Abstract
Preclinical and clinical findings indicate that glucocorticoids (GC) induce lipid accumulation in visceral depots, while inhibiting lipid stores from subcutaneous depots. Whereas some suggest that this is due to adipose depot specific concentration of glucocorticoid receptors (GR) or 11beta-hydroxysteroid dehydrogenase 1 (11β-HSD1), others demonstrate these events emerge from increases in interleukin-1 beta (IL-1β) from macrophages within distinct depots. Regardless of the mechanisms, most of these studies occur in males and thus lack evaluation of sex differences. Here, we examined the impact of 2-week corticosterone (CORT) (3 mg/kg/day) or saline treatment on GR, 11β-HSD1 and IL-1β protein concentration in intra-abdominal (epididymal/parametrial, and visceral) and subcutaneous (inguinal) depots in male and female Sprague Dawley rats. The objective was to examine if factors that regulate GC-induced adipose depot metabolism and distribution, differ between males and females. CORT inhibited, but did not decrease, body weight gain in both sexes. 11β-HSD1 was similar between the sexes in all adipose depots. CORT increased IL-1β in both sexes only in gonadal adipose tissue. Overall, males had greater GR protein concentration in all adipose depots, whereas females had more IL-1β in intra-abdominal adipose depots. Given the male-biased increase in intra-abdominal GR protein concentration, the data suggest that males may be more prone to CORT-induced increases in visceral obesity, which may have implications for increased risk for metabolic diseases. Overall, the data suggest that the effects of GC signaling in adipose tissue are multifaceted, dependent on sex, and the inherent adipocyte characteristics.
Research supports that glucocorticoids (GC) induce visceral adipose tissue accumulation, however few studies have examined if these GC-mediated outcomes are similar between males and females. This study investigates if female rats differentially respond to corticosterone treatment. Results indicate that male rats may have an increased susceptibility to CORT-induced accumulation of visceral adipose tissue compared with females, which may have implication for sex-specific risk for metabolic diseases.
Lay summary
Introduction
Obesity and associated metabolic co-morbidities such as cardiovascular disease, insulin resistance, and type 2 diabetes are a worldwide public health problem (Hill, Solt, & Foster, Citation2018). Increases in circulating glucocorticoids (GC) associated with psychosocial stressors (Bjorntorp, Citation2001) or synthetic treatment (John, Marino, Sanchez, & Hinds, Citation2016) are specifically linked to deleterious intra-abdominal (visceral) adipose tissue deposition. This is especially concerning because visceral adiposity is highly associated with disease risk (Hill et al., Citation2018), while subcutaneous adipose tissue deposition is protective against metabolic dysregulation (White & Tchoukalova, Citation2014). Although the contribution of GCs in the development of visceral adipose tissue accumulation has been vastly investigated (Lee, Pramyothin, Karastergiou, & Fried, Citation2014), few studies have examined the impact of GCs on adipose tissue protein concentration in both sexes. In the current study, we examined whether established factors that contribute to GC-induced lipid shunting in male rats are similar or different in females.
Materials and methods
Animals
Adult male (n = 14) and female (n = 14) Sprague Dawley rats (∼12 weeks of age) (Harlan Laboratories, Indianapolis, IN; 250–275 g) were housed in standard rat shoebox cages and acclimated for 1 week prior to the study. Rats were maintained in a temperature and humidity-controlled environment, on a 12:12 light/dark cycle with ad libitum access to food and water for the duration of the 2-week study. All experimental manipulations were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Glucocorticoid treatment
Adrenal intact adult male and female rats were divided into control (CTRL) and corticosterone (CORT) treated groups (female CTRL (n = 7), female CORT (n = 7), male CTRL (n = 7), and male CORT (n = 7)). Common methods of CORT delivery include subcutaneous pellets, drinking water or subcutaneous injection (Akana, Cascio, Shinsako, & Dallman, Citation1985; Akana, Jacobson, Cascio, Shinsako, & Dallman, Citation1988; Zhang, Packard, Tauchi, D'Alessio, & Herman, Citation2009). In this study rats daily received one subcutaneous injection of corticosterone (3 mg/kg/day), or saline injection for two weeks. This method was chosen to eliminate the variability of CORT ingestion associated with differential water intake as well as chronic release of CORT from pellets. The dose was chosen based on previous studies (Liston & Gan, Citation2011; Piazza et al., Citation1991; Wentworth-Eidsaune, Hennessy, & Claflin, Citation2016) because it is considered a relatively lower dose that mimics a mild stressor and does not induce pharmacologically elevated levels of corticosterone. Injections were given at endogenous CORT nadir (Lights ON).
Terminal measures
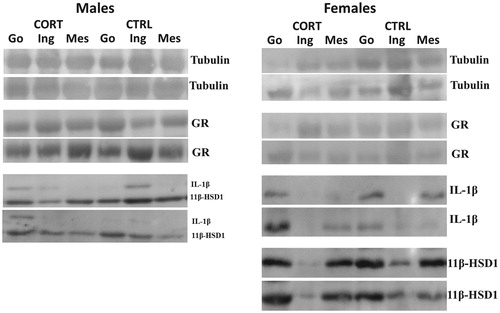
At the end of the 2-week study, rats were terminated via rapid decapitation. Gonadal (epididymal in males, and parametrial in females), visceral (mesenteric) and subcutaneous (inguinal) depots were collected for protein analysis. Western blot antibodies included: glucocorticoid receptor (FiGR) [SC-12763] mouse-monoclonal (1:200, Santa Cruz Biotechnology; Santa Cruz, CA); 11β-hydroxysteroid dehydrogenase 1 [ab39364] rabbit-polyclonal (1:200, Abcam; Cambridge, UK); interleukin-1β [ab9722] rabbit-polyclonal (1:500, Abcam; Cambridge, UK); and α-tubulin (B-7) [sc-5286] mouse-monoclonal (1:500, Santa Cruz Biotechnology; Santa Cruz, CA). Images were made using gel-based western blot chemiluminescence and ImageJ software was used for density quantification.
Statistics
Statistical analyses were performed in SPSS version 25. All data are presented as means ± SEM, and statistical significance was established at p ≤ .05. Protein concentration data were analyzed by one-way ANOVA and Tukey’s HSD Post Hoc test. Body weights over time were compared using repeated measures ANOVA, with the multivariate Wilks’ Lambda method used for denoting significant differences between groups (i.e. sex or drug treatment).
Results
Body mass
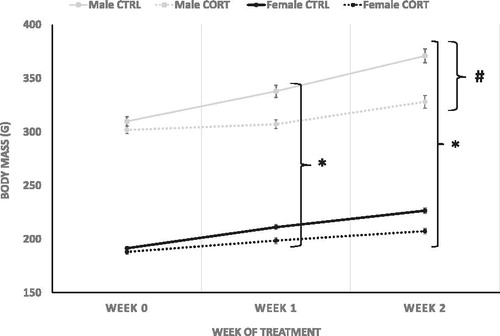
Total body weight (g) was measured prior to treatment (week 0), and post-treatment (weeks 1 and 2; ). Overall, males were significantly larger than females at all time points (week 0 F (1,40)=1537.95, p = .0001) (week 2; df = 1, F (1,40)=892.39, p = .0001) (week 3, F (1,40)=758.52, p = .0001). CORT treatment attenuated weight gain following a week of treatment (week 2; F (1,40)=30.213, p = .0001) and continued through the second week of treatment (week 3 F (1,40)=41.308, p = .0001). Attenuation of weight gain was greater in males than females at week 2 (Treatment * Sex Interaction: week 2; F (1,40)=5.381, p = .026) and 3 (Treatment * Sex Interaction: week 3 F (1,40)=6.10, p = .018).
Protein concentration
Steroid treatment is reported to reduce subcutaneous adipose depot stores while enhancing visceral depot accumulation (John et al., Citation2016). The impact of CORT on Glucocorticoid receptor (GR), 11beta-hydroxysteroid dehydrogenase 1 (11β-HSD1), and interleukin-1 beta (IL-1β) protein concentration was assessed in intra-abdominal (gonadal-(epididymal/parametrial) and visceral)) and subcutaneous (inguinal) depots because of their distinct roles in adiposity regulation. contains all representative western blots images for proteins measured.
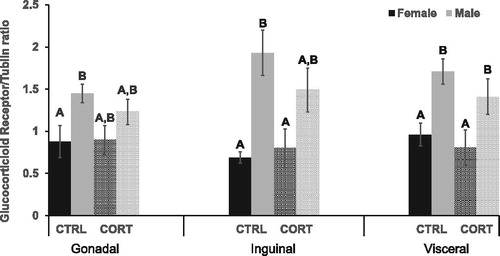
GR concentration in two of the three adipose depots differed between experimental groups (Inguinal: F (3,16)=5.680, p = .010) (Visceral: F (3,22)=5.459, p = .007) (). Specifically, post hoc analysis revealed CRTL males had significantly greater GR in the inguinal (p = .003) and visceral (p = .008) depots compare with females. Similarly, in CORT treated rats GR concentration was higher in the inguinal depot, and significantly higher in visceral depot (p = .020) of males compared with females. CORT treatment, however, did not alter GR protein.
Figure 2. Glucocorticoid Receptor. CRTL males had significantly greater GR in gonadal (p = .032), inguinal (p = .003) and visceral (p = .008) adipose depots compared with CRTL females. GR concentration was significantly higher in the visceral depot (p = .021) of CORT treated males compared with CORT treated females.
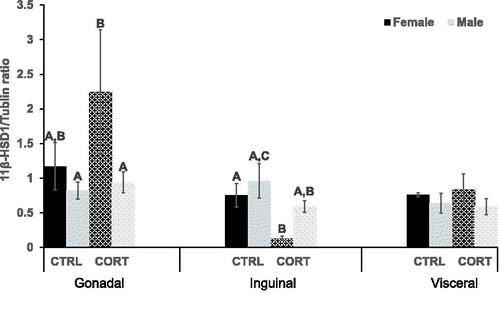
11β-HSD1 concentration was only different in the inguinal adipose depot between groups (F (3,17)=3.178, p = .05) (). Specifically, post hoc analysis revealed that CORT decreased 11β-HSD1 in the inguinal depot of female (p = .042), but not male, mice. CORT did not alter 11β-HSD1 concentration in the abdominal depots.
Figure 3. 11 beta-hydroxysteroid dehydrogenase 1. 11β-HSD1 was significantly higher (p = .028), in the gonadal depot of CORT female rats compared with CORT males. CORT treatment decreased 11β-HSD1 In the inguinal depot of females only (p = .042).
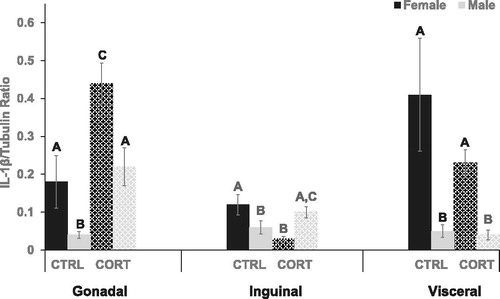
IL-1β concentration within the intra-abdominal depots was significantly different between experimental groups (Gonadal: F (3,14)=12.10, p = .001), (Visceral: F (3,19)=5.037, p = .012) (). First, post-hoc analysis revealed that gonadal depots of both CTRL (p = .05) and CORT (p = .004) females had a significantly higher (∼2-8x) IL-1β concentration than respective group males. Increased IL-1β was additionally observed in the visceral depot of females compared with males ((CTRL females vs. CTRL males; p = .004) (CORT females vs. CORT males; p = .01)). IL-1β concentration was also significantly different among groups in the inguinal (subcutaneous) depot (F (3,14)=13.215, p = .001). Specifically, IL-1β was ∼2X higher in CRTL females compared with males (p = .05). CORT treatment significantly increased IL-1β concentration within the gonadal depot of female (p = .003) and males (p = .008). In opposition, CORT treatment reduced IL-1β in the inguinal depot of female rats only (p = .005) compared with respective controls. CORT treatment did not change IL-1β concentration in the visceral adipose depots.
Figure 4. Interlukin-1 beta. Compared with male rats, IL-1β was significantly higher in the gonadal depot of females ((CTRL females vs. CTRL males; p = .05) (CORT females vs. CORT males; p = .004)). IL-1β was also significantly higher in the visceral depot of female rats compared with males ((CTRL females vs. CTRL males; p = .004) (CORT females vs. CORT males; p = .01)). CTRL female rats had significantly higher IL-1β, ∼2X, in the inguinal depot compared with males (p = .05). CORT treatment significantly increased IL-1β concentration in the gonadal depot of female (p = .003) and male (p = .008) rats, but CORT reduced IL-1β in the inguinal depot of female rats only (CORT vs. CTRL: p = .005).
Discussion
Glucocorticoid treatment in rodents causes adipose tissue redistribution to intra-abdominal adipose depots, including the gonadal (Campbell, Peckett, D'Souza A, Hawke, & Riddell, Citation2011; Yan et al., Citation2016) and visceral depot (Chimin et al., Citation2014), despite significant decreases in overall body mass. Here, we examined factors proposed to play a contributory role in CORT-induced redistribution of adipose tissue stores from subcutaneous to central adipose depots. Males and females were investigated to determine if CORT-induced adiposity alterations were different between sexes. We demonstrated that males have a greater concentration of GR within all adipose depots, whereas females have more IL-1β in intra-abdominal adipose depots compared to males. 11β-HSD1 concentration, however, was similar between the sexes. Gonadal adipose depot IL-1β was the only protein increased with CORT treatment.
In rats, adipose tissue GC signaling/action is proposed to be primarily mediated through the Type II GR (Lee & Fried, Citation2014) which regulates adipocyte differentiation/adipogenesis, lipogenesis, lipolysis, endocrine functions and inflammation (Lee et al., Citation2014). Central adipose tissue expansion is highly associated with stress because intra-abdominal adipocytes have been characterized to have more GR than subcutaneous depots (Lee et al., Citation2014). Although GCs are characterized to induce both lipogenesis and lipolysis, research supports that GCs stimulate triglyceride synthesis and lipoprotein lipase activity within intra-abdominal, but not subcutaneous adipose depots (Lee et al., Citation2014). This would result in hypertrophy of abdominal adipocytes when systemic production or local conversion of GC is increased. Here, we demonstrate that exogenous corticosterone does not change adipose depot GR, however adipocyte GR is higher in all adipose depots of male rats compared to females. This indicates that male rats may have a greater susceptibility toward CORT-induced adipose tissue redistribution. We postulate that much like human adipocytes that GR activation in intra-abdominal depots of male rats drives lipogenesis, whereas in the inguinal depot it stimulates lipolysis. Taken together this highlights that complexity of GC actions within distinct adipose depots, to both stimulate lipolysis or lipogenesis.
The enzyme 11β-HSD1 converts inactive 11-dehydrocorticosterone to active corticosterone within tissues (Shimojo, Whorwood, & Stewart, Citation1996). Rodents with adipose tissue specific overexpression of 11β-HSD1 have elevated corticosterone, visceral obesity, insulin resistance and hyperlipidemia (Peng et al., Citation2016). Exogenously administered CORT exacerbates GC signaling, in part, by stimulation of 11β-HSD1 expression and activity, further fueling GC excess (Morgan et al., Citation2014). In females only, CORT decreased 11β-HSD1 in the inguinal depot. These changes may support a sex-specific effect of CORT on fat distribution.
Last, IL-1β is emerging as a factor in GC-induced adipose tissue redistribution. Innate immune cells (i.e. macrophages and dendritic cells) within adipose tissue produce IL-1β, which becomes active with adipocyte factors (Ballak, Stienstra, Tack, Dinarello, & van Diepen, Citation2015). IL-1β is an inflammatory factor that also stimulates lipolysis and is postulated to contribute to the provision of oxidative fuels during stress (Speaker & Fleshner, Citation2012). Indeed, it is thought that increases in subcutaneous adipose tissue IL-1β contribute to CORT mediated redistribution of lipids to the visceral depot (Speaker & Fleshner, Citation2012). In male rats the stress of tail shock increases IL-1β in subcutaneous adipose tissue. The authors postulate that IL-1β shunts lipids to the intra-abdominal cavity because of subcutaneous depot dysfunction, lipolysis, and inhibited hyperplasia. Here we demonstrate that CORT treatment increased IL-1β in the gonadal, but not subcutaneous, depot of male and female rats. We extend this previous study by demonstrating sex differences in adipose tissue IL-1β. The intra-abdominal depots of female rats contain more IL-1β than males, indicating that these tissues likely have a greater susceptibility to stress-induced lipolysis, especially within the visceral depot where IL-1β is ∼5–8x higher in females than in males. Therefore, we predict that female rats may be less susceptible to glucocorticoid-induced adipose tissue redistribution and metabolic disease. In support of this, others demonstrate an interplay between GC and sex hormones, proposing they play a role in differential adipose tissue distribution, specifically visceral deposition, between sexes (Veilleux et al., Citation2012).
In conclusion, chronic GC production systemically or through local adipose tissue conversion is linked to decreases in subcutaneous adipose tissue and increases in central adiposity. These alterations contribute to stress-induced metabolic derangements, yet factors causing adipose redistribution remain unclear. Differences in GR number among distinct adipose depots are suggested to play a pivotal role in tissue responsiveness to steroids, yet outcomes of GC signaling within distinct depots is multifaceted and complex, as they are dependent upon the adipocyte and immune milieu. We further support that metabolic alterations associated with increasing GC is likely different between male and female rats, where females may have an attenuated response to CORT-induced adipose redistribution compared to males.
Disclosure statement
Authors do not have conflicts of interest to report.
Additional information
Funding
Notes on contributors
Jessica L. Hill
Jessica Hill L did lab procedures, prepared figures, ran statistics and wrote publication.
Matia B. Solomon
Matia B. Solomon contributed to study design, ran animal experiments, collected data, ran statistics, produced graphs and edited manuscript.
Elizabeth T. Nguyen
Elizabeth T. Nguyen ran animal experiments, collected data, ran statistics, produced graphs and edited manuscript.
Jody L. Caldwell
Jody J. Caldwell ran animal experiments and collected data.
Yuren Wei
Yuren Wei did laboratory procedures.
Michelle T. Foster
Michelle T. Foster contributed to study design, collected data, ran statistics, produced graphs and contributed to writing manuscript.
References
- Akana, S.F., Cascio, C.S., Shinsako, J., & Dallman, M.F. (1985). Corticosterone: narrow range required for normal body and thymus weight and ACTH. The American Journal of Physiology, 249, R527–532. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2998210. doi:10.1152/ajpregu.1985.249.5.R527
- Akana, S.F., Jacobson, L., Cascio, C.S., Shinsako, J., & Dallman, M.F. (1988). Constant corticosterone replacement normalizes basal adrenocorticotropin (ACTH) but permits sustained ACTH hypersecretion after stress in adrenalectomized rats. Endocrinology, 122, 1337–1342. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2831027. doi:10.1210/endo-122-4-1337
- Ballak, D.B., Stienstra, R., Tack, C.J., Dinarello, C.A., & van Diepen, J.A. (2015). IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine, 75, 280–290. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26194067. doi:10.1016/j.cyto.2015.05.005
- Bjorntorp, P. (2001). Do stress reactions cause abdominal obesity and comorbidities? Obesity Reviews, 2, 73–86. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12119665 doi:10.1046/j.1467-789x.2001.00027.x
- Campbell, J.E., Peckett, A.J., D'Souza, A. M., Hawke, T.J., & Riddell, M.C. (2011). Adipogenic and lipolytic effects of chronic glucocorticoid exposure. American Journal of Physiology-Cell Physiology, 300, C198–209. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20943959. doi:10.1152/ajpcell.00045.2010
- Chimin, P., Farias, T. D S. M., Torres-Leal, F.L., Bolsoni-Lopes, A., Campaña, A.B., Andreotti, S., & Lima, F.B. (2014). Chronic glucocorticoid treatment enhances lipogenic activity in visceral adipocytes of male Wistar rats. Acta Physiologica, 211, 409–420. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24410866. doi:10.1111/apha.12226
- Hill, J.H., Solt, C., & Foster, M.T. (2018). Obesity associated disease risk: the role of inherent differences and location of adipose depots. Hormone Molecular Biology and Clinical Investigation, 33, Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29547393. doi:10.1515/hmbci-2018-0012
- John, K., Marino, J.S., Sanchez, E.R., & Hinds, T.D. Jr. (2016). The glucocorticoid receptor: cause of or cure for obesity? American Journal of Physiology-Endocrinology and Metabolism, 310, E249–257. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26714851. doi:10.1152/ajpendo.00478.2015
- Lee, M.J., & Fried, S.K. (2014). The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. International Journal of Obesity, 38, 1228–1233. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24430397. doi:10.1038/ijo.2014.6
- Lee, M.J., Pramyothin, P., Karastergiou, K., & Fried, S.K. (2014). Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochimica et Biophysica Acta (Bba) - Molecular Basis of Disease, 1842, 473–481. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23735216. doi:10.1016/j.bbadis.2013.05.029
- Liston, C., & Gan, W.B. (2011). Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proceedings of the National Academy of Sciences USA, 108, 16074–16079. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21911374. doi:10.1073/pnas.1110444108
- Morgan, S.A., McCabe, E.L., Gathercole, L.L., Hassan-Smith, Z.K., Larner, D.P., Bujalska, I.J., … Lavery, G.G. (2014). 11beta-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proceedings of the National Academy of Sciences USA, 111, E2482–2491. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24889609. doi:10.1073/pnas.1323681111
- Peng, K., Pan, Y., Li, J., Khan, Z., Fan, M., Yin, H., … Zheng, C. (2016). 11beta-Hydroxysteroid Dehydrogenase Type 1(11beta-HSD1) mediates insulin resistance through JNK activation in adipocytes. Scientific Reports, 6, 37160. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27841334. doi:10.1038/srep37160
- Piazza, P.V., Maccari, S., Deminiere, J.M., Le Moal, M., Mormede, P., & Simon, H. (1991). Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proceedings of the National Academy of Sciences USA, 88, 2088–2092. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2006148. doi:10.1073/pnas.88.6.2088
- Shimojo, M., Whorwood, C.B., & Stewart, P.M. (1996). 11 beta-Hydroxysteroid dehydrogenase in the rat adrenal. Journal of Molecular Endocrinology, 17, 121–130. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8938587. doi:10.1677/jme.0.0170121
- Speaker, K.J., & Fleshner, M. (2012). Interleukin-1 beta: A potential link between stress and the development of visceral obesity. BMC Physiology, 12, 8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22738239. doi:10.1186/1472-6793-12-8
- Veilleux, A., Côté, J.-A., Blouin, K., Nadeau, M., Pelletier, M., Marceau, P., … Tchernof, A. (2012). Glucocorticoid-induced androgen inactivation by aldo-keto reductase 1C2 promotes adipogenesis in human preadipocytes. American Journal of Physiology-Endocrinology and Metabolism, 302, E941–949. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22275760. doi:10.1152/ajpendo.00069.2011
- Wentworth-Eidsaune, C.L., Hennessy, M.B., & Claflin, D.I. (2016). Short-term, high-dose administration of corticosterone by injection facilitates trace eyeblink conditioning in young male rats. Behavioural Brain Research, 298, 62–68. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26239002. doi:10.1016/j.bbr.2015.07.051
- White, U.A., & Tchoukalova, Y.D. (2014). Sex dimorphism and depot differences in adipose tissue function. Biochimica et Biophysica Acta (Bba) - Molecular Basis of Disease, 1842, 377–392. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23684841. doi:10.1016/j.bbadis.2013.05.006
- Yan, C., Yang, H., Wang, Y., Dong, Y., Yu, F., Wu, Y., … Liu, Y. (2016). Increased glycogen synthase kinase-3beta and hexose-6-phosphate dehydrogenase expression in adipose tissue may contribute to glucocorticoid-induced mouse visceral adiposity. International Journal of Obesity, 40, 1233–1241. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27102048. doi:10.1038/ijo.2016.57
- Zhang, R., Packard, B.A., Tauchi, M., D'Alessio, D.A., & Herman, J.P. (2009). Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proceedings of the National Academy of Sciences USA, 106, 5913–5918. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19307579. doi:10.1073/pnas.0808716106