Abstract
Extensive evidence indicates that noradrenergic activation is essentially involved in mediating the enhancing effects of emotional arousal on memory consolidation. Our current understanding of the neurobiological mechanisms underlying the memory-modulatory effects of the noradrenergic system is primarily based on pharmacological studies in rats, employing targeted administration of noradrenergic drugs into specific brain regions. However, the further delineation of the specific neural circuitry involved would benefit from experimental tools that are currently more readily available in mice. Previous studies have not, as yet, investigated the effect of noradrenergic enhancement of memory in mice, which show different cognitive abilities and higher endogenous arousal levels induced by a training experience compared to rats. In the present study, we investigated the effect of posttraining noradrenergic activation in male C57BL/6J mice on the consolidation of object recognition and object location memory. We found that the noradrenergic stimulant yohimbine (0.3 or 1.0 mg/kg) administered systemically immediately after an object training experience dose-dependently enhanced 24-h memory of both the identity and location of the object. Thus, these findings indicate that noradrenergic activation also enhances memory consolidation processes in mice, paving the way for a systematic investigation of the neural circuitry underlying these emotional arousal effects on memory.
LAY SUMMARY: The current study successfully validated the effect of noradrenergic activation on both object recognition and object location memory in mice. This study thereby provides a fundamental proof-of-principle for the investigation of the neural circuitry underlying noradrenergic and arousal effects on long-term memory in mice.
Introduction
Emotionally arousing training conditions enhance noradrenergic activity (McGaugh, Citation2004). Animal studies have provided extensive evidence that such noradrenergic activation, arising from catecholaminergic cell bodies in the locus coeruleus (LC), is crucially involved in strengthening the consolidation of long-term memory (McGaugh, Citation2004; Roozendaal & McGaugh, Citation2011; Sara, Citation2009; Takeuchi et al., Citation2016). Our current understanding of the neurobiological mechanisms underlying the memory-modulatory effects of the noradrenergic system is primarily based on pharmacological studies in rats, employing either systemic administration of noradrenergic drugs or targeted administration into specific brain regions. For example, norepinephrine or noradrenergic agents administered into the basolateral amygdala (BLA), or other brain regions such as the hippocampus or prefrontal cortex, were found to enhance long-term memory of emotionally arousing training experiences (Bevilaqua et al., Citation1997; Ferry & McGaugh, Citation1999; Hatfield & McGaugh, Citation1999; Introini-Collison et al., Citation1991; LaLumiere et al., Citation2003; Liang et al., Citation1990). Conversely, posttraining infusions of β-adrenoceptor antagonists into these brain regions were shown to impair retention and block the memory-enhancing effects of co-administered norepinephrine (Berman et al., Citation2000; Bevilaqua et al., Citation1997; Hatfield & McGaugh, Citation1999). Noteworthy, noradrenergic activation does not only enhance memory for highly arousing events that are known to induce the release of high levels of norepinephrine throughout the brain (Hatfield & McGaugh, Citation1999; McIntyre et al., Citation2002; Quirarte et al., Citation1998), but also for low-arousing experiences such as different forms of object recognition training (McReynolds et al., Citation2014; Roozendaal et al., Citation2008).
Human research supports the findings from animal studies that an activation of the noradrenergic system induces better memory (Cahill et al., Citation1994, Citation2003; O’Carroll et al., Citation1999; Southwick et al., Citation2002). Accumulating evidence from human neuroimaging studies, however, indicates that emotional arousal and noradrenergic activation are associated with widespread changes in functional connectivity and the activation of large-scale neural networks (Hermans et al., Citation2011; Hermans, Battaglia, et al., Citation2014; Hermans, Henckens, et al., Citation2014; Murty et al., Citation2010; Seeley et al., Citation2007). A recent neuroimaging study in mice indicated that direct chemogenetic stimulation of the LC induces a highly comparable large-scale reconfiguration of neural network activity (Zerbi et al., Citation2019). However, how such changes in network activity by norepinephrine could contribute to enhancement of memory for emotional experiences remains to be elucidated. A further delineation of this specific neural circuitry would benefit from novel experimental tools such as optogenetics and chemogenetics. Many laboratories are using mice for such circuitry-based investigations because of the availability of a wide variety of transgenic lines. However, previous studies have not investigated whether noradrenergic activation by exogenous drug administration further enhances memory in this species, which shows different cognitive abilities and higher endogenous arousal levels induced by a training experience compared to rats (Hok et al., Citation2016; Stepanichev et al., Citation2016).
In the present study, we investigated the effect of posttraining noradrenergic activation in male C57BL/6J mice on object recognition memory (ORM) and object location memory (OLM) (Leger et al., Citation2013; Roozendaal et al., Citation2010; Vogel-Ciernia & Wood, Citation2014). Several findings suggest that memory performance in these two tasks is supported by distinct neural substrates. (Balderas et al., Citation2008; Barker & Warburton, Citation2011), memory for the location on an object relies on the hippocampus (Balderas et al., Citation2008). Standard ORM and OLM have been successfully tested using mice (Vogel-Ciernia & Wood, Citation2014), but the effect of posttraining noradrenergic activation in mice on ORM and OLM has not yet been investigated. Here we found that, similar to rats, systemic posttraining injection of yohimbine, a noradrenergic stimulant which increases noradrenergic signaling (Nirogi et al., Citation2012; Szemeredi et al., Citation1991), induces dose-dependent enhancement of memory consolidation on both the ORM and OLM tasks. These findings thus pave the way for a systematic investigation of the neural circuitry underlying emotional arousal effects on memory.
Material and methods
Animals
One-hundred-and-five male CB57BL/6J mice (8 weeks old at the time of behavioral experiments) from Charles River Breeding Laboratories (Kisslegg, Germany) were group housed (3 animals per cage) in a temperature-controlled (22 °C) vivarium room with a regular 12-h/12-h light/dark cycle (lights on between 7:00 and 19:00 h). The vivarium room had a light intensity of 47 lux and humidity of 72%. Mice had ad libitum access to food and water. Object recognition memory differs between sexes (Sutcliffe et al., Citation2007), is modulated by stress exposure in a sex-specific manner (Coutellier & Würbel, Citation2009; Luine, Citation2002), and varies with the estrous cycle phase in females (e.g. Minni et al., Citation2014; Graham & Scott, Citation2018; Do Nascimento et al., Citation2019; Kirry et al., Citation2019). Since we aimed at replicating previous rat studies in our lab (e.g. Roozendaal et al., Citation2006; Barsegyan et al., Citation2014; Atucha et al., Citation2017; Chen et al., Citation2018), in which only male rats were used, we restricted our studies to male mice only. Training and testing was performed during the light phase of the cycle, between 10:00 and 15:00 h. All experimental procedures were in compliance with European Union Directive 2010/63/EU and approved by the Institutional Animal Care and Use Committee of Radboud University, Nijmegen, The Netherlands. All efforts were made to minimize animal suffering and to reduce the number of animals.
Experimental apparatus and behavioral procedures
The experimental apparatus used for both the ORM and OLM tasks was a gray open-field box (40 cm × 40 cm × 40 cm) with the floor covered with sawdust. One side of the box was marked with a line of white tape through the midline of the wall, serving as an internal cue. The objects to be discriminated were white glass light bulbs (6 cm diameter, 11 cm length) and transparent glass vials (5.5 cm diameter, 5 cm height), secured to the floor of the box with Velcro tape. The behavior of the animals was videotaped by a camera mounted above the box, which was connected to a laptop computer.
Mice were first handled for 1 min each for 3 consecutive days. Subsequently, the animals underwent a 5-min habituation procedure to the experimental box for another 3 days prior to training. Habituation to the box is required to guarantee sufficient exploration of the objects by the mice, necessary to form long-term ORM (Stefanko et al., Citation2009). During this habituation phase, mice could freely explore the training apparatus without the objects. Training and testing on the ORM and OLM tasks was according to Leger et al. (Citation2013) and Vogel-Cierna and Wood (2014) with slight modifications. On the training trial, the mouse was placed in the experimental apparatus and allowed to explore two identical objects (A1 and A2), placed 5 cm away from the corners of the apparatus, for 3 min. Drug administration occurred immediately after the training trial, after which the animals were placed back into their home cages. To avoid the presence of olfactory trails, sawdust was stirred, feces were removed, and the objects were thoroughly cleaned with 70% ethanol in between trials. Retention was tested 1 h or 24 h after the training trial. For the ORM task, one exemplar of the familiar object (A3) and a novel object (B) were placed at the same locations as during the training trial (). For the OLM task, both objects were familiar (A3 and A4), yet one was placed at a novel location (). All combinations of locations and objects were used in a balanced manner to reduce potential biases due to preference for a particular location or object. For testing, the mouse was placed in the experimental apparatus and allowed to explore the objects for 5 min. Behavioral videos of the training and test sessions were analyzed offline by a trained observer blind to treatment condition, and the time spent exploring the novel and familiar object (or location) and the total time spent exploring both objects were scored. Part of the videos was analyzed by a second independent and blinded rater. Reliability of scoring was confirmed by high intra (r(42) = 0.804, p < .001) and inter-rater (r(42) = 0.670, p < .001) correlations in object exploration times. Object exploration was defined as actual active interaction with an object, i.e. pointing the nose to the object at a distance of <1 cm and/or touching it with the nose (Leger et al., Citation2013; Okuda et al., Citation2004). Turning around, climbing or sitting on an object per se was not included in exploration times as the animals then often are not actively engaged in exploring the object but rather exhibit grooming behavior or are using the object to scan the environment (Bianchi et al., Citation2006; Leger et al., Citation2013; Li et al., Citation2011; Pezze et al., Citation2017; Roozendaal et al., Citation2006; Vogel-Ciernia & Wood, Citation2014; Wimmer et al., Citation2012). In order to analyze memory performance, a discrimination index was calculated as the difference in time exploring the novel and familiar object (or location), expressed as the ratio of the total time spent exploring both objects (i.e. [(time novel − time familiar)/(time novel + time familiar)] × 100%). Since low object exploration during training might result in poor long-term memory unrelated to the drug condition, mice showing a total exploration time of <4 s on the training trial (n = 3) were removed from analyses. Furthermore, three mice showing a clear preference for one of the objects or locations during the training trial (defined as a discrimination index deviating more than two standard deviations from the mean) were removed (Leger et al., Citation2013; Vogel-Ciernia & Wood, Citation2014). Video analysis software (EthoVision XT, Noldus Information Technology, Wageningen, The Netherlands) was used to also measure total distance moved by the mice in the experimental apparatus during both training and retention testing.
Figure 1. Posttraining administration of the noradrenergic stimulant yohimbine dose-dependently enhances consolidation of object recognition memory. Data are shown as mean ± SEM. (A) Experimental design of the object recognition memory (ORM) task. Mice were trained for 3 min followed immediately by a subcutaneous injection of yohimbine (YOH, 0.3 or 1.0 mg/kg) or saline. Object recognition memory was tested 24 h later during which one of the objects was replaced by a novel object. (B) The higher dose of yohimbine improved memory performance on the object recognition retention test compared to saline-treated animals. (C) Yohimbine treatment did not affect total exploration time of the two objects during the retention test. (D) Yohimbine treatment did not affect the total distance moved during the retention test. saline: n = 14, YOH 0.3 mg/kg: n = 14, YOH 1.0 mg/kg: n = 13. ◆ p < .05, main effect of drug administration; ## p < .01, difference from saline; ** p < .01, difference from chance level.
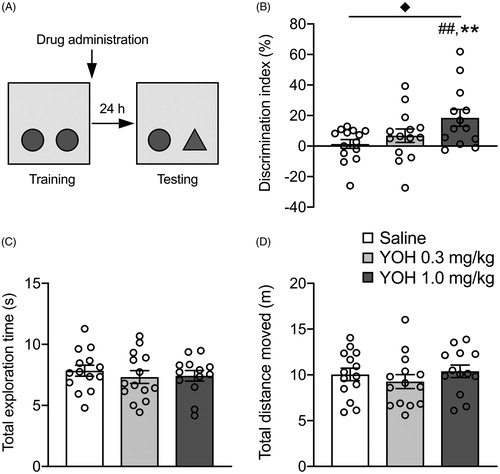
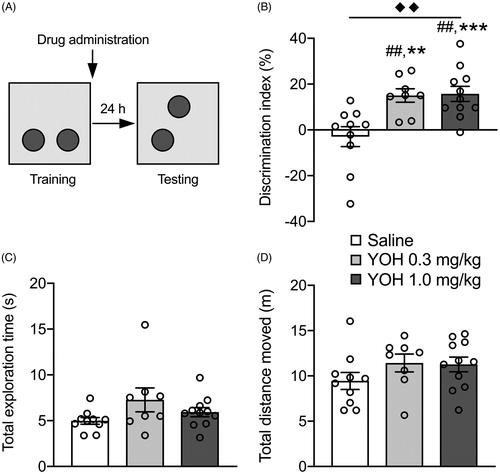
Figure 2. Posttraining administration of the noradrenergic stimulant yohimbine dose-dependently enhances the consolidation of object location memory. Data are shown as mean ± SEM. (A) Experimental design of the object location memory (OLM) task. Mice were trained for 3 min followed immediately by a subcutaneous injection of yohimbine (YOH, 0.3 or 1.0 mg/kg) or saline. Object location memory was tested 24 h later during which one of the objects was relocated to a novel location. (B) Both the higher and lower dose of yohimbine improved memory performance on the object location retention test compared to saline. (C) Yohimbine treatment did not affect total exploration time of the two objects during the retention test. (D) Yohimbine treatment did not affect the total distance moved during the retention test. saline: n = 10, YOH 0.3 mg/kg: n = 8, YOH 1.0 mg/kg: n = 11. ◆◆ p < .01, main effect of drug administration; ## p < .01, difference from saline; ** p < .01,*** p < .001, difference from chance level.
Systemic drug administration
Yohimbine (17-hydroxyyohimban-16-carboxylic acid methyl ester hydrochloride; 0.3 or 1.0 mg/kg; Sigma-Aldrich), an α2-adrenoceptor antagonist which increases noradrenergic activity (Szemeredi et al., Citation1991), was dissolved in saline and administered subcutaneously, in a volume of 0.1 mL/10 g of body weight, immediately after the training trial. The two doses were selected based on previous studies in rats (Roozendaal et al., Citation2006) and pilot data in mice (Supplementary Fig. 1). Drug solutions were freshly prepared before each experiment.
Statistics
Data are expressed as mean ± SEM. The discrimination index, total exploration time of the objects and total distance moved were analyzed with one-way ANOVAs with drug condition as between-subject variable. When appropriate, Tuckey post-hoc analyses were used to determine the source of significance in the ANOVA. One-sample t-tests were used to determine whether the discrimination index was different from zero (i.e. chance level) and thus whether learning had occurred. For all comparisons, p < 0.05 was accepted as statistical significance. The number of mice per group is indicated in the figure legends.
Results
Posttraining noradrenergic stimulation dose-dependently enhances object recognition memory
In this experiment, we first determined, in non-injected control mice, whether 3 min of object training was sufficient to induce successful acquisition of the identity of the training object in the ORM task. With these training conditions, we found that the discrimination index was significantly greater than zero at 1 h following training (M = 20.02, SEM = 6.14; t(11) = 3.26, p < .01), but not 24 h later (M = 3.64, SEM = 2.16; t(10) = 1.69, p = .12, Supplementary Fig. 2). Thus, these findings indicate that 3 min of object training is sufficient to induce short-term, but not long-term, memory.
Next, we investigated whether yohimbine (0.3 or 1.0 mg/kg) administered immediately after a 3-min training trial enhanced 24-h memory for the object in the ORM task. Total exploration time of the two identical objects (F(2,38) = 0.85, p = .69, ) or the total distance moved (F(2,38) = 3.11, p = .06, ) during the training trial did not differ between later drug treatment groups. During the 24-h retention test, the discrimination index showed a significant effect of yohimbine treatment (F(2,38) = 3.95, p = .03, ). Tukey’s post-hoc analysis revealed that mice treated with the higher dose of yohimbine (1.0 mg/kg) had a significantly greater discrimination index than that of the saline group (p < .05), whereas the discrimination index of mice treated with the lower dose of yohimbine (0.3 mg/kg) did not differ from that of saline-treated animals (p = .65). The discrimination index of both saline-treated mice (t(13) = 0.47, p = .65) and those treated with the lower dose of yohimbine (t(13) = 1.53; p = .15) was not significantly different from zero, indicating that a 3-min training trial was not sufficient to induce long-term memory of the training object in these groups. Mice treated with the higher dose of yohimbine, however, exhibited a significant exploration preference for the novel object (t(12) = 3.35; p < .01). Yohimbine treatment did not affect total exploration time of the two objects (F(2,38) = 0.34, p = .54, ) or total distance moved in the apparatus during the retention test (F(2,38) = 0.65, p = .53, ).
Table 1. Training data of object recognition memory (ORM) and object location memory (OLM).
Posttraining noradrenergic stimulation dose-dependently enhances object location memory
Next, we investigated, in separate groups of mice, whether posttraining systemic yohimbine (0.3 or 1.0 mg/kg) treatment also enhanced 24-h retention for the location of the object in the OLM task. Total exploration time of the two identical objects (F(2,26) = 2.75, p = .08, ) and the total distance moved (F(2,26) = 1.80, p = .19, ) during the training trial did not differ between later drug treatment groups. The discrimination index during the retention test, however, indicated a significant effect of yohimbine on memory performance (F(2,26) = 8.52, p < .001, ). Tukey’s post-hoc analysis revealed that the discrimination index of mice treated with either the 0.3 or 1.0 mg/kg dose of yohimbine was significantly greater than that of the saline group (p < .01). Whereas the discrimination index of saline-treated mice did not significantly differ from zero (t(9) = 0.67, p = .52), indicating that they did not express memory of the location of the training object, mice treated with either dose of yohimbine exhibited a significant exploration preference for the object located in the novel position (0.3 mg/kg: t(7) = 5.15; p < .01; 1.0 mg/kg: t(10) = 4.77; p < .001). Yohimbine treatment did not affect total exploration time of the two objects (F(2,26) = 2.27, p = .12, ) or total distance moved in the apparatus during the retention test (F(2,26) = 1.49, p = .24, ).
Discussion
The current study successfully validated the memory-enhancing effect of posttraining noradrenergic stimulation with systemic yohimbine in both the ORM and OLM tasks in mice. As such, this study provides a fundamental proof-of-principle for future investigation of the neural circuits underlying the effects of noradrenergic arousal on long-term memory in this species.
Similar to previous findings in rats (Dornelles et al., Citation2007; Jurado-Berbel et al., Citation2010; Nirogi et al., Citation2012), the main finding of the present study is that, in mice given 3 min of object training, noradrenergic activation immediately after the training trial induces dose-dependent enhancement of 24-h memory on both the ORM and OLM tasks. We found that 3 min of object training was insufficient to induce 24-h memory in saline-treated controls, but that such training conditions were sufficient to enable posttraining systemic yohimbine administration to enhance memory of both the identity and location of the object. Yohimbine is an α2-adrenoceptor antagonist which, by blocking receptors located on noradrenergic terminals, elevates norepinephrine levels and its metabolites in the brain and in blood (Szemeredi et al., Citation1991). As the yohimbine was administered immediately after the training experience, the retention improvement effects cannot be attributed to memory-encoding effects or to nonspecific influences on attentional or locomotor effects during the training trial. Furthermore, the yohimbine treatment did not affect total exploration of the two objects or total distance moved during the retention test. These findings are thus consistent with the view that the noradrenergic stimulation enhances consolidation processes on both versions of the object memory task.
Extensive evidence from pharmacological manipulation studies in rats indicates that (nor)adrenergic agonists administered systemically or directly into specific brain regions enhance memory consolidation on a wide variety of emotionally arousing training tasks, including inhibitory avoidance, active avoidance discrimination learning, contextual fear conditioning, water-maze spatial learning and appetitive tasks (Bevilaqua et al., Citation1997; Costa-Miserachs et al., Citation1994; Ferry & McGaugh, Citation1999; Gold & Van Buskirk, Citation1975; Hatfield & McGaugh, Citation1999; Introini-Collison et al., Citation1991; Introini-Collison & McGaugh, Citation1986; Izquierdo & Dias, Citation1985; LaLumiere et al., Citation2003; Liang et al., Citation1986, Citation1990; Sternberg et al., Citation1985). Noradrenergic activation also enhances recognition memory in rats (Dornelles et al., Citation2007; Jurado-Berbel et al., Citation2010). In the experiments by Dornelles et al. (Citation2007), the adrenomedullary hormone epinephrine injected systemically immediately after the training session increased the retention delay at which memory was still present (Dornelles et al., Citation2007). Another study confirmed the finding that posttraining systemic epinephrine administration improves long-term memory on both the ORM and OLM tasks (Jurado-Berbel et al., Citation2010). Other studies support the view that posttraining systemic yohimbine administration increases norepinephrine levels in the medial temporal lobe (Nirogi et al., Citation2012), and enhances memory on the ORM task (Nirogi et al., Citation2012; Roozendaal et al., Citation2006). Norepinephrine administration directly into the BLA also enhances the consolidation of ORM as well as of the association of an object with its context (Barsegyan et al., Citation2014; Roozendaal et al., Citation2008). With such targeted pharmacological manipulation studies in rats, considerable knowledge has been gained regarding the neural mechanisms by which norepinephrine facilitates long-term memory formation, particularly by its actions on the BLA (McGaugh, Citation2000, Citation2004; Roozendaal & McGaugh, Citation2011), subsequently modulating neural plasticity and information storage processes in its projection regions, including the hippocampus, perirhinal cortex, medial prefrontal cortex and insular cortex (Barsegyan et al., Citation2019; Beldjoud et al., Citation2015; Chen et al., Citation2018; Laing & Bashir, Citation2014; McIntyre et al., Citation2005; McReynolds et al., Citation2014; Roozendaal et al., Citation1999).
Neuroimaging studies in humans, however, have indicated that emotional arousal triggers dynamic shifts in network balance throughout the brain, leading to a large-scale neural network reconfiguration (Murty et al., Citation2010; Seeley et al., Citation2007). Moreover, exposure to emotional arousal induces complex temporal dynamics in neural activity. Emotional arousal, in a norepinephrine-dependent fashion, first rapidly increases salience network activity, while simultaneously suppressing central executive network activity (Hermans et al., Citation2011; Seeley et al., Citation2007). Later, when the arousing situation subsides, resource allocation to these two networks reverses: the salience network shuts off and the central executive network becomes active, which normalizes emotional reactivity and enhances higher-order cognitive processes (Hermans, Henckens, et al., Citation2014; Van Leeuwen et al., Citation2018). Studies have shown that the LC noradrenergic system has the ability to rapidly rearrange neural activity within and between large-scale neural systems to optimize cognitive processes relevant for task performance or adaptive behaviors (Aston-Jones & Cohen, Citation2005; Bullmore & Sporns, Citation2009; Van Den Heuvel & Pol, Citation2010; Zerbi et al., Citation2019). However, it remains unknown how noradrenergic activation by emotional arousal might achieve both spatial and temporal specificity in regulating large-scale neural network activity. Such effects might depend on brain region- and time-specific effects of norepinephrine on excitatory and inhibitory subpopulations of neurons. Further, it is poorly understood how such changes in network activity by norepinephrine could contribute to enhancement of memory for emotional experiences.
Dedicated studies allowing for tight experimental control over neuronal subpopulations and neural circuit activity are required to elucidate these exact mechanistic underpinnings. New technologies such as optogenetics and chemogenetics could be optimally combined with the use of a variety of readily available transgenic lines of mice, to decipher these mechanisms. Validated behavioral tasks and effects of norepinephrine are a prerequisite to conduct such studies. The present findings indicating that noradrenergic activation enhances memory for ORM and OLM in mice, pave the way for a further investigation of the specific neural circuits and molecular mechanisms that regulate emotional arousal effects on memory consolidation.
Song_SupplementaryInformation.docx
Download MS Word (7.6 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aston-Jones, G., & Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience, 28(1), 403–450. doi:10.1146/annurev.neuro.28.061604.135709
- Atucha, E., Vukojevic, V., Fornari, R. V., Ronzoni, G., Demougin, P., Peter, F., Atsak, P., Coolen, M. W., Papassotiropoulos, A., McGaugh, J. L., de Quervain, D. J.-F., & Roozendaal, B. (2017). Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proceedings of the National Academy of Sciences, 114(34), 9176–9181. doi:10.1073/pnas.1710819114
- Balderas, I., Rodriguez-Ortiz, C. J., Salgado-Tonda, P., Chavez-Hurtado, J., Mcgaugh, J. L., & Bermudez-Rattoni, F. (2008). The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learning & Memory, 15(9), 618–624. doi:10.1101/lm.1028008
- Barker, G. R., & Warburton, E. C. (2011). When is the hippocampus involved in recognition memory? Journal of Neuroscience, 31(29), 10721–10731.
- Barsegyan, A., McGaugh, J. L., & Roozendaal, B. (2014). Noradrenergic activation of the basolateral amygdala modulates the consolidation of object-in-context recognition memory. Frontiers in Behavioral Neuroscience, 8, 160. doi:10.3389/fnbeh.2014.00160
- Barsegyan, A., Mirone, G., Ronzoni, G., Guo, C., Song, Q., van Kuppeveld, D., Schut, E. H. S., Atsak, P., Teurlings, S., McGaugh, J. L., Schubert, D., & Roozendaal, B. (2019). Glucocorticoid enhancement of recognition memory via basolateral amygdala-driven facilitation of prelimbic cortex interactions. Proceedings of the National Academy of Sciences, 116(14), 7077–7082. doi:10.1073/pnas.1901513116
- Beldjoud, H., Barsegyan, A., & Roozendaal, B. (2015). Noradrenergic activation of the basolateral amygdala enhances object recognition memory and induces chromatin remodeling in the insular cortex. Frontiers in Behavioral Neuroscience, 9(108), 108. doi:10.3389/fnbeh.2015.00108
- Berman, D. E., Hazvi, S., Neduva, V., & Dudai, Y. (2000). The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: Activation of erk1–2 and formation of a memory trace. The Journal of Neuroscience, 20(18), 7017–7023. doi:10.1523/JNEUROSCI.20-18-07017.2000
- Bevilaqua, L., Ardenghi, P., Shröder, N., Bromberg, E., Schmitz, P., Schaeffer, E., Quevedo, J., Bianchin, M., Walz, R., & Medina, J. (1997). Drugs acting upon the cyclic adenosine monophosphate/protein kinase a signalling pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behavioural Pharmacology, 8(4), 331–338.
- Bianchi, M., Fone, K., Azmi, N., Heidbreder, C., Hagan, J., & Marsden, C. (2006). Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. European Journal of Neuroscience, 24(10), 2894–2902. doi:10.1111/j.1460-9568.2006.05170.x
- Bullmore, E., & Sporns, O. (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186–198. doi:10.1038/nrn2575
- Cahill, L., Gorski, L., & Le, K. (2003). Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory, 10(4), 270–274. doi:10.1101/lm.62403
- Cahill, L., Prins, B., Weber, M., & McGaugh, J. L. (1994). Β-adrenergic activation and memory for emotional events. Nature, 371(6499), 702–704. doi:10.1038/371702a0
- Chen, Y., Barsegyan, A., Nadif Kasri, N., & Roozendaal, B. (2018). Basolateral amygdala noradrenergic activity is required for enhancement of object recognition memory by histone deacetylase inhibition in the anterior insular cortex. Neuropharmacology, 141, 32–41. doi:10.1016/j.neuropharm.2018.08.018
- Costa-Miserachs, D., Portell-Cortés, I., Aldavert-Vera, L., Torras-García, M., & Morgado-Bernal, I. (1994). Long-term memory facilitation in rats by posttraining epinephrine. Behavioral Neuroscience, 108(3), 469–474. doi:10.1037/0735-7044.108.3.469
- Coutellier, L., & Würbel, H. (2009). Early environmental cues affect object recognition memory in adult female but not male c57bl/6 mice. Behavioural Brain Research, 203(2), 312–315. doi:10.1016/j.bbr.2009.05.001
- Do Nascimento, E. B., Dierschnabel, A. L., de Macêdo Medeiros, A., Suchecki, D., Silva, R. H., & Ribeiro, A. M. (2019). Memory impairment induced by different types of prolonged stress is dependent on the phase of the estrous cycle in female rats. Hormones and Behavior, 115, 104563. doi:10.1016/j.yhbeh.2019.104563
- Dornelles, A., de Lima, M. N., Grazziotin, M., Presti-Torres, J., Garcia, V. A., Scalco, F. S., Roesler, R., & Schroder, N. (2007). Adrenergic enhancement of consolidation of object recognition memory. Neurobiology of Learning and Memory, 88(1), 137–142. doi:10.1016/j.nlm.2007.01.005
- Ferry, B., & McGaugh, J. L. (1999). Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of Learning and Memory, 72(1), 8–12. doi:10.1006/nlme.1998.3904
- Gold, P. E., & Van Buskirk, R. B. (1975). Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behavioral Biology, 13(2), 145–153. doi:10.1016/S0091-6773(75)91784-8
- Graham, B. M., & Scott, E. (2018). Effects of systemic estradiol on fear extinction in female rats are dependent on interactions between dose, estrous phase, and endogenous estradiol levels. Hormones and Behavior, 97, 67–74. doi:10.1016/j.yhbeh.2017.10.009
- Hatfield, T., & McGaugh, J. L. (1999). Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiology of Learning and Memory, 71(2), 232–239. doi:10.1006/nlme.1998.3875
- Hermans, E. J., Battaglia, F. P., Atsak, P., de Voogd, L. D., Fernandez, G., & Roozendaal, B. (2014). How the amygdala affects emotional memory by altering brain network properties. Neurobiology of Learning and Memory, 112, 2–16. doi:10.1016/j.nlm.2014.02.005
- Hermans, E. J., Henckens, M. J., Joels, M., & Fernandez, G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences, 37(6), 304–314. doi:10.1016/j.tins.2014.03.006
- Hermans, E. J., van Marle, H. J. F., Ossewaarde, L., Henckens, M. J. A. G., Qin, S., van Kesteren, M. T. R., Schoots, V. C., Cousijn, H., Rijpkema, M., Oostenveld, R., & Fernandez, G. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science, 334(6059), 1151–1153. doi:10.1126/science.1209603
- Hok, V., Poucet, B., Duvelle, E., Save, E., & Sargolini, F. (2016). Spatial cognition in mice and rats: Similarities and differences in brain and behavior. Wiley Interdisciplinary Reviews: Cognitive Science, 7(6), 406–421. doi:10.1002/wcs.1411
- Introini-Collison, I. B., & McGaugh, J. L. (1986). Epinephrine modulates long-term retention of an aversively motivated discrimination. Behavioral and Neural Biology, 45(3), 358–365. doi:10.1016/S0163-1047(86)80024-3
- Introini-Collison, I. B., Miyazaki, B., & McGaugh, J. L. (1991). Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology, 104(4), 541–544. doi:10.1007/BF02245663
- Izquierdo, I., & Dias, R. D. (1985). Influence on memory of posttraining or pre-test injections of acth, vasopressin, epinephrine, and β-endorphin, and their interaction with naloxone. Psychoneuroendocrinology, 10(2), 165–172. doi:10.1016/0306-4530(85)90054-X
- Jurado-Berbel, P., Costa-Miserachs, D., Torras-Garcia, M., Coll-Andreu, M., & Portell-Cortes, I. (2010). Standard object recognition memory and “what” and “where” components: Improvement by post-training epinephrine in highly habituated rats. Behavioural Brain Research, 207(1), 44–50. doi:10.1016/j.bbr.2009.09.036
- Kirry, A. J., Durigan, D. J., Twining, R. C., & Gilmartin, M. R. (2019). Estrous cycle stage gates sex differences in prefrontal muscarinic control of fear memory formation. Neurobiology of Learning and Memory, 161, 26–36. doi:10.1016/j.nlm.2019.03.001
- Laing, M., & Bashir, Z. (2014). Β-adrenoceptors and synaptic plasticity in the perirhinal cortex. Neuroscience, 273, 163–173. doi:10.1016/j.neuroscience.2014.04.070
- LaLumiere, R. T., Buen, T.-V., & McGaugh, J. L. (2003). Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. The Journal of Neuroscience, 23(17), 6754–6758. doi:10.1523/JNEUROSCI.23-17-06754.2003
- Leger, M., Quiedeville, A., Bouet, V., Haelewyn, B., Boulouard, M., Schumann-Bard, P., & Freret, T. (2013). Object recognition test in mice. Nature Protocols, 8(12), 2531–2537. doi:10.1038/nprot.2013.155
- Li, J.-T., Su, Y.-A., Guo, C.-M., Feng, Y., Yang, Y., Huang, R.-H., & Si, T.-M. (2011). Persisting cognitive deficits induced by low-dose, subchronic treatment with mk-801 in adolescent rats. European Journal of Pharmacology, 652(1–3), 65–72. doi:10.1016/j.ejphar.2010.10.074
- Liang, K. C., Juler, R. G., & McGaugh, J. L. (1986). Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Research, 368(1), 125–133. doi:10.1016/0006-8993(86)91049-8
- Liang, K., McGaugh, J. L., & Yao, H.-Y. (1990). Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Research, 508(2), 225–233. doi:10.1016/0006-8993(90)90400-6
- Luine, V. (2002). Sex differences in chronic stress effects on memory in rats. Stress, 5(3), 205–216. doi:10.1080/1025389021000010549
- McGaugh, J. L. (2000). Memory–a century of consolidation. Science, 287(5451), 248–251. doi:10.1126/science.287.5451.248
- McGaugh, J. L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27(1), 1–28. doi:10.1146/annurev.neuro.27.070203.144157
- McIntyre, C. K., Hatfield, T., & McGaugh, J. L. (2002). Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience, 16(7), 1223–1226. doi:10.1046/j.1460-9568.2002.02188.x
- McIntyre, C. K., Miyashita, T., Setlow, B., Marjon, K. D., Steward, O., Guzowski, J. F., & McGaugh, J. L. (2005). Memory-influencing intra-basolateral amygdala drug infusions modulate expression of arc protein in the hippocampus. Proceedings of the National Academy of Sciences, 102(30), 10718–10723. doi:10.1073/pnas.0504436102
- McReynolds, J. R., Anderson, K. M., Donowho, K. M., & McIntyre, C. K. (2014). Noradrenergic actions in the basolateral complex of the amygdala modulate arc expression in hippocampal synapses and consolidation of aversive and non-aversive memory. Neurobiology of Learning and Memory, 115, 49–57. doi:10.1016/j.nlm.2014.08.016
- Minni, A., de Medeiros, G., Helbling, J.-C., Duittoz, A., Marissal-Arvy, N., Foury, A., De Smedt-Peyrusse, V., Pallet, V., & Moisan, M.-P. (2014). Role of corticosteroid binding globulin in emotional reactivity sex differences in mice. Psychoneuroendocrinology, 50, 252–263. doi:10.1016/j.psyneuen.2014.07.029
- Murty, V. P., Ritchey, M., Adcock, R. A., & LaBar, K. S. (2010). Fmri studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia, 48(12), 3459–3469. doi:10.1016/j.neuropsychologia.2010.07.030
- Nirogi, R., Abraham, R., Jayarajan, P., Medapati, R. B., Shanmuganathan, D., Kandikere, V., Irappanavar, S., Saralaya, R., Benade, V., Bhyrapuneni, G., & Muddana, N. (2012). Difference in the norepinephrine levels of experimental and non-experimental rats with age in the object recognition task. Brain Research, 1453, 40–45. doi:10.1016/j.brainres.2012.03.013
- O’Carroll, R. E., Drysdale, E., Cahill, L., Shajahan, P., & Ebmeier, K. P. (1999). Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychological Medicine, 29(5), 1083–1088. doi:10.1017/S0033291799008703
- Okuda, S., Roozendaal, B., & McGaugh, J. L. (2004). Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy of Sciences, 101(3), 853–858. doi:10.1073/pnas.0307803100
- Pezze, M. A., Marshall, H. J., Fone, K. C., & Cassaday, H. J. (2017). Role of the anterior cingulate cortex in the retrieval of novel object recognition memory after a long delay. Learning & Memory, 24(7), 310–317. doi:10.1101/lm.044784.116
- Quirarte, G. L., Galvez, R., Roozendaal, B., & McGaugh, J. L. (1998). Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Research, 808(2), 134–140. doi:10.1016/S0006-8993(98)00795-1
- Roozendaal, B., Castello, N. A., Vedana, G., Barsegyan, A., & McGaugh, J. L. (2008). Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning and Memory, 90(3), 576–579. doi:10.1016/j.nlm.2008.06.010
- Roozendaal, B., Hernandez, A., Cabrera, S. M., Hagewoud, R., Malvaez, M., Stefanko, D. P., Haettig, J., & Wood, M. A. (2010). Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. Journal of Neuroscience, 30(14), 5037–5046. doi:10.1523/JNEUROSCI.5717-09.2010
- Roozendaal, B., & McGaugh, J. L. (2011). Memory modulation. Behavioral Neuroscience, 125(6), 797–824. doi:10.1037/a0026187
- Roozendaal, B., Nguyen, B. T., Power, A. E., & McGaugh, J. L. (1999). Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proceedings of the National Academy of Sciences, 96(20), 11642–11647. doi:10.1073/pnas.96.20.11642
- Roozendaal, B., Okuda, S., Van der Zee, E. A., & McGaugh, J. L. (2006). Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences, 103(17), 6741–6746.
- Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience, 10(3), 211–223. doi:10.1038/nrn2573
- Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., Reiss, A. L., & Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007
- Southwick, S. M., Davis, M., Horner, B., Cahill, L., Morgan, C. A., III, Gold, P. E., Bremner, J. D., & Charney, D. C. (2002). Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. American Journal of Psychiatry, 159(8), 1420–1422. doi:10.1176/appi.ajp.159.8.1420
- Stefanko, D. P., Barrett, R. M., Ly, A. R., Reolon, G. K., & Wood, M. A. (2009). Modulation of long-term memory for object recognition via hdac inhibition. Proceedings of the National Academy of Sciences, 106(23), 9447–9452. doi:10.1073/pnas.0903964106
- Stepanichev, M., Markov, D., Pasikova, N., & Gulyaeva, N. (2016). Behavior and the cholinergic parameters in olfactory bulbectomized female rodents: Difference between rats and mice. Behavioural Brain Research, 297, 5–14. doi:10.1016/j.bbr.2015.09.033
- Sternberg, D., Isaacs, K., Gold, P. E., & McGaugh, J. (1985). Epinephrine facilitation of appetitive learning: Attenuation with adrenergic receptor antagonists. Behavioral and Neural Biology, 44(3), 447–453. doi:10.1016/S0163-1047(85)90856-8
- Sutcliffe, J., Marshall, K., & Neill, J. (2007). Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behavioural Brain Research, 177(1), 117–125. doi:10.1016/j.bbr.2006.10.029
- Szemeredi, K., Komoly, S., Kopin, I. J., Bagdy, G., Keiser, H. R., & Goldstein, D. S. (1991). Simultaneous measurement of plasma and brain extracellular fluid concentrations of catechols after yohimbine administration in rats. Brain Research, 542(1), 8–14. doi:10.1016/0006-8993(91)90990-D
- Takeuchi, T., Duszkiewicz, A. J., Sonneborn, A., Spooner, P. A., Yamasaki, M., Watanabe, M., Smith, C. C., Fernandez, G., Deisseroth, K., Greene, R. W., & Morris, R. G. (2016). Locus coeruleus and dopaminergic consolidation of everyday memory. Nature, 537(7620), 357–362. doi:10.1038/nature19325
- Van Den Heuvel, M. P., & Pol, H. E. H. (2010). Exploring the brain network: A review on resting-state fmri functional connectivity. European Neuropsychopharmacology, 20(8), 519–534. doi:10.1016/j.euroneuro.2010.03.008
- Van Leeuwen, J., Vink, M., Fernández, G., Hermans, E., Joëls, M., Kahn, R., & Vinkers, C. (2018). At-risk individuals display altered brain activity following stress. Neuropsychopharmacology, 43(9), 1954–1960. doi:10.1038/s41386-018-0026-8
- Vogel-Ciernia, A., & Wood, M. A. (2014). Examining object location and object recognition memory in mice. Current Protocols in Neuroscience, 69(1), 17–31. 8.31doi:10.1002/0471142301.ns0831s69
- Wimmer, M. E., Hernandez, P. J., Blackwell, J., & Abel, T. (2012). Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiology of Aging, 33(9), 2220–2224. doi:10.1016/j.neurobiolaging.2011.07.007
- Zerbi, V., Floriou-Servou, A., Markicevic, M., Vermeiren, Y., Sturman, O., Privitera, M., von Ziegler, L., Ferrari, K. D., Weber, B., De Deyn, P. P., Wenderoth, N., & Bohacek, J. (2019). Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron, 103(4), 702–718 e705. doi:10.1016/j.neuron.2019.05.034
