, B.A., Gennings, C., Wright, R.O., & Wright, R.J. (2016). Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress, 19(1), 45–52. doi:10.3109/10253890.2015.1117447
- Schubert, K.O., Air, T., Clark, S.R., Grzeskowiak, L.E., Miller, E., Dekker, G.A., … Clifton, V.L. (2017). Trajectories of anxiety and health related quality of life during pregnancy. PLoS One, 12, e0181149 doi:10.1371/journal.pone.0181149
- Shannon, M., King, T.L., & Kennedy, H.P. (2007). Allostasis: A theoretical framework for understanding and evaluating perinatal health outcomes. Journal of Obstetric, Gynecologic, and Neonatal Nursing: JOGNN, 36, 125–134. doi:10.1111/j.1552-6909.2007.00126.x
- Stalder, T., & Kirschbaum, C. (2012). Analysis of cortisol in hair-state of the art and future directions. Brain Behavior and Immunity, 26, 1019–1029. doi:10.1016/j.bbi.2012.02.002
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., … Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. doi:10.1016/j.psyneuen.2016.12.017
- Steudte, S., Stalder, T., Dettenborn, L., Klumbies, E., Foley, P., Beesdo-Baum, K., & Kirschbaum, C. (2011). Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Research, 186, 310–314. doi:10.1016/j.psychres.2010.09.002
- Trickett, P.K., Noll, J.G., Susman, E.J., Shenk, C.E., & Putnam, F.W. (2010). Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology, 22, 165–175. doi:10.1017/S0954579409990332
- Tworoger, S.S., & Hankinson, S.E. (2006). Use of biomarkers in epidemiologic studies: Minimizing the influence of measurement error in the study design and analysis. Cancer Causes & Control, 17, 889–899. doi:10.1007/s10552-006-0035-5
- Vedhara, K., Miles, J., Bennett, P., Plummer, S., Tallon, D., Brooks, E., … Farndon, J. (2003). An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biological Psychology, 62, 89–96. doi:10.1016/S0301-0511(02)00128-X
- Wadhwa, P.D., Entringer, S., Buss, C., & Lu, M.C. (2011). The contribution of maternal stress to preterm birth: Issues and considerations. Clinics in Perinatology, 38, 351–384. doi:10.1016/j.clp.2011.06.007
- Wikenius, E., Moe, V., Kjellevold, M., Smith, L., Lyle, R., Waagbo, R., … Myhre, A.M. (2016). The association between hair cortisol and self-reported symptoms of depression in pregnant women. PloS One, 11, e0161804 doi:10.1371/journal.pone.0161804
- Zhong, Q., Gelaye, B., Rondon, M., Sanchez, S.E., Garcia, P.J., Sanchez, E., … Williams, M.A. (2014). Comparative performance of patient health questionnaire-9 and Edinburgh postnatal depression scale for screening antepartum depression. Journal of Affective Disorders, 162, 1–7. doi:10.1016/j.jad.2014.03.028
- Zhong, Q.Y., Gelaye, B., Zaslavsky, A.M., Fann, J.R., Rondon, M.B., Sanchez, S.E., & Williams, M.A. (2015). Diagnostic validity of the generalized anxiety disorder - 7 (GAD-7) among pregnant women. PloS One, 10, e0125096. doi:10.1371/journal.pone.0125096
