Abstract
We report the effects of acute and chronic stress on the expression of selective immune-related genes and markers of neuronal function in the brain of the zebrafish (Danio rerio). Fish were distributed into three groups: the non-stressed control group; the acute stress (AS) group, submitted to a single stressing episode; and the unpredictable chronic stress (UCS) group, submitted to two daily stressing episodes of alternating times and types of stress. The stressing protocols were applied for a period of 14 days. The UCS protocol triggered the expression of the pro-inflammatory cytokine genes IL-1β and TNF-α, the anti-inflammatory cytokine IL-10 (negative feedback from the immune system), reduction in cFOS gene expression, and caused neuro-inflammation. The AS protocol had no effect on gene expression. Altered expression of cytokine genes, as observed in our study, correlates with several pathologies associated with neuro-inflammation, and the reduction of cFOS gene expression may indicate the occurrence of reduced neuronal plasticity. Our study further extends our knowledge about the interaction of the immune system and the different forms of stress.
1. Introduction
Stress is a growing condition of significant concern to public health, mainly in developed countries. Stressing episodes induce metabolic changes that allow individuals to appropriately respond to stressor stimuli (Dhabhar, Citation2014). However, intensive and/or chronic stressing episodes may exceed the responding capacity and trigger several pathologies (Calcia et al., Citation2016). Cardiovascular diseases (Steptoe & Kivimäki, Citation2012) and pathologies that affect the central nervous system, like anxiety and depression, may be associated with stress (Baxter et al., Citation2013). The role of chronic stress on disease development includes deregulation of the hypothalamic-pituitary-adrenal (HPA) axis, activation of neuro-inflammation, and neuronal apoptosis (Pijanowski et al., Citation2015).
Stress is associated with elevated pro-inflammatory cytokines (Calcia et al., Citation2016). The immune system responds to stressors and communicates with the central nervous system through different mechanisms including cytokine signaling, vagal innervation, and the lymphatic system (Louveau et al., Citation2015). High circulating levels of cytokines induce changes in microglia and astrocyte functions, leading to neuro-inflammation and neurodegeneration (Calcia et al., Citation2016) which, in turn, might be associated with structural and functional changes in the brain that predispose humans to mental illness (Réus et al., Citation2015). To better understand the biological effects of stress, animal models such as the zebrafish (Danio rerio) are widely used in translational research (Piato et al., Citation2011; Glovatchcka, Ennes, & Mayer, Citation2012). The zebrafish brain is neuroanatomically and functionally comparable to the mammal brain (Vargas, Jóhannesdóttir, Sigurgeirsson, Torsteinsson, & Karlsson, Citation2011). In addition, zebrafish stress response is mediated by the hypothalamus–pituitary–interrenal (HPI) axis, which is similar to the mammalian hypothalamus–pituitary–adrenal (HPA) axis (Abreu et al., Citation2018) and culminates with the production of cortisol.
The deleterious effects of stress have been intensively studied using zebrafish and, for that, different stressing protocols were developed, like the unpredictable chronic stress (UCS) (Piato et al., Citation2011) and several acute stress (AS) protocols (Abreu et al., Citation2018). Previous studies related to the effect of stressing analyzed behavioral alterations, cortisol levels, and inflammatory response by measuring levels of selected cytokines (Marcon et al., Citation2016; Piato et al., Citation2011); however, there is a lack of information on a wider range of cytokine gene expression in the brain during acute and chronic stress. Thus, here we evaluated the expression of cytokine genes and biomarkers of neuronal function in the brain of zebrafish undergoing acute and chronic stress.
2. Methods
2.1. Ethical and legal note
This study was approved by the Ethics Committee on the Use of Animals (CEUA), Universidade de Passo Fundo, Rio Grande do Sul, Brazil (Protocol #019/2018-CEUA), and abided by the guidelines of the National Council for the Control of Animal Experimentation (CONCEA). In addition, this research was registered in the SisGen (Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado) and complied with their guidelines (registration code A14E252).
2.2. Animals and maintenance
We used wild type zebrafish (Danio rerio) from our stock population; fish were kept in a glass aquarium (14 × 40 × 30 cm; width × depth × height) under constant water flux and a 14/10 h light/dark cycle. The experiment was carried out using 96 mixed sex adults that were transferred to the experimental tanks (24 glass tanks: 20 × 15 × 15 cm; width × depth × height; 3 fish per tank) and acclimatized for seven days prior to stressing.
Water temperature was maintained at 28 ± 2 °C, dissolved oxygen concentration was 6.0 ± 0.4 mg/L, pH was 7.0 ± 0.25, and the total ammonia concentration was less than 0.5 mg/L.
2.3. Study strategy
After acclimatizing, zebrafish were allocated into three groups of 24, as follows: a non-stressed control group (CG); an acute stress group (AS) that was submitted to a single episode of stress; an unpredictable chronic stress group (UCS) that was submitted to a 14-day stressing protocol. After the stressing protocols, we analyzed the expression of selective cytokine genes and markers of neuronal activity in the fish brain.
2.4. Acute stress protocol
The single stress episode consisted of exposing the fish to the air for one minute (Song et al., Citation2018). The fish was then returned to the aquarium and sacrificed one hour after the stressing episode to evaluate gene expression. This time point was chosen because it is the estimated time for a possible alteration of gene expression after stimulation (Vojtech et al., Citation2009).
2.5 Unpredictable chronic stress protocol
The UCS protocol was adapted from Piato et al. (Piato et al., Citation2011) and consisted of two daily stressing episodes over 14 days. The complete UCS protocol, with the time and sequence of each stressor, is shown in . Fish were sacrificed 12 hours after the last stress episode to assure that the analysis of gene expression represented the cumulative effect of the UCS and not the last stress episode.
Table 1. Procedure of the unpredictable chronic stress protocol.
2.6. Euthanasia, brain, and RNA extraction
We anesthetized fish by immersion in eugenol (50 mg/L) prior to killing by sectioning the spinal cord. Then, the skull was opened and the cranial nerves severed to carefully remove the brain (Vargas et al., Citation2011). For RNA extraction, the brains of three fish were pooled per sample (total of 6 samples per 18-fish group).
Pooled brain samples were lysed using the Tissuelyser LT® (Qiagen, Hilden, Germany). RNA extraction was then carried out using the RNeasy® Mini Kit (Qiagen, Hilden, Germany) and treated with DNAse I amplification grade® (Invitrogen, Carlsbad, CA, USA) to eliminate genomic DNA. The RNA quality and concentration were measured by spectrophotometry (Nanophotometer Pearl®; IMPLEN, Munich, Germany).
2.7. cDNA synthesis and gene expression analysis
Total RNA (500 ng) was used for cDNA synthesis in the reverse transcription assay (QuantiTect® III Reverse Transcription kit, Qiagen, Hilden, Germany). A real time PCR (qPCR) was performed on the Rotor-Gene Q (Qiagen, Hilden, Germany), using GoTaq® qPCR Master Mix (Promega, Madison, Wisconsin, USA), cDNA (diluted 1:10), and specific primers. The genes analyzed, their respective primers, and the efficiency of each reaction are described in . All samples were analyzed in triplicate. Non-template controls and the expression of a housekeeping gene (β-actin) were also analyzed for comparison purposes. The relative quantification of gene expression was carried out using the 2−ΔΔct formula.
Table 2. Genes amplified, qPCR primers, and efficiency of amplification.
2.8. Statistical analysis
Data were analyzed using ordinary one-way ANOVA followed by Tukey’s multiple comparison test or using the Kruskal-Wallis test followed by Dunn’s multiple comparison test, depending on data normality, as assessed by Brown-Forsythe and Bartlett’s tests. In all comparisons, significance was set at <0.05.
3. Results
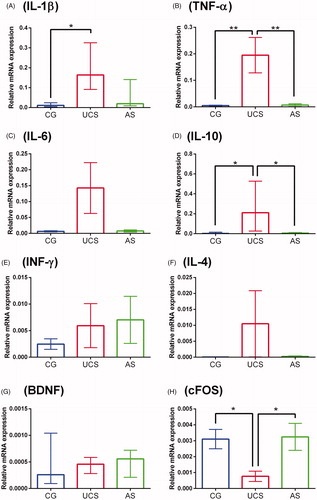
The mRNA levels of the pro-inflammatory cytokines IL-1β and TNF-α, and the anti-inflammatory cytokine IL-10, were highest in the brains of fish from the UCS group (). The mRNA levels of cFOS were lowest in the UCS group (). No significant differences were found in the mRNA levels of the other genes tested, nor for any of the genes tested in the AS group.
Figure 1. Gene expression in the brains of fish from the control (CG), unpredictable chronic stress (UCS) and acute stress (AS) groups. Data are expressed as mean ± SEM (panels B, C, E, F and H) or median ± interquartile range (panels A, D and G), depending on the data normality, which was assessed using Bartlett’s test. The asterisks indicate significant differences (*p = < 0.05, **p = < 0.01).
4. Discussion
Here we show that acute and chronic stressing differently affect the expression of cytokine genes and neuronal markers in the zebrafish brain. The UCS increased the expression of the pro-inflammatory cytokine genes (IL-1β and TNF-α) and the anti-inflammatory cytokine gene IL-10. In addition, UCS induced a reduction in the expression of the cFOS gene, which may indicate reduction of neuronal plasticity (Jaworski, Kalita, & Knapska, Citation2018). On the other hand, a single isolated stressing episode (AS) had no effect on the expression of the evaluated genes.
In the UCS group of fish, the increased expression of pro-inflammatory cytokine genes in the brain is consistent with an inflammatory reaction. As commented above, fish submitted to the UCS protocol had increased levels of IL-1β and TNF-α mRNA, both pro-inflammatory cytokines produced by microglia in response to chronic stress (Calcia et al., Citation2016). Previous studies using the UCS protocol in zebrafish reported increased levels of some pro-inflammatory cytokines in the body (Song et al., Citation2018) and brain (Marcon et al., Citation2016). However, here we measured a wider range of cytokines in the brain that allowed us to better assess the neuroimmunological phenomena after chronic stress.
Interestingly, in the UCS group, we also observed increased expression of the gene encoding the anti-inflammatory cytokine IL-10, which is produced by astrocytes and has the potential to act by protecting neurons against the potential neurodegeneration caused by stress (Palmer & Ousman, Citation2018). The increase of IL-10 is a response to inflammation and a mechanism of neuroprotection (Garcia, Stillings, Leclerc, & Phillips, Citation2017).
In contrast to the findings in the UCS group, the expression of pro-inflammatory cytokine genes was not altered in fish from the AS group, indicating that an isolated stressing episode might not be enough to cause alterations in pro-inflammatory cytokines, or that the post-stress evaluation time (one hour) chosen to collect samples did not overlap with the alteration in cytokine expression (Vojtech et al., Citation2009).
Chronic stress can promote microglia activation and cytokine production in human and rodent models (Glovatchcka et al., Citation2012). Microglia are immune cells present in the brain and represent the main line of defense in the central nervous system; additionally, these cells modulate neuronal function in illness and health (Calcia et al., Citation2016). Chronic neuro-inflammation causes changes in microglia, which in turn may be associated with structural and functional changes (CITAR) in the brain, predisposing individuals to mental illnesses (Réus et al., Citation2015) like depression, anxiety, schizophrenia and autism spectrum disorders (Calcia et al., Citation2016). The upregulation of cytokine gene expression, as demonstrated in the present study, and their role in neuro-inflammation, reinforces the translational potential of the zebrafish for studying the effects of stress in the brain.
In a neuroimmunological context, each cytokine has unique characteristics, and several were altered in our study. In rodents, increased expression of TNF-α is involved in fear memory (Yu et al., Citation2017). Similarly, in our study, the repeated episodes of stress might have induced a fear memory and upregulation of TNF-α (Yu et al., Citation2017). Furthermore, increased levels of IL-β in the brain can cause impairments to memory and learning, as it has a deleterious effect on neuronal plasticity (Avital et al., Citation2003). Here, we also found reduced levels of cFOS expression which, as discussed below, is involved in memory and learning processes (Velazquez, Caputto, & Boussin, Citation2015). Behavioral tests have shown that stress impairs memory and learning in zebrafish (Manuel et al., Citation2014), which corroborates the molecular findings of our study.
Analyzing the gene expression of neuronal markers, the nerve growth factor, brain-derived neurotrophic factor (BDNF) and cFOS gene, we found a reduction in cFOS gene expression in the UCS group. The cFOS is an early expression gene that is a well-known marker of neuronal activity (Velazquez et al., Citation2015). The cFOS protein is involved in key cellular events, including cell proliferation, differentiation, and survival. Also, it is involved in the processes of memory and learning (Velazquez et al., Citation2015). Previous studies in zebrafish showed that the expression of cFOS can be induced by acute stress (Pavlidis, Theodoridi, & Tsalafouta, Citation2015). However, the expression of the cFOS gene in our AS group of fish was not altered, probably because we evaluated the expression one hour after the stress episode and, as cFOS is an early expression gene, its value probably returned to basal level during this time (Jaworski et al., Citation2018; Pavlidis et al., Citation2015). On the other hand, the UCS group showed a down regulation of the cFOS gene, indicating a possible reduction of neuronal plasticity in fish exposed to chronic stress (Jaworski et al., Citation2018).
BDNF is a neurotrophic factor involved in maturation, differentiation and survival of neurons in the nervous system, and depicts a neuroprotective effect under adverse conditions, such as chronic stress (Lucini, Angelo, Cacialli, Palladino, & Girolamo, Citation2018). Chronic stress might alter BDNF (Manuel et al., Citation2014; Pavlidis et al., Citation2015) but, conversely, here we could not find any alterations in the expression of the BDNF gene in the stressed groups. The fact that we analyzed gene expression rather than protein concentration may have been a limitation for detecting possible alterations. Moreover, in fish submitted to UCS, cFOS gene expression was reduced but BDNF gene expression was not. Although correlated, BDNF and cFOS may have different gene expression patterns, which may also vary according to the post-stress assessment time (Pavlidis et al., Citation2015).
In conclusion, our most important finding is the different pattern of expression of cytokine genes and neuronal markers caused by acute and chronic stress in the zebrafish brain. Chronic stress caused significant alterations in the expression of genes linked to inflammation and neuronal plasticity, while acute stress did not change the expression of these genes, suggesting that a single stressing episode does not impair cerebral homeostasis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abreu, M.S., Giacomini, A.V., Zanandrea, R., Santos, B.E., Genario, R., & Oliveira, G.G.D. (2018). Psychoneuroimmunology and immunopsychiatry of zebrafish. Psychoneuroendocrinology, 92, 1–12. doi:10.1016/j.psyneuen.2018.03.014
- Abreu, M.S., Koakoski, G., Barreto, R.E., & Barcellos, L. (2018). Modulation of cortisol responses to an acute stressor in zebrafish visually exposed to heterospecific. Zebrafish, 00, 1–6. doi:10.1089/zeb.2017.1509
- Avital, A., Goshen, I., Kamsler, A., Segal, M., Iverfeldt, K., Richter-Levin, G., & Yirmiya, R. (2003). Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus, 13, 826–834. doi:10.1002/hipo.10135
- Baxter, A.J., Scott, K.M., Vos, T., Whiteford, H.A., Baxter, A.J., & Scott, K.M. (2013). Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychological Medicine, 43, 897–910. doi:10.1017/S003329171200147X
- Calcia, M.A., Bonsall, D.R., Bloomfield, P.S., Selvaraj, S., Barichello, T., & Howes, O.D. (2016). Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology, 233, 1637–1650. doi:10.1007/s00213-016-4218-9
- Dhabhar, F.S. (2014). Effects of stress on immune function: The good, the bad, and the beautiful. Immunologic Research, 58, 193–210. doi:10.1007/s12026-014-8517-0
- Réus, G.Z., Fries, G.R., Stertz, L., Badawy, M., Passos, I.C., Barichello, T., Kapczinski, F. & Quevedo, J. (2015). Review the role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience, 300, 141–154. doi:10.1016/j.neuroscience.2015.05.018
- Garcia, J.M., Stillings, S.A., Leclerc, J.L., & Phillips, H. (2017). Role of interleukin-10 in acute brain injuries. Frontiers in Neurology, 8, 1–17.
- Jaworski, J., Kalita, K., & Knapska, E. (2018). c-Fos and neuronal plasticity: The aftermath of Kaczmarek‘s theory. Acta Neurobiologiae Experimentalis, 78, 287–296. doi:10.21307/ane-2018-027
- Louveau, A., Smirnov, I., Keyes, T.J., Eccles, J.D., Rouhani, S.J., Peske, J.D., … Kipnis, J. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523, 337–341. doi:10.1038/nature14432
- Lucini, C., Angelo, L.D., Cacialli, P., Palladino, A., & Girolamo, P.D. (2018). BDNF, brain, and regeneration: Insights from Zebrafish. International Journal of Molecular Sciences, 19, 1–16.
- Manuel, R., Gorissen, M., Zethof, J., Ebbesson, L.O.E., van de Vis, H., Flik, G., & van den Bos, R. (2014). Unpredictable chronic stress decreases inhibitory avoidance learning in Tuebingen long-fin zebrafish: Stronger effects in the resting phase than in the active phase. Journal of Experimental Biology, 217, 3919–3928. doi:10.1242/jeb.109736
- Marcon, M., Herrmann, A.P., Mocelin, R., Rambo, C.L., Koakoski, G., Abreu, M.S., … Piato, A.L. (2016). Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology, 233, 3815–3824. doi:10.1007/s00213-016-4408-5
- Palmer, A.L., & Ousman, S.S. (2018). Astrocytes and aging. Frontiers in Aging Neuroscience, 10, 1–14.
- Pavlidis, M., Theodoridi, A., & Tsalafouta, A. (2015). Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 60, 121–131. doi:10.1016/j.pnpbp.2015.02.014
- Piato, Â.L., Capiotti, K.M., Tamborski, A.R., Oses, J.P., Barcellos, L.J.G., Bogo, M.R., … Bonan, C.D. (2011). Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35, 561–567. doi:10.1016/j.pnpbp.2010.12.018
- Pijanowski, L., Jurecka, P., Irnazarow, I., Kepka, M., Szwejser, E., Verburg-van Kemenade, B.M.L., & Chadzinska, M. (2015). Activity of the hypothalamus–pituitary–interrenal axis (HPI axis) and immune response in carp lines with different susceptibility to disease. Fish Physiology and Biochemistry, 41, 1261–1278. doi:10.1007/s10695-015-0084-3
- Song, C., Liu, B.-P., Zhang, Y.-P., Peng, Z., Wang, J., Collier, A.D., … Kalueff, A.V. (2018). Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 384–394. doi:10.1016/j.pnpbp.2017.08.021
- Steptoe, A., & Kivimäki, M. (2012). Stress and cardiovascular disease. Nature Reviews Cardiology, 9, 360–370. doi:10.1038/nrcardio.2012.45
- Glovatchcka, H., Ennes, E.A., & Mayer, S.B. (2012). Chronic stress-induced changes in pro-inflammatory cytokines and spinal glia markers in the rat: A time course study. Neuroimmunomodulation, 19, 367–376. doi:10.1159/000342092
- Vargas, R., Jóhannesdóttir, I. T., Sigurgeirsson, B., Torsteinsson, H., & Karlsson, K.AE. (2011). The zebrafish brain in research and teaching: A simple in vivo and in vitro model for the study of spontaneous neural activity. Advances in Physiology Education, 35, 188–196. doi:10.1152/advan.00099.2010
- Velazquez, F.N., Caputto, B.L., & Boussin, F.D. (2015). c-Fos importance for brain development. Aging, 7, 1028–1029. doi:10.18632/aging.100862
- Vojtech, L.N., Sanders, G.E., Conway, C., Ostland, V., & Hansen, J.D. (2009). Host immune response and acute disease in a zebrafish model of francisella pathogenesis. Infection and Immunity, 77, 914–925. doi:10.1128/IAI.01201-08
- Yu, Z., Fukushima, H., Ono, C., Sakai, M., Kasahara, Y., Kikuchi, Y., … Tomita, H. (2017). Microglial production of TNF-alpha is a key element of sustained fear memory. Brain, Behavior, and Immunity, 59, 313–321. doi:10.1016/j.bbi.2016.08.011
