Abstract
Prolonged or repeated activation of the stress response can have negative psychological and physical consequences. The prefrontal cortex (PFC) is thought to exert an inhibitory influence on the activity of autonomic and neuroendocrine stress response systems. In this study, we further investigated this hypothesis by increasing PFC excitability using transcranial direct current stimulation (tDCS). Healthy male participants were randomized to receive either anodal (excitatory) tDCS (n = 15) or sham stimulation (n = 15) over the left dorsolateral prefrontal cortex (DLPFC) immediately before and during the exposure to a psychosocial stress test. Autonomic (heart rate (HR) and its variability) and neuroendocrine (salivary cortisol) parameters were assessed. One single session of excitatory tDCS over the left DLPFC (i) reduced HR and favored a larger vagal prevalence prior to stress exposure, (ii) moderated stress-induced HR acceleration and sympathetic activation/vagal withdrawal, but (iii) had no effect on stress-induced cortisol release. However, anodal tDCS over the left DLPFC prevented stress-induced changes in the cortisol awakening response. Finally, participants receiving excitatory tDCS reported a reduction in their levels of state anxiety upon completion of the psychosocial stress test. In conclusion, this study provides first insights into the efficacy of one single session of excitatory tDCS over the left DLPFC in attenuating autonomic and neuroendocrine effects of psychosocial stress exposure. These findings might be indicative of the important role of the left DLPFC, which is a cortical target for noninvasive brain stimulation treatment of depression, for successful coping with stressful stimuli.
Introduction
Exposure to psychological stress is a constant characteristic of everyday life in our society, and may adversely affect the mental and physical health of vulnerable individuals. Research into the link between stress and disease outcomes has shown that frequent elevations of physiological responses during the occurrence of stressful events (reactivity hypothesis) and/or persistent emotional or physiological activation during episodes in which stress is cognitively represented, but not necessarily present (perseverative cognition hypothesis; Brosschot, Gerin, & Thayer, Citation2006), trigger several pathogenic pathways that may ultimately lead to mental and/or somatic disease (Carnevali, Thayer, Brosschot, & Ottaviani, Citation2018; Treiber et al., Citation2003). Physiological stress responses include parasympathetic (vagal) withdrawal and sympathetic activation, resulting in increased heart rate (HR) and reduced HR variability (HRV), and activation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in the secretion of glucocorticoids (in humans mainly cortisol). The Neurovisceral Integration Model posits that the prefrontal cortex (PFC) regulates and inhibits the activity of limbic structures which act to suppress vagal tone and activate sympathetic circuits (Thayer & Lane, Citation2009). Similarly, animal studies have demonstrated an inhibitory role of the PFC in the regulation of the HPA axis stress response (Gilabert-Juan, Castillo-Gomez, Guirado, Molto, & Nacher, Citation2013; Herman et al., Citation2003). Notably, a “bottom-up” pattern of limbic hyperactivity and PFC hypoactivity is observed in mood and anxiety disorders (Britton, Lissek, Grillon, Norcross, & Pine, Citation2011; Drevets, Price, & Furey, Citation2008), potentially representing a neural substrate for the persistent autonomic dysfunction that characterizes these disorders (Makovac et al., Citation2016; Sgoifo, Carnevali, Alfonso, & Amore, Citation2015).
Transcranial direct current stimulation (tDCS) is a form of noninvasive brain stimulation and represents an interesting tool for the modulation of PFC excitability. TDCS is a neuromodulatory technique that consists in applying a direct electric current over the scalp to increase (anodal tDCS) or decrease (cathodal tDCS) cortical excitability (Priori, Berardelli, Rona, Accornero, & Manfredi, Citation1998). The dorsolateral PFC (DLPFC) has been the subject of much tDCS research due to its relevance for psychiatric disorders (Berlim, Van den Eynde, & Daskalakis, Citation2013; Brunoni et al., Citation2016). Specifically, anodal tDCS over the left DLPFC brain region exerts beneficial effects on clinical symptoms of major depression (Berlim et al., Citation2013). Besides its role in emotion regulation, studies have shown a potential involvement of the DLPFC in the top-down regulation of autonomic and neuroendocrine stress responses (Baeken et al., Citation2014, Brunoni et al., Citation2013). For example, anodal tDCS over the left DLPFC was found to induce a higher vagally mediated HRV and lower cortisol levels during the presentation of emotional negative images in healthy individuals (Brunoni et al., Citation2013). Importantly, other forms of noninvasive brain stimulation (i.e. transcranial magnetic stimulation) of the left DLPFC proved to be effective in counteracting the reduction in vagally mediated HRV observed in healthy participants during the execution of a stressful task (Remue et al., Citation2016). However, it remains unclear whether increased excitability of the left DLPFC via anodal tDCS can affect autonomic and neuroendocrine responses to a psychosocial stress test. The Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, Citation1993) is a psychosocial stress paradigm that evokes potent, but relatively short-lasting, autonomic (e.g. increased HR and reduced HRV) and neuroendocrine (e.g. increases in cortisol levels) responses in healthy individuals (for a recent review see Allen et al., Citation2017). Notably, an extensive activation of the DLPFC has been documented during TSST exposure (Rosenbaum et al., Citation2018), supporting the notion that the DLPFC is one of the most stress-sensitive brain areas (McEwen & Morrison, Citation2013).
In the present study in healthy adult males, we tested the hypothesis that a single session of anodal tDCS performed over the left DLPFC immediately before and during the exposure to a psychosocial stress test (i.e. a modified version of the TSST) would attenuate autonomic (as indexed by HR and HRV) and HPA axis (as indexed by salivary cortisol levels) stress responses. To the best of our knowledge, no studies have investigated whether autonomic and neuroendocrine perturbations induced by this psychosocial stress paradigm persist long after its conclusion. Therefore, our secondary aim was to explore potential long-lasting consequences of psychosocial stress exposure on 24-hour HR and HRV, as indicators of resting autonomic function, and the cortisol awakening response (CAR), and to investigate whether these changes would be moderated by previous excitatory stimulation of the left DLPFC, providing that the neuroplastic effects of tDCS can last for up to 1 h (Liebetanz, Nitsche, Tergau, & Paulus, Citation2002; Nitsche et al., Citation2004).
Methods
Subjects
Thirty healthy male participants with no psychiatric or clinical conditions were recruited by the use of flyers among university students. Exclusion criteria included age younger than 18 years, self-reported history of head injury, current or past neurological, psychiatric, and cardiac disorders, cognitive impairment, substance or alcohol abuse or dependence, body mass index ≥30 kg/m2, and ambidexterity or left handedness. None of the participants had metallic implants/implanted electric devices, nor took any medication regularly or in the preceding 2 weeks. The study conformed to the Declaration of Helsinki, and the protocol was approved by the Bioethical Committee of the Santa Lucia Foundation, Rome, Italy. All volunteers gave written informed consent and were compensated (30 €) for their participation.
Procedure
A randomized, sham-controlled, single-blind design with parallel groups was used. Twenty-four hours before the laboratory session, participants completed a series of socio-demographic, lifestyle, and psychometric questionnaires, including the trait version of the State-Trait Anxiety Inventory (STAI) (Spielberg, Gorsuch, Lushene, Vagg, & Jacobs, Citation1983), and the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, Citation1977). Then, they were fitted with a wearable HR monitoring device (Bodyguard 2, Firstbeat) for 24-h beat-to-beat interval recording. Finally, they were given oral swabs and clearly marked swab storage tubes (Salimetrics, Cambridge, UK), received oral and written instructions on how to collect and store saliva samples at home on the next morning (between 7:00 and 8:00 h) immediately after awaking and 30 min later (day 0). On the day of the laboratory session, participants were randomly assigned to two groups (tDCS or sham) and submitted to a psychosocial stress test (see below), which took place between 14:30 and 16:30 h in a quiet room at a comfortable temperature (21 ± 2 °C). After the test, participants were again fitted with the HR monitoring device and invited to collect saliva samples at home on the next morning with the same modalities described above (i.e. immediately after awaking and 30 min later; day +1). Twenty-four hours later, participants returned to the lab, handed in the saliva samples, received monetary compensation, and were de-briefed. Detailed experimental procedures are described below.
tDCS
Participants were randomly allocated to receive either active (n = 15) or sham (n = 15) anodal tDCS over the left DLPFC (F3 according to the International EEG 10-20 system). For the anodic stimulation, a current intensity of 2 mA was applied for 15 min through saline-soaked sponges measuring 5 × 7 cm. The anode was placed over F3 and the cathode was located over the right DLPFC (F4). The anodal and cathodal electrodes were fixed by elastic bands and connected to the BrainSTIM device (EMS s.r.l., Bologna, Italy). The same electrode disposition was used to perform the sham stimulation; however, the stimulator was turned off after 30 s to preserve participant blinding. Thus, the subjects reported to feel a tingling or itching sensation coming from the initial electrical stimulation, but they did not receive any further current.
Laboratory session
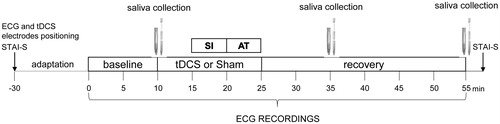
Subjects were asked to avoid intense physical training the day before the experiment, and to refrain from drinking coffee and energizing drinks, eating a meal, and smoking for at least two hours prior to their arrival to the lab. depicts the sequence of events that occurred during the laboratory session. Initially, participants were instrumented with a BT16Plus device (Francesco Marazza Hardware & Software, Monza, Italy), which allows real time acquisition of the ECG signal (sampling frequency: 250 Hz) through three electrodes secured to the right and left parasternal regions and the left groin area, respectively. Subsequently, the electrodes for tDCS were positioned as described above. Then, participants completed the state version of the STAI (Spielberg et al., Citation1983) and were allowed to settle down in the new environment while sitting on a comfortable chair in front of two familiar experimenters. After baseline ECG recordings, a saliva sample was collected from each participant using oral swabs and swab storage tubes (Salimetrics, Cambridge, UK). Subsequently, participants received either active or sham anodal tDCS for 15 min. After the first 5 min of stimulation, they were submitted to a psychosocial stress test that was based on an adapted version of the TSST (Kirschbaum et al., Citation1993). During the 5-min stress interview (SI) phase, they were asked to answer a series of questions about how they behave and feel in different social contexts. Subsequently, they were asked to complete a 5-min mental arithmetic task (AT) by counting aloud backwards from 2083 by 13’s. The SI and AT were administered by an unfamiliar female interviewer, with a small unfamiliar audience (two people) sitting behind the participants. Upon completion of the stress phases, the stimulator was turned off, and the unfamiliar interviewer and audience left the room, while the participants remained seated and quiet in the presence of the two familiar experimenters for the following 30-min recovery phase. Additional saliva samples were collected 20 and 40 minutes after the beginning of the SI. At the end of the recovery phase, participants completed the state version of the STAI (Spielberg et al., Citation1983).
Questionnaires
The severity of trait anxiety was measured using the trait version of the STAI (Spielberg et al., Citation1983), which is a quadruplet Likert type scale consisting of 20 items assessing how the patient feels, independent from the current status and circumstances. The validity of the scale has been repeatedly confirmed, with reliability coefficients ranging from 0.71 to 0.86 and good internal consistency and homogeneity coefficients between 0.83 and 0.87. State anxiety was measured using the state version of the STAI (Spielberg et al., Citation1983), which asks how respondents feel “right now” using four-point Likert scale items that measure subjective feelings of apprehension, tension, nervousness, worry, and activation/arousal of the autonomic nervous system. Greater scores indicate greater anxiety levels and lower scores indicate lower levels of anxiety. The reliability coefficient is 0.62.
The CES-D is a 20-item self-report scale designed to measure depressive symptomatology during the past week in the general population (Radloff, Citation1977). The total score ranges from 0 to 60. Standard cutoffs are >16 for mild depression and >23 for clinical depression. Cronbach’s alphas are above .85 in the general population and .90 in depressed patients confirming high reliability (Radloff, Citation1977).
Heart rate and heart rate variability data
Twenty-four-hour beat-to-beat interval recordings
Twenty-four-hour raw beat-to-beat intervals obtained with the wearable HR monitoring device were arranged in 5-min epochs. Outlier and artifact detection as well as HR (reported in beats per minute; bpm) and time- and frequency-domain HRV analyses were performed using Kubios HRV software. In the time-domain, we computed the root mean square of successive beat-to-beat interval differences (RMSSD, ms), which reflects vagal regulation of HR (Laborde, Mosley, & Thayer, Citation2017) and is less susceptible to respiratory and movement artifacts compared to the alternative frequency-domain high frequency (HF) activity (Hill & Siebenbrock, Citation2009). In the frequency domain, we quantified the power of the low frequency (LF; 0.04–0.15 Hz) and HF (0.15–0.4 Hz) bands in normalized units (nu), and calculated the LF to HF ratio (LF/HF). The LF band reflects a mix between sympathetic and vagal influences, whereas the HF band carries information about the relative contribution of vagal influence (Laborde et al., Citation2017). The LF/HF ratio estimates the fractional distribution of power and is taken as a surrogate measure of cardiac sympathovagal balance (Montano et al., Citation1994); although, this stance is not without controversy (Laborde et al., Citation2017).
HR and HRV data from each 5-min epoch were further averaged for waking and sleep hours, respectively, based on self-reports of bed and wake up times. Accelerometer data were collected alongside beat-to-beat interval recordings. This allowed the exclusion of epochs where movement was excessive from ECG analysis (Hansen et al., Citation2013).
ECG recordings during the laboratory session
ECG signals were acquired (BT16Plus Acquisition Software 1.9.0, Francesco Marazza), converted to digital, pre-processed in a Matlab environment, and analyzed by means of Chart5 software (ADInstruments, Sydney, Australia). Initially, each raw ECG signal was manually inspected to ensure that all R-waves were correctly detected. For each recording period, ECGs were split in 5-min epochs. For each epoch, we then calculated HR and time (RMSSD)- and frequency (LFnu, HFnu, and LF/HF)-domain indexes of HRV. HR and HRV data during the experimental phases of the laboratory session () were also calculated as changes from the respective baseline value (i.e. delta values).
Saliva collection and cortisol determination
Immediately after collection, saliva samples were frozen at –20 °C until analysis. Salivary cortisol levels were determined by enzyme-linked immunosorbent assay (High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit: Salimetrics LLC, State College, PA). Samples were thawed, brought to room temperature and centrifuged (1500×g × 10 min), resulting in a clear supernatant of low-viscosity. Cortisol levels were assayed in duplicates following kit instructions with a 96-well plate, using Infinite F50 plate reader and Magellan software (TecanGroup Ltd, Männedorf, Switzerland). To avoid inter-assay variability, all samples from the same participant were assayed in the same batch. The inter-assay and intra-assay coefficients of variability were 5.1 and 8.6, respectively. Salivary cortisol levels during the experimental phases of the laboratory session () were calculated as absolute values and as changes from the respective baseline value (i.e. delta values). Salivary cortisol levels after awakening and 30 min later were computed as absolute values and also used to capture the CAR, which was calculated as the difference between the latter values and the former values, respectively. We asked participants to record the actual time when saliva samples were collected. Samples from two non-compliant participants (one per group) were excluded from the analysis.
Statistical analyses
All statistical analyses were performed using SPSS 25 software package (SPSS Inc., Chicago, IL). Socio-demographic, anthropometric, and lifestyle factors were compared between the two groups using independent t-tests and Chi-square tests, as appropriate. STAI-T and CES-D scores were compared between the two groups using independent t-tests. To test for the effects of tDCS on baseline physiological parameters and stress responses, two-way ANOVAs for repeated measures, with “group” as the between-subject factor (two levels: active tDCS and sham tDCS) and “time” as the within-subject factor were applied for: (i) HR and HRV data, (ii) cortisol levels, and (iii) STAI-S scores during the laboratory session. To test for the enduring effects of tDCS and stress exposure on physiological parameters, two-way ANOVAs for repeated measures, with “group” as the between-subject factor (two levels: active tDCS and sham tDCS) and “phase” (two levels: wake and sleep or minute 0 and minute 30) and “time” (two levels: before and after) as the within-subject factors were applied for: (i) HR and HRV data and (ii) cortisol levels collected before and after the laboratory session. Follow-up analyses were conducted using Student's t-tests, with a Bonferroni correction for multiple comparisons. Statistical significance was set at p < .05.
Results
Socio-demographic, anthropometric, lifestyle, and psychological data
The two groups were well matched regarding all socio-demographic, anthropometric, lifestyle, and psychological characteristics, which are listed in .
Table 1. Socio-demographic, anthropometric, lifestyle, and psychological characteristics.
Blinding
Participants recruited to this study were naïve to tDCS and were only exposed to one condition (i.e. either active or sham tDCS). All of the subjects tolerated the stimulation well; no adverse effects occurred during or after the stimulation with the exception of commonly reported short-lasting side effects as tingling and itching. At the completion of the laboratory session, participants were asked to guess whether they had received active or sham tDCS. Analysis of their guesses using Chi-square test revealed no significant group differences (p = .409).
Effects of tDCS on baseline physiological parameters and stress responses
Heart rate and heart rate variability
During the baseline period that preceded active or sham tDCS, the two groups showed similar HR and HRV values, which are listed in .
Table 2. Baseline heart rate and heart rate variability parameters during the laboratory session.
The two-way ANOVA applied on delta HR values yielded a significant effect of “time” (F(8,224) = 68.1, p < .001, ηp2 = 0.709) and a significant “time × group” interaction (F(8,224) = 4.5, p = .011, ηp2 = 0.086). As represented in , tDCS provoked a significant reduction in resting HR compared to sham stimulation (p < .01, ηp2 = 0.295). Stress exposure provoked an increase in HR in both groups, with the magnitude of this HR acceleration being significantly smaller during the SI phase (p = .037, ηp2 = 0.151) and marginally smaller during the AT (p = .081, ηp2 = 0.109) in the tDCS group compared to the sham group. During the recovery phase, we observed a similar return of HR towards the respective baseline values in the two groups.
Figure 2. Time course of changes in delta values of heart rate and heart rate variability parameters during the psychosocial stress test, in the tDCS (n = 15) and sham (n = 15) group. Data are reported as means ± standard errors. *A significant difference between the two groups (p values are reported in the text). tDCS: transcranial direct current stimulation; SI: stress interview; AT: arithmetic task; HR: heart rate; RMSSD: root mean square of successive beat-to-beat interval differences; LF: low frequency; HF: high frequency.
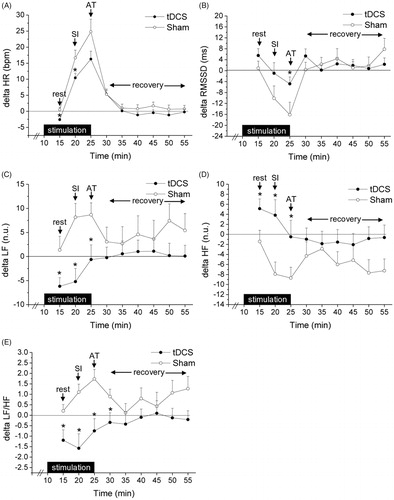
In the same way, delta RMSSD values significantly varied over time (F(8,224) = 9.5, p < .001, ηp2 = 0.261) and showed a significant “time × group” interaction (F(8,224) = 2.9, p < .01, ηp2 = 0.100). As shown in , there was no significant difference in the changes induced by tDCS or sham stimulation on resting RMSSD values. However, the magnitude of the stress-induced reduction in RMSSD values was smaller in the tDCS group compared to the sham group, reaching statistical significance specifically during the AT (p = .042, ηp2 = 0.145). No group differences were observed in delta RMSSD values during the recovery phase.
For delta LF values, we found a significant variation over time (F(8,224) = 2.1, p < .05, ηp2 = 0.075), and a marginally significant “time × group” interaction (F(8,224) = 1.8, p = .069, ηp2 = 0.062). As depicted in , a reduction in resting LF values was observed only during tDCS stimulation (p = .033, ηp2 = 0.152). Compared to baseline, stress exposure provoked an increase in LF values in the sham, but not in the tDCS, group. This group difference was statistically significant both during the SI (p < .01, ηp2 = 0.290) and the AT (p = .025, ηp2 = 0.196). No group differences were observed in delta LF values during the recovery phase.
For delta HF values, we found a significant variation over time (F(8,224) = 2.8, p < .01, ηp2 = 0.089) and between groups (F(1,28) = 4.2, p = .044, ηp2 = 0.127), and a marginally significant “time × group” interaction (F(8,224) = 1.8, p = .074, ηp2 = 0.061). As depicted in , an increase in resting HF values was observed only during tDCS stimulation (p = .035, ηp2 = 0.150). Compared to baseline, stress exposure provoked a reduction in HF values only in the sham group, with this group difference being statistically significant both during the SI (p < .01, ηp2 = 0.242) and the AT (p = .046, ηp2 = 0.135). No group differences were observed in delta HF values during the recovery phase.
For delta LF/HF values, we found a significant effect of “group” (F(1,28) = 7.4, p = .011, ηp2 = 0.209) and a significant “time × group” interaction (F(8,224) = 2.0, p = .043, ηp2 = 0.068). As represented in , a reduction in resting LF/HF values was observed only during tDCS stimulation (p = .022, ηp2 = 0.173). Compared to baseline, stress exposure provoked an increase in LF/HF values in the sham, but not in the tDCS, group. This group difference was statistically significant both during the SI (p < .01, ηp2 = 0.292) and the AT (p < .01, ηp2 = 0.285), and persisted during the first 5 min of the recovery phase (p = .043, ηp2 = 0.138).
Cortisol
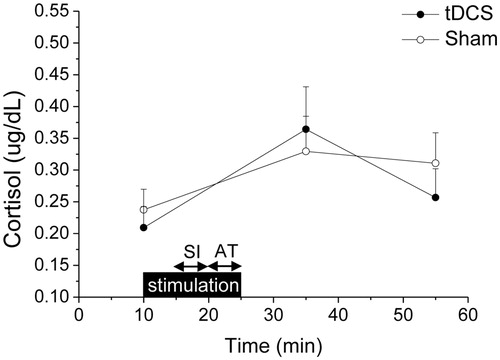
Cortisol levels varied significantly over time (F(2,56) = 6.8, p < .01, ηp2 = 0.196). As represented in , there were no group differences in baseline cortisol levels. Stress exposure provoked an increase in cortisol levels, with the magnitude of this increment being similar between the two groups (min 35: tDCS = +0.15 ± 0.05 g/dL vs. sham = +0.09 ± 0.04 µg/dL, p = .299, ηp2 = 0.038; min 55: tDCS = +0.05 ± 0.04 µg/dL vs. sham = +0.07 ± 0.05 µg/dL, p = .696, ηp2 = 0.006).
State anxiety
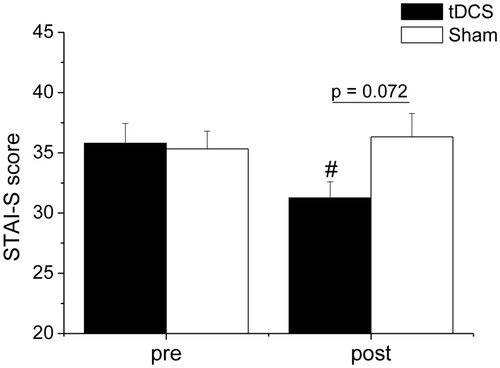
The two-way ANOVA applied on STAI-S scores yielded a significant “time × group” interaction (F(1,28) = 6.2, p = .019, ηp2 = 0.181). As depicted in , the two groups reported similar levels of state anxiety at the beginning of the laboratory session. At the end of the psychosocial stress procedure, the tDCS, but not the sham, group reported significantly lower levels of state anxiety compared to pre-stress levels (p = .011, ηp2 = 0.211). Moreover, post-stress state anxiety levels were marginally lower in the tDCS group compared to the sham group (p = .072, ηp2 = 0.111).
Figure 4. Levels of state anxiety reported by the tDCS (n = 15) and sham (n = 15) group prior to (pre) and after (post) the psychosocial stress test. Data are reported as means ± standard errors. #A significant difference between post-stress value and the corresponding pre-stress value (p value is reported in the text). tDCS: transcranial direct current stimulation; STAI-S: State-Trait Anxiety Inventory, State version.
Performance on the arithmetic task
There was no difference in the performance of the two groups on the AT (number of errors: tDCS = 6.3 ± 0.8 vs. sham = 7.2 ± 0.7, p = .406).
Long-lasting effects of tDCS and stress exposure on physiological parameters
Heart rate and heart rate variability
We did not find any significant effect of group or time on HR and HRV data collected during the 24 h that preceded and 24 h that followed the laboratory session, which are listed in .
Table 3. 24-Heart rate and heart rate variability parameters before (pre) and after (post) the laboratory session.
Cortisol
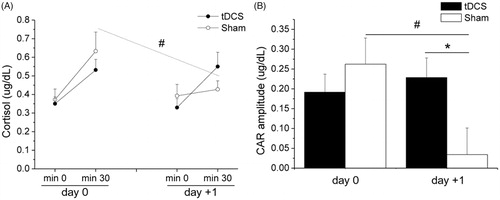
The two-way ANOVA applied on absolute cortisol levels revealed a significant “group × phase × time” interaction (F(1,26) = 6.9, p = .014, ηp2 = 0.209). As represented in , the two groups showed similar cortisol levels on both days. However, the sham, but not the tDCS, group showed significantly lower cortisol levels at the 30-min assessment of day +1 compared to the respective value of day 0 (p = .014, ηp2 = 0.212). Moreover, for CAR amplitude, we found a significant “time × group” interaction (F(1,26) = 6.9, p = .014, ηp2 = 0.209). As shown in , there was no group difference in CAR amplitude on day 0. However, CAR amplitude was significantly smaller in the sham, but not tDCS, group on day +1 compared to day 0 (p < .01, ηp2 = 0.281). Consequently, the tDCS group showed a larger CAR amplitude than the sham group on day +1 (p = .033, ηp2 = 0.163).
Figure 5. Salivary cortisol levels at awakening (min 0) and 30 min later (panel A) and cortisol awakening response (panel B) on the day of the laboratory assessment (prior to psychosocial stress exposure, day 0) and on the following day (day +1), in the tDCS (n = 15) and sham (n = 15) group. Data are reported as means ± standard errors. *A significant difference between the two groups; #A significant difference between post-stress value and the corresponding pre-stress value (p values are reported in the text). tDCS: transcranial direct current stimulation; CAR: cortisol awakening response.
Discussion
The major findings of the present study are that one single session of anodal tDCS over the left DLPFC, which was performed immediately before and during psychosocial stress exposure, (i) moderated stress-induced short-lasting autonomic responses, (ii) had no effect on stress-induced cortisol release, (iii) favored a reduction in self-report levels of state anxiety, and (iv) prevented stress-induced changes in the CAR. Moreover, our results are in agreement with previous studies documenting links between noninvasive PFC stimulation and reduced HR and increased HRV at rest (Makovac, Thayer, & Ottaviani, Citation2017).
Effects of tDCS on resting cardiac autonomic parameters
A series of meta-analyses demonstrated the existence of associations between noninvasive brain stimulation (including transcranial magnetic stimulation and tDCS) and diminished HR and increased vagally mediated HRV at rest (Makovac et al., Citation2017), which would be indicative of preferential activation of vagal neural circuitry via increased prefrontal activity. However, previous studies which have investigated the effects of noninvasive brain stimulation over the left DLPFC specifically on HRV indexes of resting vagal tone (i.e. HF and RMSSD absolute values) reported mixed results. While a study described a significant increase in HF values during resting conditions (Nikolin, Boonstra, Loo, & Martin, Citation2017), others did not find any specific effects on resting values of HF (Brunoni et al., Citation2013) or RMSSD (Remue et al., Citation2016). Quite surprisingly, none of these studies reported HR results. Our data seem to be in agreement with the latter studies, given that no significant effects were found on resting values of the vagal index RMSSD after excitatory stimulation of the left DLPFC. Therefore, we suggest that the present results on the effects of anodal tDCS over the left DLPFC on resting cardiac autonomic function should be more cautiously interpreted as a shift of the sympathovagal balance towards a larger vagal prevalence (as indirectly indexed by higher HFnu and lower LF/HF values), rather than increased vagal modulation per se, which consequently resulted in lower resting HR. Regardless, these findings are in agreement with the Neurovisceral Integration Model, which links inhibitory prefrontal-subcortical circuits to autonomic motor circuits responsible for both the sympatho-excitatory and vagal-inhibitory effects on the heart (Thayer & Lane, Citation2009). It must be noted, however, that a clear picture regarding the effects of increased prefrontal activity via tDCS on resting autonomic function (sympathetic inhibition, or vagal potentiation, or a combination or both) is yet to emerge, largely due to the high heterogeneity in experimental designs and adopted autonomic measures (Makovac et al., Citation2017). Moreover, while it has been proposed that left-sided forebrain structures are predominantly involved in vagal regulation and right-sided forebrain structures primarily control sympathetic tone and responses (Craig, Citation2005), the lateralization model of autonomic control of the heart remains controversial (Carnevali, Koenig, Sgoifo, & Ottaviani, Citation2018; Thayer, Åhs, Fredrikson, Sollers, & Wager, Citation2012).
Effects of tDCS on stress responses
As expected, psychosocial stress exposure evoked a potent, but short-lasting, autonomic activation (Hermann, Biallas, Predel, & Petrowski, Citation2019). Specifically, anodal tDCS over the DLPFC moderated the HR acceleration and counteracted the vagal withdrawal and larger sympathetic prevalence observed in the sham group during the stressful tasks. Similar to the point, we have discussed above, our data do not allow to conclusively demonstrate whether the effects of excitatory tDCS over the left DLPFC on autonomic stress responsiveness were mediated predominantly by sympathetic or vagal pathways, or, more likely, by a combination of both. However, these results extend previous findings documenting that noninvasive brain stimulation of left DLPFC with tDCS or transcranial magnetic stimulation results in higher vagally mediated HRV specifically during emotional negative stimuli (Brunoni et al., Citation2013) and a stressful mental task (Remue et al., Citation2016), respectively. Together, these results suggest that the left DLPFC may be a critical area in the neurocircuitry underlying autonomic stress reactivity. The left DLPFC has been implicated in cognitive appraisal and working memory (Shin et al., Citation2005). Notably, a number of studies have identified a subset of mental workload components (i.e. executive demands) as key to understand the autonomic-cognitive processing link, with larger sympathetic activation and/or vagal withdrawal as executive demands increase (e.g. Duschek, Muckenthaler, Werner, & del Paso, Citation2009; Luft, Takase, & Darby, Citation2009). In this scenario, building from the common neural basis for cognitive and autonomic regulation, the above-mentioned Neurovisceral Integration Model predicts an inverse relationship between executive task demands and vagal indexes of HRV (Thayer & Lane, Citation2009). Therefore, it is reasonable to hypothesize that by increasing the activity of the left DLPFC, cognitive control of stress may be positively affected, and that this would be physiologically reflected in reduced autonomic activation. Indeed, it is not surprising that autonomic responsiveness was higher during the stressful task that presumably demanded greater dorsolateral prefrontal cortical processing (i.e. the AT), thus amplifying tDCS effects on vagally mediated HRV (RMSSD index). This interpretation is further supported by (i) findings of an association between high levels of vagally mediated HRV at rest and enhanced performance on cognitive control tasks that require working memory, attentional control, and inhibition in healthy individuals (Gillie & Thayer, Citation2014; Ottaviani et al., Citation2018) and (ii) research that suggests that noninvasive brain stimulation of the left DLPFC ameliorates cognitive functions that have been found to be impaired in depressed individuals and linked to the emergence and recurrence of depression (Snyder, Citation2013). DLPFC activity has also been related to the processing of incorrect mathematical equations (Menon, Mackenzie, Rivera, & Reiss, Citation2002). Interestingly, excitatory stimulation of the left DLPFC by tDCS was found to reduce reaction times in arithmetic tests and cortisol levels in high math anxious individuals (Sarkar, Dowker, & Cohen Kadosh, Citation2014). However, here we did not find any specific effect of anodal tDCS over the left DLPFC on the performance on the AT. Nevertheless, the tDCS, but not the sham, group showed a significant reduction in self-report levels of state anxiety at the end of the psychosocial stress procedure, further supporting the view that left DLPFC activity may be critical for successful coping with stressful stimuli. TDCS has been shown to be capable of inducing changes in cortical excitability lasting for up to 1 h after the end of the stimulation (Liebetanz et al., Citation2002; Nitsche et al., Citation2004). Yet, differences between the two groups on autonomic parameters evanished soon after active stimulation. It is plausible that the potent vagal rebound observed after completion of the stressful tasks might have masked enduring subtle differences in resting autonomic parameters induced by tDCS.
Contrary to our initial hypothesis, we found that the magnitude of stress-induced HPA-axis activation, as measured by salivary cortisol levels, was not modulated by excitatory tDCS over the left DLPFC. Decreased cortisol levels were described in individual receiving left anodal tDCS over this cortical region while viewing emotional negative images (Brunoni et al., Citation2013). Moreover, transcranial magnetic stimulation over the left DLPFC prior to a stress-inducing task resulted in decreases in cortisol levels, as compared to stimulation over the right DLPFC and/or sham stimulation (Baeken et al., Citation2014). Previous models have suggested that HPA axis activity decreases or increases according to left and right prefrontal activity, respectively (Cerqueira, Almeida, & Sousa, Citation2008). However, to the best of our knowledge, in the only study that had investigated the effects of tDCS on cortisol responses to the TSST, pre-stress cathodal stimulation over the right medial-PFC was associated with higher stress-induced cortisol release (Antal et al., Citation2014). Clearly, further investigation is required to explore top-down modulation of cortisol responses to stressful challenges using tDCS.
A secondary objective of the present study was to investigate potential long-lasting effects of acute psychosocial stress exposure on resting autonomic function and the HPA axis. While no changes were found in 24-h HR and HRV measures between pre- and post-stress assessments in both groups, a stress-induced reduction in the CAR was observed in the sham, but not tDCS, group. The CAR is the distinct rise in cortisol levels within the first hour of awakening and is influenced by psychosocial as well as genetic factors. However, the relationship between stress exposure and CAR has yet to be fully elucidated. For example, a meta-analysis revealed that several psychosocial factors are differently associated with the CAR: for example, job stress, and general life stress are associated with increased CAR, while fatigue, burnout, and exhaustion with reduced CAR (Chida & Steptoe, Citation2009). Moreover, a previous study has documented the presence of reduced CAR in the morning after a mental stressor in healthy young adult men (Fornari, Carnevali, & Sgoifo, Citation2017). Therefore, the current results may warrant further investigation of the effects of acute psychosocial stress exposure on the CAR.
Limitations
The results of this exploratory study should be carefully interpreted, taking several limitations into account. First, we included only a small sample of young healthy men, thereby increasing the risks of false negative and effect size inflation. Future work with larger samples is thus needed to test whether the present results generalize to women or to a population with a higher variation in age and health (e.g. patients suffering from depression). Second, tDCS has a disadvantage of a relatively low spatial resolution (Datta et al., Citation2009). Combined tDCS and functional magnetic resonance imaging studies in both animals (e.g. Takano et al., Citation2011) and humans (e.g. Orlov et al., Citation2017) support that stimulation of the DLPFC is effective in activating the region beneath the anodal electrode. For example, the ventromedial PFC is localized immediately below the DLPFC and plays an important role in emotional and autonomic regulation and stress reactivity (Hansel & von Kanel, Citation2008). Therefore, it must be considered that the activity of other brain regions besides the left DLPFC could also have been modulated, but without concomitant neuroimaging data this interpretation remains to some extent speculative. Third, participants received only active anodal or sham tDCS over the left DLPFC in a between-subjects design. Unfortunately, the nature of the psychosocial stress challenge adopted here prevented us from choosing a within-subject design to increase statistical power. Moreover, we decided to focus on excitatory tDCS over the left DLPFC because hypoactivity of the left side of this cortical region has been specifically implicated in stress-related psychiatric disorders, including depression (Davidson et al., Citation2002). Lastly, this study did not include an objective measure to assess the delay between awakening and the collection of the first salivary sample. This methodological issue may affect the reliability of the results concerning the CAR (Stalder et al., Citation2016), which therefore should be interpreted with particular caution. Moreover, a more detailed assessment of salivary cortisol levels throughout the day would have provided a clearer picture of the long-lasting effects of acute psychosocial stress exposure on the basal state of the HPA axis.
Conclusions
Recent meta-analyses and other reviews of brain imaging studies of stressor-evoked cardiovascular changes have implicated functional divisions of a distributed network of cortical and subcortical brain regions in the regulation of autonomic and neuroendocrine stress responses (e.g. Beissner, Schumann, Brunn, Eisentrager, & Bar, Citation2014; Gianaros & Wager, Citation2015; Myers, Citation2017; Shoemaker & Goswami, Citation2015; Thayer et al., Citation2012). These brain regions, which include the medial PFC, the anterior cingulate cortex, insula, and hippocampus are also specifically viewed to play a role in stress appraisal processes (Gianaros & Wager, Citation2015). The present study provides first insights into the efficacy of one single session of excitatory tDCS over the left DLPFC in moderating autonomic, but not cortisol, reactivity to psychosocial stress exposure in healthy participants. Speculatively, this may indicate a role for the left DLPFC in the neurobiological modulation of the autonomic stress response system, whereas simultaneous bifrontal modulation might be necessary to regulate the HPA response to acute stress, as previously suggested (Cerqueira et al., Citation2008). This buffering effect on autonomic stress responsiveness might have played a role in facilitating the deactivation of the anxious response after stress exposure in individuals receiving tDCS. These findings might indicate that by increasing the activity of the left DLPFC, we can possibly augment the cognitive control to successfully cope with stressful stimuli, with important implications for the prevention and timely treatment of stress-related disorders.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
Notes on contributors
Luca Carnevali
Luca Carnevali, Andrea Sgoifo, Cristina Ottaviani conceived and designed the experiments.
Elena Pattini
Luca Carnevali, Elena Pattini performed the experiments and analyzed the data.
Andrea Sgoifo
Luca Carnevali, Elena Pattini performed the experiments and analyzed the data.
Cristina Ottaviani
Andrea Sgoifo, Cristina Ottaviani revised the article critically for important intellectual content.
References
- Allen, A.P., Kennedy, P.J., Dockray, S., Cryan, J.F., Dinan, T.G., & Clarke, G. (2017). The Trier Social Stress Test: Principles and practice. Neurobiology of Stress, 6, 113–126. doi:10.1016/j.ynstr.2016.11.001
- Antal, A., Fischer, T., Saiote, C., Miller, R., Chaieb, L., Wang, D.J., … Kirschbaum, C. (2014). Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Human Brain Mapping, 35, 3750–3759. doi:10.1002/hbm.22434
- Baeken, C., Vanderhasselt, M.A., Remue, J., Rossi, V., Schiettecatte, J., Anckaert, E., & De Raedt, R. (2014). One left dorsolateral prefrontal cortical HF-rTMS session attenuates HPA-system sensitivity to critical feedback in healthy females. Neuropsychologia, 57, 112–121. doi:10.1016/j.neuropsychologia.2014.02.019
- Beissner, F., Schumann, A., Brunn, F., Eisentrager, D., & Bar, K.J. (2014). Advances in functional magnetic resonance imaging of the human brain. NeuroImage, 86, 91–98. doi:10.1016/j.neuroimage.2013.07.081
- Berlim, M.T., Van den Eynde, F., & Daskalakis, Z.J. (2013). Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: A systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Journal of Psychiatric Research, 47, 1–7. doi:10.1016/j.jpsychires.2012.09.025
- Britton, J.C., Lissek, S., Grillon, C., Norcross, M.A., & Pine, D.S. (2011). Development of anxiety: The role of threat appraisal and fear learning. Depression and Anxiety, 28, 5–17. doi:10.1002/da.20733
- Brosschot, J.F., Gerin, W., & Thayer, J.F. (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60, 113–124. doi:10.1016/j.jpsychores.2005.06.074
- Brunoni, A.R., Moffa, A.H., Fregni, F., Palm, U., Padberg, F., Blumberger, D.M., … Loo, C.K. (2016). Transcranial direct current stimulation for acute major depressive episodes: Meta-analysis of individual patient data. British Journal of Psychiatry, 208, 522–531. doi:10.1192/bjp.bp.115.164715
- Brunoni, A.R., Vanderhasselt, M.A., Boggio, P.S., Fregni, F., Dantas, E.M., Mill, J.G., … Bensenor, I.M. (2013). Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology, 38, 58–66. doi:10.1016/j.psyneuen.2012.04.020
- Carnevali, L., Koenig, J., Sgoifo, A., & Ottaviani, C. (2018). Autonomic and brain morphological predictors of stress resilience. Frontiers in Neuroscience, 12, 228. doi:10.3389/fnins.2018.00228
- Carnevali, L., Thayer, J.F., Brosschot, J.F., & Ottaviani, C. (2018). Heart rate variability mediates the link between rumination and depressive symptoms: A longitudinal study. International Journal of Psychophysiology, 131, 131–138. doi:10.1016/j.ijpsycho.2017.11.002
- Cerqueira, J.J., Almeida, O.F., & Sousa, N. (2008). The stressed prefrontal cortex. Left? Right! Brain, Behavior, and Immunity, 22, 630–638. doi:10.1016/j.bbi.2008.01.005
- Chida, Y., & Steptoe, A. (2009). Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology, 80, 265–278. doi:10.1016/j.biopsycho.2008.10.004
- Craig, A.D. (2005). Forebrain emotional asymmetry: A neuroanatomical basis? Trends in Cognitive Sciences, 9, 566–571. doi:10.1016/j.tics.2005.10.005
- Datta, A., Bansal, V., Diaz, J., Patel, J., Reato, D., & Bikson, M. (2009). Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation, 2, 201–207, 207.e1. doi:10.1016/j.brs.2009.03.005
- Davidson, R.J., Lewis, D.A., Alloy, L.B., Amaral, D.G., Bush, G., Cohen, J.D., … Peterson, B.S. (2002). Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry, 52, 478–502. doi:10.1016/S0006-3223(02)01458-0
- Drevets, W.C., Price, J.L., & Furey, M.L. (2008). Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Structure and Function, 213, 93–118. doi:10.1007/s00429-008-0189-x
- Duschek, S., Muckenthaler, M., Werner, N., & del Paso, G.A. (2009). Relationships between features of autonomic cardiovascular control and cognitive performance. Biological Psychology, 81, 110–117. doi:10.1016/j.biopsycho.2009.03.003
- Fornari, M., Carnevali, L., & Sgoifo, A. (2017). Single osteopathic manipulative therapy session dampens acute autonomic and neuroendocrine responses to mental stress in healthy male participants. The Journal of the American Osteopathic Association, 117, 559–567. doi:10.7556/jaoa.2017.110
- Gianaros, P.J., & Wager, T.D. (2015). Brain–body pathways linking psychological stress and physical health. Current Directions in Psychological Science, 24, 313–321. doi:10.1177/0963721415581476
- Gilabert-Juan, J., Castillo-Gomez, E., Guirado, R., Molto, M.D., & Nacher, J. (2013). Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Structure and Function, 218, 1591–1605. doi:10.1007/s00429-012-0479-1
- Gillie, B.L., & Thayer, J.F. (2014). Individual differences in resting heart rate variability and cognitive control in posttraumatic stress disorder. Frontiers in Psychology, 5, 758.
- Hansel, A., & von Kanel, R. (2008). The ventro-medial prefrontal cortex: A major link between the autonomic nervous system, regulation of emotion, and stress reactivity? BioPsychoSocial Medicine, 2, 21. doi:10.1186/1751-0759-2-21
- Hansen, A.L., Carstensen, B., Helge, J.W., Johansen, N.B., Gram, B., Christiansen, J.S., … Witte, D.R. (2013). Combined heart rate- and accelerometer-assessed physical activity energy expenditure and associations with glucose homeostasis markers in a population at high risk of developing diabetes: The ADDITION-PRO study. Diabetes Care, 36, 3062–3069. doi:10.2337/dc12-2671
- Herman, J.P., Figueiredo, H., Mueller, N.K., Ulrich-Lai, Y., Ostrander, M.M., Choi, D.C., & Cullinan, W.E. (2003). Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology, 24, 151–180. doi:10.1016/j.yfrne.2003.07.001
- Hermann, R., Biallas, B., Predel, H.G., & Petrowski, K. (2019). Physical versus psychosocial stress: Effects on hormonal, autonomic, and psychological parameters in healthy young men. Stress, 22, 103–112.
- Hill, L.K., & Siebenbrock, A. (2009). Are all measures created equal? Heart rate variability and respiration – biomed 2009. Biomedical Sciences Instrumentation, 45, 71–76.
- Kirschbaum, C., Pirke, K.M., & Hellhammer, D.H. (1993). The 'Trier Social Stress Test' – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. doi:10.1159/000119004
- Laborde, S., Mosley, E., & Thayer, J.F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research – Recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213.
- Liebetanz, D., Nitsche, M.A., Tergau, F., & Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125, 2238–2247. doi:10.1093/brain/awf238
- Luft, C.D., Takase, E., & Darby, D. (2009). Heart rate variability and cognitive function: Effects of physical effort. Biological Psychology, 82, 164–168. doi:10.1016/j.biopsycho.2009.07.007
- Makovac, E., Meeten, F., Watson, D.R., Herman, A., Garfinkel, S.N., D. Critchley, H., & Ottaviani, C. (2016). Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biological Psychiatry, 80, 786–795. doi:10.1016/j.biopsych.2015.10.013
- Makovac, E., Thayer, J.F., & Ottaviani, C. (2017). A meta-analysis of non-invasive brain stimulation and autonomic functioning: Implications for brain–heart pathways to cardiovascular disease. Neuroscience & Biobehavioral Reviews, 74, 330–341. doi:10.1016/j.neubiorev.2016.05.001
- McEwen, B.S., & Morrison, J.H. (2013). The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron, 79, 16–29. doi:10.1016/j.neuron.2013.06.028
- Menon, V., Mackenzie, K., Rivera, S.M., & Reiss, A.L. (2002). Prefrontal cortex involvement in processing incorrect arithmetic equations: Evidence from event-related fMRI. Human Brain Mapping, 16, 119–130. doi:10.1002/hbm.10035
- Montano, N., Ruscone, T.G., Porta, A., Lombardi, F., Pagani, M., & Malliani, A. (1994). Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation, 90, 1826–1831. doi:10.1161/01.CIR.90.4.1826
- Myers, B. (2017). Corticolimbic regulation of cardiovascular responses to stress. Physiology & Behavior, 172, 49–59. doi:10.1016/j.physbeh.2016.10.015
- Nikolin, S., Boonstra, T.W., Loo, C.K., & Martin, D. (2017). Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PLoS One, 12, e0181833. doi:10.1371/journal.pone.0181833
- Nitsche, M.A., Niehaus, L., Hoffmann, K.T., Hengst, S., Liebetanz, D., Paulus, W., & Meyer, B.U. (2004). MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clinical Neurophysiology, 115, 2419–2423. doi:10.1016/j.clinph.2004.05.001
- Orlov, N.D., O’Daly, O., Tracy, D.K., Daniju, Y., Hodsoll, J., Valdearenas, L., … Shergill, S.S. (2017). Stimulating thought: A functional MRI study of transcranial direct current stimulation in schizophrenia. Brain, 140, 2490–2497. doi:10.1093/brain/awx170
- Ottaviani, C., Zingaretti, P., Petta, A.M., Antonucci, G., Thayer, J.F., & Spitoni, G.F. (2018). Resting heart rate variability predicts inhibitory control above and beyond impulsivity. Journal of Psychophysiology, 1. doi:10.1027/0269-8803/a000222
- Priori, A., Berardelli, A., Rona, S., Accornero, N., & Manfredi, M. (1998). Polarization of the human motor cortex through the scalp. Neuroreport, 9, 2257–2260. doi:10.1097/00001756-199807130-00020
- Radloff, L.S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi:10.1177/014662167700100306
- Remue, J., Vanderhasselt, M.A., Baeken, C., Rossi, V., Tullo, J., & De Raedt, R. (2016). The effect of a single HF-rTMS session over the left DLPFC on the physiological stress response as measured by heart rate variability. Neuropsychology, 30, 756–766. doi:10.1037/neu0000255
- Rosenbaum, D., Hilsendegen, P., Thomas, M., Haeussinger, F.B., Metzger, F.G., Nuerk, H.C., … Ehlis, A.C. (2018). Cortical hemodynamic changes during the Trier Social Stress Test: An fNIRS study. Neuroimage, 171, 107–115. doi:10.1016/j.neuroimage.2017.12.061
- Sarkar, A., Dowker, A., & Cohen Kadosh, R. (2014). Cognitive enhancement or cognitive cost: Trait-specific outcomes of brain stimulation in the case of mathematics anxiety. The Journal of Neuroscience, 34, 16605–16610. doi:10.1523/JNEUROSCI.3129-14.2014
- Sgoifo, A., Carnevali, L., Alfonso, M.D.L.A.P., & Amore, M. (2015). Autonomic dysfunction and heart rate variability in depression. Stress (Amsterdam, Netherlands), 18, 343–352. doi:10.3109/10253890.2015.1045868
- Shin, L.M., Wright, C.I., Cannistraro, P.A., Wedig, M.M., McMullin, K., Martis, B., … Rauch, S.L. (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry, 62, 273–281. doi:10.1001/archpsyc.62.3.273
- Shoemaker, J.K., & Goswami, R. (2015). Forebrain neurocircuitry associated with human reflex cardiovascular control. Frontiers in Physiology, 6, 240.
- Snyder, H.R. (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. doi:10.1037/a0028727
- Spielberg, C.D., Gorsuch, R.L., Lushene, R., Vagg, P.R., & Jacobs, G.A. 1983. Manual for the state-trait inventory. Palo Alto, CA: Consulting Psychologist Press.
- Stalder, T., Kirschbaum, C., Kudielka, B.M., Adam, E.K., Pruessner, J.C., Wüst, S., … Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. doi:10.1016/j.psyneuen.2015.10.010
- Takano, Y., Yokawa, T., Masuda, A., Niimi, J., Tanaka, S., & Hironaka, N. (2011). A rat model for measuring the effectiveness of transcranial direct current stimulation using fMRI. Neuroscience Letters, 491, 40–43. doi:10.1016/j.neulet.2011.01.004
- Thayer, J.F., Åhs, F., Fredrikson, M., Sollers, J.J., & Wager, T.D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36, 747–756. doi:10.1016/j.neubiorev.2011.11.009
- Thayer, J.F., & Lane, R.D. (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33, 81–88. doi:10.1016/j.neubiorev.2008.08.004
- Treiber, F.A., Kamarck, T., Schneiderman, N., Sheffield, D., Kapuku, G., & Taylor, T. (2003). Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine, 65, 46–62. doi:10.1097/00006842-200301000-00007