Abstract
Successful shooting performance in competition is reliant on several factors such as shooting techniques and competition-associated psychological stresses. This study examined the hypothalamus-pituitary-adrenal (HPA) axis response to upcoming competition and its association with shooting outcomes in elite junior shooting players. The cortisol awakening response (CAR) and dehydroepiandrosterone (DHEA) secretion after awakening were measured for two consecutive days (the day before and on the day of competition for the selection of national shooting team members) in 19 junior men and 21 junior women players, and the shooting scores of the individual players were obtained. The total cortisol secretion during the CAR period (CARauc) increased, but total DHEA secretion during the post-awakening period (Daucawk) decreased on the day of competition, compared with one day before competition. The CARauc was higher in women than in men players, whereas Daucawk was higher in men than in women players across the two consecutive days. Cortisol and DHEA levels were comparable between low-scored (below the mean scores for air pistol or air rifle players) and high-scored players one day before competition. However, the CARauc on the day of competition was higher and the variations in the CARauc and molar CARauc/Daucawk ratios across the two consecutive days were greater in low-scored than in high-scored men and women players. These results indicated that upcoming competition involves alterations of the CAR and DHEA secretion after the awakening period, and greater HPA response to the upcoming competition was adversely associated with shooting scores in junior shooting athletes.
An important upcoming competition was perceived as a strong stressor on awakening that induced alteration in CAR and DHEA secretion after the awakening period in elite shooting players. This study observed that, irrespective of their age and period of shooting practice, the HPA axis function on the day of competition was associated with shooting outcomes in elite shooting players.
Lay summary
Introduction
Ten-meter air pistol and 10-meter air rifle are Olympic shooting events in which players try to hit a stationary target composed of 10 concentric scoring rings with a score grade from one to 10 from the outside ring to the center ring. Studies analyzing the mechanical factors of the shooting techniques identified that stability of hold, aiming accuracy, timing of triggering, and cleanness of triggering are the major determinants of the shooting scores in elite-level air pistol and rifle shooters (Hawkins, Citation2011; Ihalainen, Kuitunen, Mononen, & Linnamo, Citation2016a). Although technical factors are essentially required for successful shooting performance, the shooting scores in the competition are not entirely determined by the technical factors in elite-level athletes. For example, a recent longitudinal study observed that testing situation shooting scores and technical variables further improved with an increase in the years of intense shooting training even in elite-level air rifle shooters, but competition shooting scores did not significantly change with the duration of training (Ihalainen, Linnamo, Mononen, & Kuitunen, Citation2016b). A negative or inverted-U shaped association between shooting scores and pre-competition anxiety (Gould, Petlichkoff, Simons, & Vevera, Citation1987; Sade, Bar-Eli, Bresler, & Tenenbaum, Citation1990) suggests that psychological stress imposed by competition may have a negative effect on performance outcomes in elite shooters.
The hypothalamus-pituitary-adrenal (HPA) axis is the primary constituent of the neuroendocrine response to various stimuli, including anticipatory stress (Herman et al., Citation2003). A robust increase in cortisol levels within the first hour after waking, which is called the cortisol awakening response (CAR), is considered a reliable index of the HPA axis function (Adam & Kumari, Citation2009). The CAR is confounded not only by various types of perceived physical and psychological stress but also by upcoming anticipatory stress (Fries, Dettenborn, & Kirschbaum, Citation2009). Upcoming anticipatory stress can induce day-to-day variations in an individual’s CAR. Higher cortisol levels during the CAR period have been observed in circumstances that require additional demands such as the day of feeling more stress compared to less stressful days (Kunz-Ebrecht, Kirschbaum, Marmot, & Steptoe, Citation2004) and the day of a stressful event compared to a regular workday (Elder, Barclay, Wetherell, & Ellis, Citation2018; Wetherell, Lovell, & Smith, Citation2015; Wolfram, Bellingrath, Feuerhahn, & Kudielka, Citation2013).
The CAR has been shown to be associated with the HPA axis and brain functions of the same day in healthy subjects. For example, the total amount of cortisol released during the CAR period is positively correlated with the mean cortisol concentration throughout the following 12 hours (Edwards, Clow, Evans, & Hucklebridge, Citation2001). The magnitude of the CAR has been demonstrated to be associated with same-day functional connectivity of the prefrontal cortex with the other brain regions (Wu et al., Citation2015), synaptic plasticity of the motor cortex (Clow et al., Citation2014), and cognitive and executive functions such as leaning and performance memory (Hodyl et al., Citation2016; Moriarty et al., Citation2014) and executive function-related performance (Evans, Hucklebridge, Loveday, & Clow, Citation2012) in healthy subjects.
Along with cortisol, dehydroepiandrosterone (DHEA) is produced in the adrenal cortex in response to the adrenocorticotropic hormone (ACTH) and exhibits myriad physiological anti-glucocorticoid actions (Kalimi, Shafagoj, Loria, Padgett, & Regelson, Citation1994). The concentrations of DHEA in the blood oscillate in parallel with those of cortisol under ACTH stimulation and acute stress conditions (Arvat et al., Citation2000; Lennartsson, Kushnir, Bergquist, & Jonsdottir, Citation2012), but elevation in the cortisol to DHEA (C/D) ratio resulting from reduced DHEA concentrations with either no change or elevation in cortisol concentrations has been observed in subjects experiencing severe stress events, chronic stress, and medical illness (Kamin & Kertes, Citation2017). Longitudinal studies have also reported that healthy subjects have a higher C/D ratio during more stressful periods than less stressful periods (Warnock et al., Citation2010) and during workdays compared with non-workdays (Kim, Lee, & Ahn, Citation2010).
Given the HPA axis response to upcoming stress and the negative association between psychological stress imposed by competition and shooting scores, it is reasonable to hypothesize that the HPA axis response to upcoming competition may influence the shooting outcomes in competition. The present study examined differences in the HPA axis function, with a particular emphasis on CAR and DHEA secretion in the post-awakening period between the day before and on the day of competition and determined the association between the HPA axis function response and shooting outcomes in elite junior shooting players.
Subjects and methods
Subjects
The Korea Shooting Federation (KSF) selects new members (n = 16) of the national junior shooting team every year through four rounds of competition. To accomplish this, each of the high-ranked 12 junior men and women air pistol players and the same number of air rifle junior players are preselected as candidates (total number, 44) based on their outcomes during the previous year among the junior players registered in the KSF. A total of 3217 junior air pistol and air rifle players were registered in the KSF in 2017 (35.1% were junior women). The preselected players stayed at the national athletes’ village and trained for three weeks at the Taereung International Shooting Range. All of the preselected players participated in the shooting competition, which was held on the final day of 3 weeks of training. The scores and ranks were not given to the individual players until four rounds of competition were completed. The present study was carried out during the second round of the shooting competition.
A preselected 44 air pistol and rifle junior players were initially enrolled in the present study. All of the preselected players gave their consent to participate in the present study. This study was approved by the Institutional Review Board of Sahmyook University (2-1040781-AB-N-01-2017119HR). The locations where the players stayed and conducted shooting competitions were the same, and the daily schedules for the 3-week training and shooting competition were identical to those previously done. The participants were not taking any drugs or medication and had no history of endocrine disorders before or during this study.
Air pistol and air rifle shooting competition
The 10-m air pistol and air rifle shootings were performed at the Taereung International Shooting Range following the rules and restrictions of the International Shooting Sports Federation. The Taereung International Shooting Range has 90 shooting stations with Olympic standards. The players used validated equipment such as a pistol, rifle, pellet, clothing, and shoes. Air pistol and air rifle junior men players were allowed 75 min for 60 shots (maximum score, 600), and air pistol and air rifle junior women players were allowed 60 min for 40 shots (maximum score, 400). The shooting competition was started at 10.00 am, and all of the players started shooting at the same time as previously done.
Saliva collection
The players were familiarized with saliva sampling methods and procedures 1 or 2 days prior to the competition. Saliva samples were collected from each individual player during the post-awakening period (immediately upon awakening, 30 and 60 min after awakening) on two consecutive days (the day before and on the day of competition). The players fell asleep at 11.00 pm each night and were awakened by an alarm at 6.00 am when they stayed at the national athletes’ village. Twelve research assistants, six males and six females, supported the players in the collection of saliva samples to avoid delays in providing the first saliva sample after awakening.
The CAR is characterized by an increase in cortisol levels from 50% to 75% within the first 30 min after waking up in the morning (Pruessner et al., Citation1997). However, the collection of the first saliva sample after a delay of more than 10 min after awakening (that is, non-adherent subjects) is known to be a primary cause of failure to capture the typical CAR in healthy subjects (Okun et al., Citation2010). Because the measurement of the CAR critically relies on the times of sample collection, the use of electric devices to monitor the awakening and sample-collecting times is helpful to increase participant adherence and sampling accuracy (Stalder et al., Citation2016). The typical CAR is defined as an increase in cortisol levels to at least 2.5 nmol/L above an individual’s baseline (that is, CARi >2.5 nmol/L) in healthy subjects (Wust et al., Citation2000). An electric monitoring device was not used in the present study; therefore, a set of saliva samples with CARi less than 2.5 nmol/L was considered as the samples of non-adherent subjects and excluded from further analysis. The typical CAR was not observed in samples collected on one of two consecutive days in three junior men and one junior women player after hormone assay procedures. A set of data obtained from the player was excluded from this study. Finally, data obtained from 19 junior men and 21 junior women players were analyzed in the present study.
Measurement of salivary cortisol and DHEA
Salivary cortisol and DHEA concentrations were determined using a radioimmunoassay as described in our previous studies (Ahn, Lee, Choi, Kwon, & Chun, Citation2007; Kang et al., Citation2014). Cortisol antisera were purchased from Accurate Chemical & Scientific Co. (Westbury, NY, USA). DHEA antisera were purchased from United States Biological (Salem, MA, USA). Cortisol antiserum cross-reacts with cortisone, 11-deoxycortisol, prednisolone, cortisol-21-glucosiduronate, cortisol-21-sulphate, and other steroids, with cross-reaction levels of 1.0%, 8.9%, 31.6%, 1.3%, <0.01%, and <0.01%, respectively. DHEA antiserum cross-reacts with androstenedione, epiandrosterone, dihydrotestosterone, and other steroids, with cross-reaction levels of 0.4%, 0.2%, 1.3%, and <0.01%, respectively.
Routinely, standards, quality control materials, and saliva samples were assayed in duplicate. The inter-assay coefficients of variation (CV) as assessed by quality controls with mean cortisol concentrations of 3.6 and 10.9 nmol/L were 7.4% and 8.5%, respectively (n = 26). The analytical sensitivity for cortisol was 0.4 nmol/L. The inter-assay CVs as assessed from quality controls with mean DHEA concentrations of 159.0 and 795.0 pmol/L were 12.5% and 10.1%, respectively (n = 26). The analytical sensitivity for DHEA was 0.7 pmol/L. The intra-assay CVs for the same pool were less than 10% (n = 15) in both steroids.
Classification of players
The Kolmogorov-Smirnov test revealed that the air pistol and air rifle shooting scores of the men players and those of the women players had a normal distribution. The mean shooting scores for air pistol and air rifle (air pistol, 570.7; air rifle, 590.0) were adopted as the cutoff point to divide the junior men players into low-scored men players (five air pistol players and two air rifle players) and high-scored men players (five air pistol players with seven air rifle players). The mean shooting scores for air pistol and air rifle (air pistol, 377.5; air rifle, 595.9) were used as the cutoff point to dichotomize the women players into low-scored women players (four air pistol players with four air rifle players) and high-scored women players (seven air pistol players with six air rifle players).
Data analysis
Auxiliary indices were adapted to the present study to analyze the steroid secretory activity during the post-awakening period. The total cortisol and DHEA secretions during the post-awakening period were calculated as the area under the curve (AUC) with respect to ground from the time point immediately after awakening to 60 minutes after awakening (CARauc and Daucawk, respectively) (Clow, Hucklebridge, & Thorn, Citation2010). The net increases in cortisol levels within the first 30 minutes after awakening (CARi) were determined (Wust et al., Citation2000). The percent variations in CARauc, Daucawk, and molar CARauc/Daucawk ratio across two consecutive days were calculated, Δ% = [(x0 − xreference)/(xreference)]*100, where x0 was the value determined on the day of competition and xreference was the value determined the day before competition.
The Kolmogorov-Smirnov test showed that steroid concentrations and the cortisol to DHEA (C/D) ratios at each examined time point were not normally distributed. The values were therefore log-transformed to normalize the sample distributions for parametric statistic tests. The logarithm of the molar cortisol to DHEA ratio [log (cortisol/DHEA) = log (cortisol) - log (DHEA)] was calculated for each subject. Log-transformed data were used in the statistical analyses, but for reasons of interpretational clarity, untransformed means are presented in . A repeated measured two-way ANOVA and paired t-test were performed to compare the measurements obtained in two different conditions, and a two-tailed t-test or Mann-Whitney U tests were performed for comparisons of differences between player groups. These analyses were conducted separately for the males and females due to sex differences in the magnitude of the CAR and the levels of DHEA. Statistical calculations were performed using NCSS 11 statistical software (NCSS, Kaysville, Utah, USA). A p-value <.05 was considered significant.
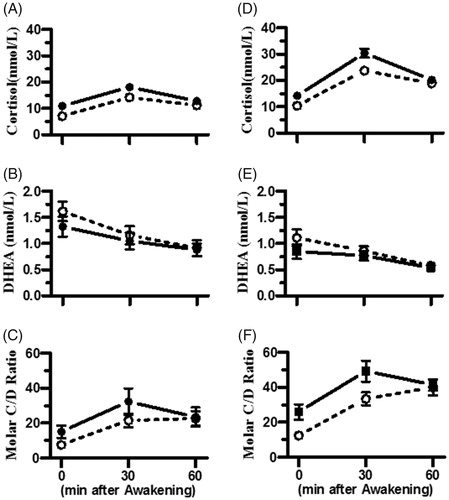
Figure 1. Cortisol, DHEA, and the molar cortisol/DHEA (C/D) ratios in the junior men and women players. Cortisol and DHEA concentrations were determined in saliva samples collected on two consecutive days (the day before and on the day of competition), and the molar C/D ratio was calculated for the individual players. The closed circle with a solid line and open circle with a dotted line in each figure represent the data collected the day before and on the day of competition, respectively. (A–C) represent the profiles of cortisol, DHEA, and molar C/D ratio in the junior men players, respectively, and (D–F) represent those values in the junior women players, respectively. Each data point represents the mean and standard error of the mean (SEM).
Results
Player characteristics
The players in the present study were composed of 40 junior shooting players (19 men and 21 women) with the following characteristics. The junior men players in the present study had a mean (range) age of 19.6 (18.5–21.5) years, height of 176.9 (170–186) cm, weight of 69.0 (57–82) kg, body mass index (BMI) of 22.1 (18.0–24.9) kg m−2, and period of shooting practice of 6.4 (4.5–8.5) years. The junior women players in the present study had a mean (range) age of 18.5 (17.1–20.5) years, height of 164.7 (156–173 cm), weight of 57.8 (53–77) kg, BMI of 21.9 (19.3–25.7) kg m−2, and period of shooting practice of 5.5 (3.5–7.5) years. The mean (range) shooting score was 570.7 (551–584) in the men players for 10-m air pistol, 590.9 (581–596) in the men players for 10-m air rifle, 377.5 (368–382) in the women players for 10-m air pistol and 395.9 (392–399) in the women players for 10-m air rifle.
Competition-related changes in the HPA axis function after the awakening period
shows the levels of cortisol and DHEA and the molar C/D ratio for two consecutive days (the day before and on the day of competition) in men and women players. Repeated measured two-way ANOVA revealed that the main effect of day on the levels of cortisol during the CAR period was significant in both the men (F1, 108 = 19.9, p < .001) and women players (F1, 120 = 15.1, p < .01) (, respectively). The levels of DHEA determined on the day of competition were lower than those determined the day before competition in both the men and women players, but the difference was not statistically significant (F1, 108 = 1.1, p > .05 for the men player; F1, 120 =2.3, p > .05 for the women players) (, respectively). The levels of the molar cortisol/DHEA (C/D) ratio determined on the day of competition were significantly higher than those determined the day before competition in both the men (F1, 108 = 6.2, p < .05) and women players (F1, 120 = 9.4, p < .01) (, respectively).
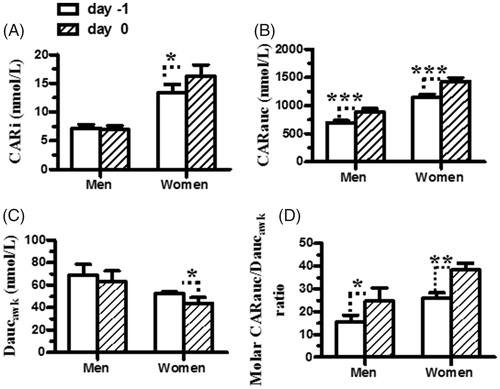
shows the differences in the auxiliary indices for cortisol and DHEA secretion between two consecutive days in the men and women players. The paired t-test revealed that the main effect of day on the CARi was significant in the women players (t = 2.3, df = 20, p < .05) but was not in the men players (t = 0.27, df = 18, p > .05) (. However, the main effect of day on CARauc, Daucawk, and the molar CARauc/Daucawk ratio was significant in both the men (all t’s >5.3, df = 18, all p’s <.01 by paired t-test) and women players (all t’s >6.6, df = 20, all p’s <.001 by paired t-test) (). There was a significant difference in HPA axis function between men and women players. The levels of CARi, CARauc, and molar CARauc/Daucawk ratio determined one day before and those determined on the day of competition were higher in the women players than in the men players (all t’s >3.8, df = 38, all p’s <.05 by two-tailed t-test) (), but the Daucawk levels determined on the day of competition were higher in the men players than the women players (t = 2.4, df = 38, p < .05 by two-tailed t-test) (. These results indicated that competitive event was perceived as a stressor on awakening, which resulted in changes in the HPA axis function, elevated cortisol secretion during the CAR period, and reduced DHEA secretion in the post-awakening period in both the men and women players.
Figure 2. The CARi, CARauc, Daucawk, and molar CARauc/Daucawk ratios of two consecutive days in the junior men and women players. Based on the determined cortisol and DHEA levels, the auxiliary indices (CARi, CARauc, Daucawk, and molar CARauc/Daucawk ratios) were calculated in the individual players. The open and closed bars represent the data obtained the day before and on the day of competition, respectively. The asterisks denote the levels of significance: *p < .05, **p < .01, and ***p < .0001. Each bar represents the mean and standard error of the mean (SEM).
The difference in HPA axis function between the low-scored and high-scored players
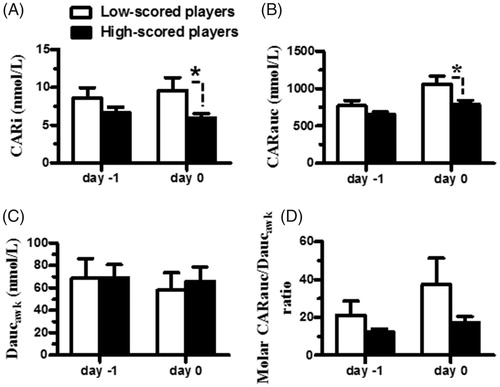
To determine whether the HPA axis function was associated with the shooting outcomes, the men players were divided into two player subgroups, as previously described, and the difference in the HPA axis function between the subgroups of the men players was determined (). The CARi, CARauc, Daucawk, and molar CARauc/Daucawk ratios determined on the day before competition were comparable between the subgroups of men players (all t’s < 0.17, df = 17, all p’s >.05 by two-tailed t-test) (). However, the CARi and CARauc determined on the day of competition were significantly higher in the low-scored than in the high-scored players (all t’s >2.6, df = 17, all p’s <.05 by two-tailed t-test) (. The Daucawk and the molar CARauc/Daucawk ratios determined on the day of competition were comparable between the subgroups of men players (all t’s <2.0, df = 17, all p’s >.05 by two-tailed t-test) (.
Figure 3. The difference in HPA axis function after the awakening period between the low-scored and high-scored junior men players. They were categorized into two subgroups based on their shooting scores: low-scored (score < mean values of the group scores, open bar) and high-scored (score > mean values of the group scores, closed bar) players; the differences in the CARi (A), CARauc (B), Daucawk (C), and molar CARauc/Daucawk ratios (D) between the low- and high-scored players were compared. The asterisk denotes the levels of significance: *p < 0.05. Each bar represents the mean and standard error of the mean (SEM).
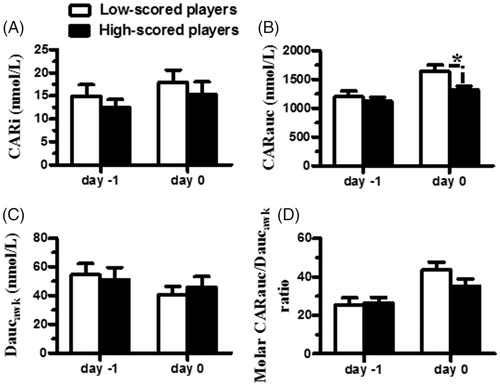
shows the difference in the HPA axis function between the subgroups of women players. The CARi, CARauc, Daucawk, and molar CARauc/Daucawk ratios determined on the day before competition were comparable between the subgroups of women players (all t’s <0.5, df = 19, all p’s >.05 by two-tailed t-test) (). The CARauc determined on the day of competition was significantly higher in the low-scored than in the high-scored women players (t = 2.3, df = 19, p < .05 by two-tailed t-test) (), but the CARi, Daucawk, and molar CARauc/Daucawk ratios determined on the day of competition were comparable between the subgroups of women players (all t’s <1.2, df = 19, all p’s >.05 by two-tailed t-test) (). These results indicated there was no difference in HPA axis function between the low- and high-scored players on the day before competition, but low-scored players had higher CAR on the day of competition than the high-scored players in both the men and women players.
Figure 4. The difference in the HPA axis function after the awakening period between the low-scored and high-scored junior women players. The women players were categorized into two subgroups based on their shooting scores: low-scored (score < mean values of the group scores, open bar) and high-scored (score > mean values of the group scores, closed bar) players; the differences in the CARi (A), CARauc (B), Daucawk (C), and molar CARauc/Daucawk ratios (D) between the low- and high-scored players were compared. The asterisk denotes the levels of significance: *p < .05. Each bar represents the mean and standard error of the mean (SEM).
Meanwhile, the mean (SD) values for age and period of shooting practice in the low-scored men players [19.4 (0.5) years and 6.4 (1.1) years, respectively] were comparable to those in the high-scored men players [19.8 (1.0) years and 6.4 (0.9) years, respectively] (all t’s <0.6, df = 17, all p’s >.05 by two-tailed t-test). The mean (SD) values for age and period of shooting practice for the low-scored women players [18.3 (0.7) years and 5.3 (1.0) years, respectively] were also comparable to those for the high-scored men players [18.5 (1.2) years and 5.7 (1.0) years, respectively] (all t’s <1.5, df = 19, all p’s >.05 by two-tailed t-test). There was no difference in the examined physical variables (that is, height, body weight, and BMI) between the low-scored and high-scored players in both the men (all t’s < 0.6, df = 17, all p’s >.05) and women (all t’s <1.5, df = 19, all p’s >.05 by two-tailed t-test (data not shown).
The difference in variations of HPA axis function across two consecutive days between the low- and high-scored players
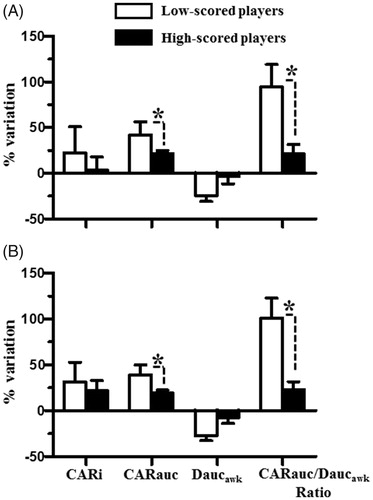
shows the percent variations in the cortisol and DHEA secretory activities across the two consecutive days in the low-scored and high-scored players. In the men players, the percent variations CARi and Daucawk across the two consecutive days in the low-scored players were similar to those observed in the high-scored players (all U’s >20, all p’s >.05 by Mann-Whitney U tests) (. However, the percent variations in the CARauc and molar CARauc/Daucawk ratios across the two consecutive days was greater in the low-scored players than in the high-scored players (all U’s <13, all p’s <.05 by Mann-Whitney U tests) (. The same results were observed in the women players; the variations in the CARi and Daucawk across the two consecutive days were comparable between the low- and high-scored players (all U’s > 25, all p’s >.05 by Mann-Whitney U tests) (), but the variations in the CARauc and molar CARauc/Daucawk ratios across the two consecutive days were greater in the low-scored players than in the high-scored players (all U’s < 17, all p’s <.05 by Mann–Whitney U tests) (). These results indicated that the low-scored players had a much greater difference in their cortisol and DHEA secretions after the awakening period between the two consecutive days compared to the high-scored players.
Figure 5. The difference in the percent variation between the low- and high-scored players. The players were categorized into two subgroups based on their shooting scores: low-scored (score < mean values of the group scores, open bar) and high-scored (score > mean values of the group scores, closed bar) players. The percent variations in the CARi, CARauc, Daucawk, and molar CARauc/Daucawk ratios across two consecutive days were calculated in the individual players. (A) represents the differences in the percent variations in the CARi, CARauc, Daucawk, and molar CARauc/Daucawk ratios between the low- and high-scored men players and (B) represents the differences in the percent variations of that between the low- and high-scored women players. The asterisk denotes the levels of significance: *p < .05. Each bar represents the mean and standard error of the mean (SEM).
Discussion
The present study examined the CAR and DHEA secretion after the awakening period with regard to an upcoming competition among elite junior shooting players. We observed that the effect of the competition day on the CAR and molar C/D ratios was significant in the elite junior shooting players, which was considered as the HPA axis response to the upcoming competition during the post-awakening period. The findings of the present study were three-fold: (1) an increase in the CARauc and a decrease in the Daucawk on the day of competition compared to the day before competition, which resulted in an increase in the molar C/D ratios in the post-awakening period in both the men and women players (2) higher CARauc levels on the day of competition in the low-scored players than in the high-scored players (3) greater variations in the CARauc and molar C/D ratios across the two consecutive days (one day before and on the day of competition) in the low-scored players than in the high-scored players.
The CAR is characterized by intra-individual stability across days (Wust et al., Citation2000), but the stability of the CAR is susceptible to short-term changes in the state of arousal (Thorn, Hucklebridge, Evans, & Clow, Citation2009) and anticipation of the demands of the respective day (Fries et al., Citation2009). We observed in the present study that the levels of cortisol during the CAR period were higher on the day of competition than on the day before competition in the shooting players, and were higher in the women players than in the men players. The results of the present study were similar to those reported in previous studies in that there was an increment of the CAR in response to an upcoming swimming competition (Meggs et al., Citation2016) and elevation of cortisol during the CAR period on the day of a tennis tournament (Filaire, Alix, Ferrand, & Verger, Citation2009). However, the results of the present study were different from other studies that observed no change in the CAR in response to an upcoming professional triathlon event (Balthazar, Garcia, & Spadari-Bratfisch, Citation2012), martial arts competition (Strahler, Ehrlenspiel, Heene, & Brand, Citation2010), or professional swimming contest (Diaz et al., Citation2013). Strahler et al. (Citation2010) suggesting that no changes in the CAR may be due to some degree of endocrine habituation in high-level athletes. Diaz et al. (Citation2013) suggested that frequent participation in higher-level competitions might contribute to no differences in the CAR between competition and control days. A review study of anticipatory cortisol responses to competition showed that cortisol was also increased several minutes (180-0 minutes) prior to competition compared with resting days in players participating in both team and individual sports (van Paridon, Timmis, Nevison, & Bristow, Citation2017). However, no change in cortisol levels was reported in professional male basketball players (Gonzalez-Bono, Salvador, Serrano, & Ricarte, Citation1999), elite junior hockey players who played an average of two games per week (Carre, Muir, Belanger, & Putnam, Citation2006), and female collegiate soccer players participating in a regular season game and a typical practice session (Haneishi et al., Citation2007). From the findings of the present and previous studies, it is considered that both an increase in the CAR and cortisol prior to competition were a manifestation of the HPA axis response to an upcoming sports competition, which was confounded by other factors such as sex and the degree of experience with competition stress or level of performance.
We observed reduced Daucawk and elevated CARauc/Daucawk ratios on the day of competition compared with the day before competition. To date, no information is available regarding DHEA secretion and the molar C/D ratios in athletes during their competition period. It is known that the levels of both cortisol and DHEA increase temporally in response to acute physical and psychological stress conditions (Marceau, Dorn, & Susman, Citation2012; Shirtcliff, Zahn-Waxler, Klimes-Dougan, & Slattery, Citation2007), whereas DHEA levels decrease due to an adrenocortical steroidogenic shift to cortisol at the expense of DHEA in stressful conditions (Kamin & Kertes, Citation2017). Previous studies have demonstrated that the cortisol/DHEA-S ratio was negatively associated with cognitive and decision-making ability during acute stress (Morgan et al., Citation2004) and lower DHEA and an elevated C/D ratio was positively associated with a negative mood (Izawa et al., Citation2008; Rasmusson et al., Citation2004). Other studies have found that higher morning cortisol and a morning C/D ratio were associated with higher anxiety (van Niekerk, Huppert, & Herbert, Citation2001). From the results of earlier studies, it is speculated that upcoming competition acts as strong stress to elite shooting players, which can influence their physical and psychological function of shooting players.
The CAR reflects the HPA axis response to the anticipated stress load of the upcoming day and plays a role in preparing for action such as attainment of alertness following awakening, physiological activation, memory retrieval, and reactivation of motor function (Clow et al., Citation2010). The difference in psychological factors such as perceived job stress, general life stress, social stress, and many other worries is known to contribute to the between-subjects variance in the CAR (Fries et al., Citation2009). We observed no significant differences in age, period of shooting practice, and the levels of cortisol and DHEA after the awakening period on the day before competition between the low- and high-scored players. However, the low-scored players had a greater CARauc than the high-scored players on the day of competition, indicating that perceived stress due to upcoming competition may have been greater in the low-scored players than in the high-scored players. Consistently, the percent variation in CARauc and molar CARauc/Daucawk between the day before and on the day of competition was greater in the low-scored players than in the high-scored players, indicating greater day-to-day variations in the HPA axis function in the low-scored players. Previous studies determining precompetitive cortisol increases have shown variable results regarding the association between cortisol responses to competition and performance outcomes; winners had lower cortisol responses to competition than losers in a female rugby league (Bateup, Booth, Shirtcliff, & Granger, Citation2002), significantly greater cortisol responses in winners than in losers in tennis (Filaire et al., Citation2009), and no significant effect of cortisol on the outcomes after basketball competition (Gonzalez-Bono et al., Citation1999). It is therefore considered that the association between HPA axis responses to upcoming competition and performance outcomes vary with the type of sports, and greater variation in HPA axis activities in relation to competition is adversely associated with the outcomes in 10 m air pistol and rifle shooting, irrespective with the age and period of shooting practice.
Animal and human studies have suggested that cortisol itself plays as a significant variable in motor system function. Single or repeated daily oral doses of corticosterone treatment causes a significant reduction in skilled limb use in the presence of elevated plasma corticosterone in rats (Metz et al., Citation2005). The acute increase in cortisol plasma levels after a burst of hydrocortisone administration leads an increase in motor cortex excitability and a decrease in short intracortical inhibition (Milani et al., Citation2010) and inhibits the neuroplasticity of the motor cortex (Sale, Ridding, & Nordstrom, Citation2008). A recent study reported that the differences in the amplitude of motor-evoked potential between pre- and post-stimulation, measured 6-7 hours after awakening, were greater on the day of a larger CAR compared with those on the days of average CARs in healthy subjects, suggesting that the daily magnitude of the CAR induces daily variations in the sensitivity of motor cortex to stimulation (Clow et al., Citation2014). We observed that the CARauc was significantly higher in the low-scored than in the high-scored men and women players. Because shooting represents a motor performance skill that demands to perform the mechanical factors of shooting accuracy such as gun stability, postural balance, and hand-eye coordination (Kayihan et al., Citation2013), it is hypothesized that a higher level of cortisol may interfere with shooting accuracy by leading to impairment in motor performance involving complex skills, fine motor movement coordination, and concentration.
The present study has some limitations. First, we did not assess anxiety and mood states in the players because the KSF considered that filling out questionnaires after the awakening period of the competition day was an additional burden to the junior players. The HPA axis response after the awakening period before the onset of competition might be moderated by competitive anxiety (Filaire et al., Citation2009). It is considered that this study needs replication in different sports and whether competition anxiety plays an important moderator role between HPA axis function and competition also must be investigated. Second, the present study analyzed the data from players during their one round competition; the variations in HPA axis function occurring in the same player cannot be properly evaluated with a one-time assessment and requires long-term follow-up studies to better understand the associations between anticipatory HPA responses and performance outcomes. For these reasons, the findings of the present study are preliminary rather than definitive and need to be replicated in a long-term follow-up study.
In summary, there was an increase in cortisol levels and a decrease in DHEA levels, which resulted in an increase in the molar cortisol to DHEA ratios during the post-awakening period on the day of competition compared with the day before competition in the junior men and women shooting players. The changes in the levels of cortisol and DHEA were considered as the HPA axis responses to upcoming competition after the awakening period. A greater anticipatory HPA axis response during the awakening period, however, was adversely associated with shooting outcomes of the players. The mechanical factors of the shooting techniques such as stability of hold, aiming accuracy, timing of triggering, and cleanness of triggering have been reported to play key roles as the major determinants of shooting scores (Hawkins, Citation2011; Ihalainen et al., Citation2016a). Assessment of the association between the shooting technical variables and HPA axis function should be included in future studies in order to improve shooting performance in competitive situations.
Acknowledgments
The authors thank Prof. Hyuk B. Kwon and Jae M. Soh in Hormone Research Center of Chonnam National University for help with steroid assays.
Disclosure statement
The authors have no conflict of interest
Additional information
Funding
References
- Adam, E.K., & Kumari, M. (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436. doi:10.1016/j.psyneuen.2009.06.011
- Ahn, R.S., Lee, Y.J., Choi, J.Y., Kwon, H.B., & Chun, S.I. (2007). Salivary cortisol and DHEA levels in the Korean population: age-related differences, diurnal rhythm, and correlations with serum levels. Yonsei Medical Journal, 48, 379–388. doi:10.3349/ymj.2007.48.3.379
- Arvat, E., Di Vito, L., Lanfranco, F., Maccario, M., Baffoni, C., Rossetto, R., … Ghigo, E. (2000). Stimulatory effect of adrenocorticotropin on cortisol, aldosterone, and dehydroepiandrosterone secretion in normal humans: dose-response study. The Journal of Clinical Endocrinology and Metabolism, 85, 3141–3146. doi:10.1210/jcem.85.9.6784
- Balthazar, C.H., Garcia, M.C., & Spadari-Bratfisch, R.C. (2012). Salivary concentrations of cortisol and testosterone and prediction of performance in a professional triathlon competition. Stress, 15, 495–502. doi:10.3109/10253890.2011.642033
- Bateup, H.S., Booth, A., Shirtcliff, E.A., & Granger, D.A. (2002). Testosterone, cortisol, and women's competition. Evolution and Human Behavior, 23, 181–192. doi:10.1016/S1090-5138(01)00100-3
- Carre, J., Muir, C., Belanger, J., & Putnam, S.K. (2006). Pre-competition hormonal and psychological levels of elite hockey players: relationship to the “ "home advantage"”. Physiology &Amp; Behavior, 89, 392–398. doi:10.1016/j.physbeh.2006.07.011
- Clow, A., Hucklebridge, F., & Thorn, L. (2010). The cortisol awakening response in context. International Review of Neurobiology, 93, 153–175. doi:10.1016/S0074-7742(10)93007-9
- Clow, A., Law, R., Evans, P., Vallence, A.M., Hodyl, N.A., Goldsworthy, M.R., … Ridding, M.C. (2014). Day differences in the cortisol awakening response predict day differences in synaptic plasticity in the brain. Stress (Amsterdam, Netherlands), 17, 219–223. doi:10.3109/10253890.2014.905533
- Diaz, M.M., Bocanegra, O.L., Teixeira, R.R., Tavares, M., Soares, S.S., & Espindola, F.S. (2013). The relationship between the cortisol awakening response, mood states, and performance. Journal of Strength and Conditioning Research, 27, 1340–1348. doi:10.1519/JSC.0b013e318267a612
- Edwards, S., Clow, A., Evans, P., & Hucklebridge, F. (2001). Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sciences, 68, 2093–2103. doi:10.1016/s0024-3205(01)00996-1
- Elder, G.J., Barclay, N.L., Wetherell, M.A., & Ellis, J.G. (2018). Anticipated next-day demand affects the magnitude of the cortisol awakening response, but not subjective or objective sleep. Journal of Sleep Research, 27, 47–55. doi:10.1111/jsr.12569
- Evans, P., Hucklebridge, F., Loveday, C., & Clow, A. (2012). The cortisol awakening response is related to executive function in older age. International Journal of Psychophysiology, 84, 201–204. doi:10.1016/j.ijpsycho.2012.02.008
- Filaire, E., Alix, D., Ferrand, C., & Verger, M. (2009). Psychophysiological stress in tennis players during the first single match of a tournament. Psychoneuroendocrinology, 34, 150–157. doi:10.1016/j.psyneuen.2008.08.022
- Fries, E., Dettenborn, L., & Kirschbaum, C. (2009). The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 72, 67–73. doi:10.1016/j.ijpsycho.2008.03.014
- Gonzalez-Bono, E., Salvador, A., Serrano, M.A., & Ricarte, J. (1999). Testosterone, cortisol, and mood in a sports team competition. Hormones and Behavior, 35, 55–62. doi:10.1006/hbeh.1998.1496
- Gould, D., Petlichkoff, L., Simons, J., & Vevera, M. (1987). Relationship between Competitive State Anxiety Inventory-2 subscale scores and pistol shooting performance. Journal of Sport Psychology, 9, 33–42. doi:10.1123/jsp.9.1.33
- Haneishi, K., Fry, A.C., Moore, C.A., Schilling, B.K., Li, Y., & Fry, M.D. (2007). Cortisol and stress responses during a game and practice in female collegiate soccer players. Journal of Strength and Conditioning Research, 21, 583–588. doi:10.1519/R-20496.1
- Hawkins, R. (2011). Identifying mechanic measures that best predict. International Journal of Performance Analysis in Sport, 11, 499–509. doi:10.1080/24748668.2011.11868568
- Herman, J.P., Figueiredo, H., Mueller, N.K., Ulrich-Lai, Y., Ostrander, M.M., Choi, D.C., & Cullinan, W.E. (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology, 24, 151–180. doi:10.1016/j.yfrne.2003.07.001
- Hodyl, N.A., Schneider, L., Vallence, A.M., Clow, A., Ridding, M.C., & Pitcher, J.B. (2016). The cortisol awakening response is associated with performance of a serial sequence reaction time task. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 100, 12–18. doi:10.1016/j.ijpsycho.2015.12.007
- Ihalainen, S., Kuitunen, S., Mononen, K., & Linnamo, V. (2016a). Determinants of elite-level air rifle shooting performance. Scandinavian Journal of Medicine & Science in Sports, 26, 266–274. doi:10.1111/sms.12440
- Ihalainen, S., Linnamo, V., Mononen, K., & Kuitunen, S. (2016b). Relation of elite rifle shooters' technique-test measures to competition performance. International Journal of Sports Physiology and Performance, 11, 671–677. doi:10.1123/ijspp.2015-0211
- Izawa, S., Sugaya, N., Shirotsuki, K., Yamada, K.C., Ogawa, N., Ouchi, Y., … Nomura, S. (2008). Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biological Psychology, 79, 294–298. doi:10.1016/j.biopsycho.2008.07.003
- Kalimi, M., Shafagoj, Y., Loria, R., Padgett, D., & Regelson, W. (1994). Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Molecular and Cellular Biochemistry, 131, 99–104. doi:10.1007/bf00925945
- Kamin, H.S., & Kertes, D.A. (2017). Cortisol and DHEA in development and psychopathology. Hormones and Behavior, 89, 69–85. doi:10.1016/j.yhbeh.2016.11.018
- Kang, J.Y., Park, J.Y., Chun, S.I., Suh, H.S., Lee, K., & Ahn, R.S. (2014). Puberty-related changes in cortisol, dehydroepiandrosterone, and estradiol-17β secretions within the first hour after waking in premenarcheal girls. Neuroendocrinology, 99, 168–177. doi:10.1159/000363368
- Kayihan, G., Ersoz, G., Zkan, A., & Koz, M. (2013). Relationship between efficiency of pistol shooting and selected physical-physiological parameters of police. Policing: An International Journal of Police Strategies & Management, 36, 819–832. doi:10.1108/PIJPSM-03-2013-0034
- Kim, M.S., Lee, Y.J., & Ahn, R.S. (2010). Day-to-day differences in cortisol levels and molar cortisol-to-DHEA ratios among working individuals. Yonsei Medical Journal, 51, 212–218. doi:10.3349/ymj.2010.51.2.212
- Kunz-Ebrecht, S.R., Kirschbaum, C., Marmot, M., & Steptoe, A. (2004). Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology, 29, 516–528. doi:10.1016/S0306-4530(03)00072-6
- Lennartsson, A.K., Kushnir, M.M., Bergquist, J., & Jonsdottir, I.H. (2012). DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biological Psychology, 90, 143–149. doi:10.1016/j.biopsycho.2012.03.003
- Marceau, K., Dorn, L.D., & Susman, E.J. (2012). Stress and puberty-related hormone reactivity, negative emotionality, and parent-adolescent relationships. Psychoneuroendocrinology, 37, 1286–1298. doi:10.1016/j.psyneuen.2012.01.001
- Meggs, J., Golby, J., Mallett, C.J., Gucciardi, D.F., & Polman, R.C. (2016). The cortisol awakening response and resilience in elite swimmers. International Journal of Sports Medicine, 37, 169–174. doi:10.1055/s-0035-1559773
- Metz, G.A., Jadavji, N.M., & Smith, L.K. (2005). Modulation of motor function by stress: a novel concept of the effects of stress and corticosterone on behavior. The European Journal of Neuroscience, 22, 1190–1200. doi:10.1111/j.1460-9568.2005.04285.x
- Milani, P., Piu, P., Popa, T., della Volpe, R., Bonifazi, M., Rossi, A., & Mazzocchio, R. (2010). Cortisol-induced effects on human cortical excitability. Brain Stimulation, 3, 131–139. doi:10.1016/j.brs.2009.07.004
- Morgan, C.A., 3rd, Southwick, S., Hazlett, G., Rasmusson, A., Hoyt, G., Zimolo, Z., & Charney, D. (2004). Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Archives of General Psychiatry, 61, 819–825. doi:10.1001/archpsyc.61.8.819
- Moriarty, A.S., Bradley, A.J., Anderson, K.N., Watson, S., Gallagher, P., & McAllister-Williams, R.H. (2014). Cortisol awakening response and spatial working memory in man: a U-shaped relationship. Human Psychopharmacology, 29, 295–298. doi:10.1002/hup.2399
- Okun, M.L., Krafty, R.T., Buysse, D.J., Monk, T.H., Reynolds, C.F., 3rd, Begley, A., & Hall, M. (2010). What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology, 35, 460–468. doi:10.1016/j.psyneuen.2009.08.017
- Pruessner, J.C., Wolf, O.T., Hellhammer, D.H., Buske-Kirschbaum, A., von Auer, K., Jobst, S., … Kirschbaum, C. (1997). Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sciences, 61, 2539–2549. doi:10.1016/s0024-3205(97)01008-4
- Rasmusson, A.M., Vasek, J., Lipschitz, D.S., Vojvoda, D., Mustone, M.E., Shi, Q., … Charney, D.S. (2004). An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 29, 1546–1557. doi:10.1038/sj.npp.1300432
- Sade, S., Bar-Eli, M., Bresler, S., & Tenenbaum, G. (1990). Anxiety, self-control and shooting performance. Perceptual and Motor Skills, 71, 3–6. doi:10.2466/pms.1990.71.1.3
- Sale, M.V., Ridding, M.C., & Nordstrom, M.A. (2008). Cortisol inhibits neuroplasticity induction in human motor cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28, 8285–8293. doi:10.1523/JNEUROSCI.1963-08.2008
- Shirtcliff, E., Zahn-Waxler, C., Klimes-Dougan, B., & Slattery, M. (2007). Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48, 580–591. doi:10.1111/j.1469-7610.2006.01723.x
- Stalder, T., Kirschbaum, C., Kudielka, B.M., Adam, E.K., Pruessner, J.C., Wüst, S., … Clow, A. (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. doi:10.1016/j.psyneuen.2015.10.010
- Strahler, K., Ehrlenspiel, F., Heene, M., & Brand, M. (2010). Competitive anxiety and cortisol awakening response in the week leading up to a competition. Psychology of Sport and Exercise, 11, 148–154. doi:10.1016/j.psychsport.2009.10.003
- Thorn, L., Hucklebridge, F., Evans, P., & Clow, A. (2009). The cortisol awakening response, seasonality, stress and arousal: a study of trait and state influences. Psychoneuroendocrinology, 34, 299–306. doi:10.1016/j.psyneuen.2008.11.005
- van Niekerk, J.K., Huppert, F.A., & Herbert, J. (2001). Salivary cortisol and DHEA: Association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology, 26, 591–612. doi:10.1016/S0306-4530(01)00014-2
- van Paridon, K.N., Timmis, M.A., Nevison, C.M., & Bristow, M. (2017). The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport &Amp; Exercise Medicine, 3, e000261. doi:10.1136/bmjsem-2017-000261
- Warnock, F., McElwee, K., Seo, R.J., McIsaac, S., Seim, D., Ramirez-Aponte, T., … Young, A.H. (2010). Measuring cortisol and DHEA in fingernails: A pilot study. Neuropsychiatric Disease and Treatment, 6, 1–7.
- Wetherell, M.A., Lovell, B., & Smith, M.A. (2015). The effects of an anticipated challenge on diurnal cortisol secretion. Stress (Amsterdam, Netherlands), 18, 42–48. doi:10.3109/10253890.2014.993967
- Wolfram, M., Bellingrath, S., Feuerhahn, N., & Kudielka, B.M. (2013). Cortisol responses to naturalistic and laboratory stress in student teachers: Comparison with a non-stress control day. Stress and Health : Journal of the International Society for the Investigation of Stress, 29, 143–149. doi:10.1002/smi.2439
- Wu, J., Zhang, S., Li, W., Qin, S., He, Y., Yang, Z., … Zhang, K. (2015). Cortisol awakening response predicts intrinsic functional connectivity of the medial prefrontal cortex in the afternoon of the same day. NeuroImage, 15, 158–165. doi:10.1016/j.neuroimage.2015.08.016
- Wust, S., Wolf, J., Hellhammer, D.H., Federenko, I., Schommer, N., & Kirschbaum, C. (2000). The cortisol awakening response - Normal values and confounds. Noise &Amp; Health, 2, 79–88.
