Abstract
Measurement of basal and stress-induced salivary alpha-amylase activity may help to understand autonomic nervous system disturbance in mental disorders. The potential sympathetic nervous system dysregulation in children and adolescent psychopathologies is mostly unknown. The present study was aimed to test the hypothesis that salivary alpha-amylase activity is higher in youths diagnosed with depression than in healthy subjects considering a part of the daily rhythm of enzyme activity and its morning to midday slope. A total of 30 children aged 15 ± 0.46 years (15 patients with depression, 4 boys, 11 girls, and 15 sex- and age-matched healthy controls) participated in the study. Two saliva samples were collected from each subject to measure activity of alpha-amylase in the morning and midday. The results of the present study revealed that the midday but not morning alpha-amylase activity was lower in patients with depression than in healthy controls. The diurnal increase in enzyme activity present in healthy subjects was absent in patients. The children and adolescents with depression exhibited flatter morning to midday slopes of alpha-amylase activity. In conclusion, the present results indicate a disturbance of alpha-amylase daily rhythm in youths with depression and motivate further studies on the relationship between sympathetic activation and mood disorders.
Introduction
Mental disorders are known to be associated with alteration of autonomic nervous system functioning. One alternative to assess its functioning is the evaluation of salivary alpha-amylase activity, which can be considered a noninvasive and easily applicable indirect marker of the sympathetic activation. The employment of basal and stress-induced salivary alpha-amylase activity, as an indicator of autonomic nervous system dysregulation in mental disorders is scarce but promising (Schumacher et al., Citation2013; Buzgoova et al., Citation2020).
Recent studies in patients with major depressive disorder suggest both increased and decreased salivary alpha-amylase activity compared to that in healthy participants. Activity of salivary alpha-amylase in patients with depression was found to be increased at the time of awakening (Bauduin et al., Citation2018) and throughout the day when the diurnal rhythm of salivary alpha-amylase activity was thoroughly investigated (Booij et al., Citation2015). On the other hand, the drug naive patients with first episode of depression exhibited lower salivary alpha-amylase activity compared to healthy subjects as measured three times around 9.00 a.m. (Szarmach et al., Citation2017).
The sympathetic nervous system dysregulation in mental disorders is even less clear in children and adolescents. There are few papers on alpha-amylase in adolescents and children related to anxiety showing either higher (Yorbik et al., Citation2016) or lower (Kapsdorfer et al., Citation2018) activity of salivary alpha-amylase in anxious subjects. Funke et al. (Citation2017) observed increased diurnal profile but blunted response to an acute stressor of salivary alpha-amylase activity in children with generalized anxiety disorder with comorbid depression compared to values in healthy children.
Apparently, research has not yet examined salivary alpha-amylase activity in children and adolescents with depressive disorder. Even with respect to depressive symptoms in healthy children, only one recent study (Khoury et al., Citation2020) describes higher baseline salivary alpha-amylase activity in children with mother-reported depressive symptoms. This study was aimed to test the hypothesis that salivary alpha-amylase activity is higher in youths diagnosed with depression than in healthy subjects considering a part of the daily rhythm of enzyme activity and its morning to midday slope.
Material and methods
Subjects
A total of thirty youths of both sexes were recruited to participate in this study. The study sample included 15 out-patients (4 boys and 11 girls) suffering from depressive disorder (n = 6) or mixed anxiety-depressive disorder (n = 9) registered at the Department of Pediatric Psychiatry, Faculty of Medicine, Comenius University and the National Institute of Children’s Diseases. Out of them, ten patients were untreated and diagnosed for the first time. The treatment was performed by standard antidepressants. The diagnoses were determined according to the International Classification of Diseases, revision 10 (ICD10). The reference control group consisted of 15 sex- and age-matched healthy subjects registered in the Pediatric Center Juvenalia. s.r.o Dunajska Streda, Slovakia. All girls included in the sample, with the exception of one healthy subject, were postmenarchal. The exclusion criteria for both groups of participants were chronic somatic diseases, endocrinopathy, dietary restrictions (vegetarians, lactose intolerance, and celiac disease), psychotic disorders, eating disorders, addiction to psychoactive compounds, personality disorders, organic mental disorders, and pervasive developmental disorders. The exclusion criteria were examined by the medical records available, the parent reports and the pedopsychiatric examination. Parents of the study participants obtained detailed information about the study and provided written informed consent with study participation. The study was approved by the Ethics Committee of the National Institute of Children’s Diseases and the Faculty of Medicine, Comenius University Bratislava (20 March 2013) in agreement with the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from parents or legal guardians and children gave verbal assent prior to participation in the study.
Study design and analysis of alpha-amylase activity
Subject characteristics (age, sex, and body mass index [BMI]) and relevant clinical variables were recorded for each participant (). The evaluation of the depressive state in children patients was performed using the self-rated scale Children’s Depression Inventory (CDI) (Kovacs et al., Citation1984; Kovacs, Citation1998).
Table 1. Characteristics of participants.
Two saliva samples were collected from each subject to measure activity of alpha-amylase during the day. The saliva samples of patients were collected during the examination in a children’s psychiatric out-patient clinic. As for control group of healthy children, saliva samples were collected during regular visit at pediatrician office. Morning saliva samples were taken between 8:00 and 8:30 a.m. and midday samples were collected between 11:00 and 11:30 a.m. The parents were asked to wake the children up 90 min before the morning sampling. Children were allowed to brush their teeth earlier than 60 min before and remained fasting until morning saliva collection. They were exposed to a short routine examination by their psychiatrist or pediatrician without any stressful procedures. Thereafter they left the office to have breakfast and perform undemanding free-time activities together with their parents in the surroundings of the clinic. With respect to the midday sampling, children were instructed to refrain from eating and drinking for 60 min before saliva collection. At both time intervals, the children were sitting for at least 10 minutes before sampling. The subjects were asked to get cotton swab out of the Salivette and to keep it in mouth for 1 min. The samples were collected into saliva sampling tubes (Salivette® device, Sarstedt, UK). Salivettes were stored at −20 °C until analyzed. The activity of salivary alpha-amylase was measured by commercially available kinetic reaction assay (Salimetrics, Suffolk, UK; coefficient of intra- and inter-assay variation was 6.7% and 3.6%, respectively).
Statistical analysis
All data were checked for normality of distribution by the Shapiro–Wilk test. Repeated-measures general linear model (GLM) with the within-subject factor time (morning vs. midday), the between-subject factor group (children with depression vs. healthy children) and the covariates sex and BMI was used to examine the effects of group, time and the interaction of group by time on alpha-amylase activity. Tukey pairwise comparisons for the interaction effect were performed.
Morning to midday slope of alpha-amylase activity for each subject was calculated as described previously (Izakova et al., Citation2020). Briefly, alpha-amylase slope was computed from the line’s equation in the slope-intercept form “y = mx + b.” Letter “m” is the angular coefficient which represents the slope of the line. Large absolute values of the angular coefficient m correspond to a steeper straight line, and smaller absolute values correspond to a flatter line. The values of angular coefficients were analyzed by t-test for independent groups. Results are expressed as dot plots with each dot representing individual subject with mean ± SEM. The overall level of statistical significance was defined as p < .05.
Results
Characteristics of patients diagnosed with depression and their reference group of healthy controls are summarized in . As expected, there was no difference in sex, age, and BMI of healthy subjects and patients.
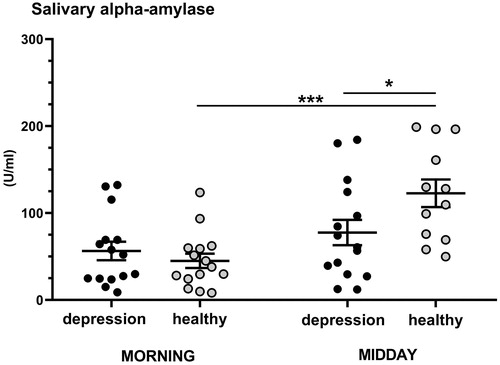
The repeated-measures GLM revealed a significant time*group interaction (F(1, 23) = 8.61, p = .007, η2 = 0.27) on alpha-amylase activity (). Post-hoc analysis showed that the midday activity of alpha-amylase was significantly lower in children with depression compared to healthy children (p = .048). There was a significant increase in alpha-amylase activity during the midday compared to morning in healthy children (p = 0.001), but not in children with depression. GLM showed that sex and BMI were not significant covariates (for sex: F(1, 23) = 0.99, p = .33, η2 = 0.04; for BMI: F(1, 23) = 1.09, p = .30, η2 = 0.05).
Figure 1. Morning and midday activity of alpha-amylase in the group of youths with depression (n = 15) and the group of healthy youths (n = 15). Results are expressed as dot plots with each dot representing individual subject with mean ± SEM represented by horizontal lines. Statistical significance as revealed by repeated-measures GLM with subsequent Tukey pairwise comparisons: *p < .05, *p < .001.
We have performed additional statistical analyses to check the potential influence of different diagnoses or the presence of treatment on salivary alpha-amylase activity. Repeated-measures ANOVA with group as between-subject factor (group of children with depressive disorder vs. group of children with mixed anxiety-depressive disorder) and time as within-subject factor time (morning vs. midday) did not reveal significant main effect of group (F(1, 13) = 1.67, p = .217, η2 = 0.114) or significant time × group interaction (F(1, 13) = 2.822, p = .116, η2 = 0.178). Statistical analysis of data on morning salivary alpha-amylase activity by t-test for independent groups did not show differences between the group of children with depressive disorder vs. group of children with mixed anxiety-depressive disorder (t13 = 0.89, p = .386). No significant effect of group was observed on midday values (t13 = 1.57, p = .154) or morning to midday alpha-amylase slope (t13 = 1.68, p = .116). Similarly, repeated-measures ANOVA with group as between-subject factor (treated vs. untreated) and time as within-subject factor time (morning vs. midday) did not reveal significant main effect of group (F(1, 13) = 2.13, p = .167, η2 = 0.141) or significant time × group interaction (F(1, 13) = 0.75, p = .401, η2 = 0.054). There were no significant differences in the morning (t13 = 1.38, p = .189) or midday salivary alpha-amylase activity (t13 = 1.42, p = .177) and morning to midday alpha-amylase slopes (t13 = 0.40, p = .401) between the treated and untreated children with depression, as revealed by t-test for independent groups.
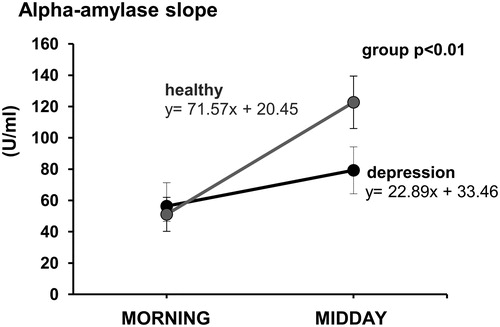
Morning to midday alpha-amylase slopes calculated from the line’s equations in the slope-intercept form is expressed as the values of angular coefficients m. A t-test for independent groups revealed a significant difference in the morning to midday alpha-amylase slope between the groups (t25 = −2.98, p = .006, ). Significantly flatter slopes of morning to midday alpha-amylase activity slopes were observed in youths with depression with angular coefficient m values of 22.89 ± 8.15 compared to those in healthy controls with m values of 71.57 ± 15.19.
Figure 2. Morning to midday alpha-amylase slopes in the group of youths with depression (n = 15) and the group of healthy youths (n = 15). Slopes calculated from the line’s equations in the slope-intercept form are expressed as the values of angular coefficients m. Results are expressed as mean ± SEM. Statistical significance as revealed by t-test for independent groups.
Discussion
The results of this study revealed differences in alpha-amylase activity between the youths with depression and healthy subjects. Concretely, midday alpha-amylase activity was lower in patients with depression and the diurnal increase in enzyme activity present in healthy subjects was absent in patients.
Lower alpha-amylase activity in youths with depression as compared to that in healthy controls was unexpected with respect to the hypothesis postulated. As the alpha-amylase activity has not yet been investigated in this context, the present data cannot be directly confronted with previous studies. The majority of the present sample was newly diagnosed adolescents and consistently, lower alpha-amylase activity was observed in drug-naive adult patients with first episode of depression compared to healthy subjects (Szarmach et al., Citation2017). The present finding is consistent also with the results obtained in anxious healthy prepubertal children exhibiting lower alpha-amylase activity compared to non-anxious children (Kapsdorfer et al., Citation2018). Thus, the low alpha-amylase activity revealed in this study may be related to age scope studied as well as to the presence of patients with mixed anxiety-depressive disorder in the present sample.
As mentioned above, alpha-amylase activity is an indirect marker of sympathetic activation and the association between depressive disorder and increased sympathetic function in adults is well established. The corresponding data in children and adolescents are, however, surprisingly rare. A few studies reported higher heart rate in youths with depression compared to healthy subjects (Byrne et al., Citation2010; Vloet et al., Citation2019), but the resting heart rate differences were not explained by increased sympathetic activity (Byrne et al., Citation2010). The present results on alpha-amylase activity are consistent with blunted sympathetic response to anxiety tasks in children with autism (Panju et al., Citation2015).
The present sample included an imbalanced proportion of boys and girls reflecting the higher prevalence of depressive disorders in females. We failed to find an influence of sex on alpha-amylase activity. Data on sex differences in adolescents are not available and the findings in adults are ambiguous. Some authors reported that men exhibited significantly higher baseline salivary alpha-amylase activity, others revealed that females had a more pronounced increase in alpha-amylase activity over the course of the day or failed to observe any differences (Nater et al., Citation2007; van Stegeren et al., Citation2008; Wingenfeld et al., Citation2010; Helpman et al., Citation2017). In small children, diurnal pattern of salivary alpha-amylase was found to be independent of sex (Lim et al., Citation2019).
The finding of significant rise in alpha-amylase activity from the morning to the midday in healthy subjects is in agreement with the known daily rhythm of this enzyme (Rohleder et al., Citation2004). As to the children and adolescents, the present results are in consistent with previous observations of Wolf et al. (Citation2008) who recorded an increase in alpha-amylase activity around midday. In young children, diurnal pattern of salivary alpha-amylase activity did not correlate with age, sex, and BMI (Lim et al., Citation2019) which was confirmed also in the present sample. Furthermore, these authors showed that alpha-amylase diurnal pattern was independent of other demographic factors and food intake. Above mentioned rise in alpha-amylase activity corresponding to the daily rhythm, was absent in youth with depression. Thus, the present findings demonstrate for the first time a disturbance of the daily rhythm of alpha-amylase activity in youth with depression.
In this study, the disturbance of diurnal pattern of alpha-amylase was confirmed also by calculation of morning to midday slopes. Significantly flatter slopes were revealed in patients compared to controls. Research reports on diurnal alpha-amylase changes expressed as slopes are very rare. Skoluda et al. (Citation2017) used diurnal alpha-amylase slopes to evaluate long-term stability pattern in adults. Other authors related alpha-amylase diurnal slopes to hyperactivity problems in preschool children (Messerli-Bürgy et al., Citation2018).
Even though we found no statistical differences in salivary alpha-amylase activity between children with depressive disorder vs. group of children with mixed anxiety-depressive disorder or between the treated and untreated children with depression, the heterogeneity of the sample remains a limitation of the present study. Other limitations are possible differences in hormonal status and the short time period investigated (morning to midday). Because of the small sample size, the results obtained must be interpreted with caution and require verification in future studies.
In conclusion, children and adolescents with depression exhibited lower values of alpha-amylase activity and flatter morning to midday slopes which may indicate a disturbance of alpha-amylase daily rhythm. The present results motivate further studies on the relationship between sympathetic activation and mood disorders.
Acknowledgments
The authors thank Zuzana Nagyova, MD, MPH from Pediatric Center Juvenalia. s.r.o Dunajska Streda, Slovakia for clinical examination and saliva collection of healthy controls as well as Ludmila Zilava for her help with analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Daniela Jezova
Prof. Daniela Jezova, PharmD is a full professor of Pharmacology at the Faculty of Medicine of Comenius University and the Head of the Laboratory of Pharmacological Neuroendocrinology being a part of the Department of Endocrine Regulations and Psychopharmacology of the IEE BMC SAS, Slovakia. She has been a vice-president of the Slovak Academy of Sciences and vice-president of All European Academies. She is serving as an international expert in European research networks including the panel of European Research Council (ERC) on Endocrinology, Pathophysiology and Physiology. Her research team focused on stress research and psychopharmacology in both humans and animals belongs to the evaluated top research teams in Slovakia. Assoc.
Jana Trebaticka
Prof. Jana Trebaticka, MD is an associated professor of psychiatry and head of the Department of Child Psychiatry of the Faculty of Medicine and National Institute of Child Diseases in Bratislava. She was the chief of Section of Child and Adolescent Psychiatry of Slovak Psychiatric Association. She is a guarantor of child psychiatry specialization study of clinicians. She leads a team focused on depressive disorders and ADHD in children and adolescents.
Katarina Buzgoova
Katarina Buzgoova, PharmD is a postdoctoral fellow at the Laboratory of Pharmacological Neuroendocrinology, Department of Endocrine Regulations and Psychopharmacology of the Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, Slovakia. She has finished her PhD in pharmacology at the Faculty of Pharmacy of Comenius University. Her research is focused on stress and psychopharmacology mainly in clinical studies in humans. Prof. dipl. Ing.
Zdenka Durackova
Zdenka Durackova, PhD. is a full professor of Biochemistry at the Faculty of Medicine, Comenius University, Bratislava. She was the head of the Department of Medical Chemistry, Biochemistry and Clinical Biochemistry of the Faculty of Medicine (2006-2012). She led the team or managed the team within the framework of EU grants from the Interreg SR-AT program (Diaplant, Nutriaging). She currently leads a team that deals with the molecular basis of depressive disorders in the co-operation with Department of Child psychiatry of Faculty of Medicine, with Department of Biomedical Physic of the Faculty of Mathematic, Physic and Informatic of Comenius University and Department of Endocrine Regulations and Psychopharmacology of the IEE BMC SAS, Slovakia.
Natasa Hlavacova
Natasa Hlavacova, PhD. is a Head of the Department of Endocrine Regulations and Psychopharmacology at the Institute of Experimental Endocrinology of the Biomedical Research Center, SAS, Slovakia. She is a member of the Scientific Committee of the Biomedical Research Center, SAS. She is serving as an Editor of the General Physiology and Biophysics. She is expert in the field of Behavioral Neuroscience.
References
- Bauduin, S. E. E. C., van Noorden, M. S., van der Werff, S. J. A., de Leeuw, M., van Hemert, A. M., van der Wee, N. J. A., & Giltay, E. J. (2018). Elevated salivary alpha-amylase levels at awakening in patients with depression. Psychoneuroendocrinology, 97, 69–77. https://doi.org/10.1016/j.psyneuen.2018.07.001
- Booij, S. H., Bos, E. H., Bouwmans, M. E., van Faassen, M., Kema, I. P., Oldehinkel, A. J., & de Jonge, P. (2015). Cortisol and α-amylase secretion patterns between and within depressed and non-depressed individuals. PLoS One, 10(7), e0131002. https://doi.org/10.1371/journal.pone.0131002
- Buzgoova, K., Balagova, L., Marko, M., Kapsdorfer, D., Riecansky, I., & Jezova, D. (2020). Higher perceived stress is associated with lower cortisol concentrations but higher salivary interleukin-1beta in socially evaluated cold pressor test. Stress, 23(3), 248–255. https://doi.org/10.1080/10253890.2019.1660872
- Byrne, M. L., Sheeber, L., Simmons, J. G., Davis, B., Shortt, J. W., Katz, L. F., & Allen, N. B. (2010). Autonomic cardiac control in depressed adolescents. Depression and Anxiety, 27(11), 1050–1056. https://doi.org/10.1002/da.20717
- Funke, R., Eichler, A., Distler, J., Golub, Y., Kratz, O., & Moll, G. H. (2017). Stress system dysregulation in pediatric generalized anxiety disorder associated with comorbid depression. Stress Health, 33(5), 518–529. https://doi.org/10.1002/smi.2736
- Helpman, L., Penso, J., Zagoory-Sharon, O., Feldman, R., & Gilboa-Schechtman, E. (2017). Endocrine and emotional response to exclusion among women and men; cortisol, salivary alpha amylase, and mood. Anxiety, Stress, and Coping, 30(3), 253–263. https://doi.org/10.1080/10615806.2016.1269323
- Izakova, L., Hlavacova, N., Segeda, V., Kapsdorfer, D., Morovicsova, E., & Jezova, D. (2020). Salivary aldosterone, cortisol and their morning to evening slopes in patients with depressive disorder and healthy subjects: Acute episode and follow up six months after reaching remission. Neuroendocrinology (in Press), https://doi.org/10.1159/000505921
- Kapsdorfer, D., Hlavacova, N., Vondrova, D., Argalasova, L., Sevcikova, L., & Jezova, D. (2018). Neuroendocrine response to school load in prepubertal children: Focus on trait anxiety. Cellular and Molecular Neurobiology, 38(1), 155–162. https://doi.org/10.1007/s10571-017-0544-7
- Khoury, J. E., Jamieson, B., Gonzalez, A., & Atkinson, L. (2020). Child depressive symptoms: Associations with salivary cortisol and alpha amylase in two distinct challenges. Biological Psychology, 149, 107808. https://doi.org/10.1016/j.biopsycho.2019.107808
- Kovacs, M. (1998). Childrens depression inventory CDI. Psychodiagnos- tika a.s., Bratislava, SR (In Slovak).
- Kovacs, M., Feinberg, T. L., Crouse-Novak, M. A., Paulauskas, S. L., & Finkelstein, R. (1984). Depressive disorders in childhood. I. A longitudinal prospective study of characteristics and recovery. Archives of General Psychiatry, 41(3), 229–237. https://doi.org/10.1001/archpsyc.1984.01790140019002
- Lim, P. W., Nambiar, S., Muhardi, L., Abdul Kader, U. H., Garssen, J., & Sandalova, E. (2019). Young children display diurnal patterns of salivary IgA and alpha-amylase expression which are independent of food intake and demographic factors. BioMed Research International, 2019, 3687416. https://doi.org/10.1155/2019/3687416
- Messerli-Bürgy, N., Arhab, A., Stülb, K., Kakebeeke, T. H., Zysset, A. E., Leeger-Aschmann, C. S., Schmutz, E. A., Ehlert, U., Kriemler, S., Jenni, O. G., Munsch, S., & Puder, J. J. (2018). Physiological stress measures in preschool children and their relationship with body composition and behavioral problems. Developmental Psychobiology, 60(8), 1009–1022. https://doi.org/10.1002/dev.21782
- Nater, U. M., Rohleder, N., Schlotz, W., Ehlert, U., & Kirschbaum, C. (2007). Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology, 32(4), 392–401. https://doi.org/10.1016/j.psyneuen.2007.02.007
- Panju, S., Brian, J., Dupuis, A., Anagnostou, E., & Kushki, A. (2015). Atypical sympathetic arousal in children with autism spectrum disorder and its association with anxiety symptomatology. Molecular Autism, 6, 64. https://doi.org/10.1186/s13229-015-0057-5
- Rohleder, N., Nater, U. M., Wolf, J. M., Ehlert, U., & Kirschbaum, C. (2004). Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Annals of the New York Academy of Sciences, 1032, 258–263. https://doi.org/10.1196/annals.1314.033
- Schumacher, S., Kirschbaum, C., Fydrich, T., & Ströhle, A. (2013). Is salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders?-a review of preliminary findings and the interactions with cortisol. Psychoneuroendocrinology, 38(6), 729–743. https://doi.org/10.1016/j.psyneuen.2013.02.003
- Skoluda, N., La Marca, R., Gollwitzer, M., Müller, A., Limm, H., Marten-Mittag, B., Gündel, H., Angerer, P., & Nater, U. M. (2017). Long-term stability of diurnal salivary cortisol and alpha-amylase secretion patterns. Physiology & Behavior, 175, 1–8. https://doi.org/10.1016/j.physbeh.2017.03.021
- Szarmach, J., Cubała, W. J., Landowski, J., & Chrzanowska, A. (2017). No relationship between baseline salivary alpha-amylase and State-Trait Anxiety Inventory Score in drug-naïve patients with short-illness-duration first episode major depressive disorder: An exploratory study. Journal of Clinical and Experimental Dentistry, 9(4), e527–e530. https://doi.org/10.4317/jced.53631
- van Stegeren, A. H., Wolf, O. T., & Kindt, M. (2008). Salivary alpha amylase and cortisol responses to different stress tasks: Impact of sex. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 69(1), 33–40. https://doi.org/10.1016/j.ijpsycho.2008.02.008
- Vloet, T. D., Jans, T., Frey, A., Häußler, M., Vloet, A., Geissler, J., Susewind, M., von Dobschütz, B., Frantz, S., & Romanos, M. (2019). Mean heart rate and parameters of heart rate variability in depressive children and the effects of antidepressant medication. Zeitschrift Fur Kinder- Und Jugendpsychiatrie Und Psychotherapie, 47(3), 253–260. https://doi.org/10.1024/1422-4917/a000672
- Wingenfeld, K., Schulz, M., Damkroeger, A., Philippsen, C., Rose, M., & Driessen, M. (2010). The diurnal course of salivary alpha-amylase in nurses: An investigation of potential confounders and associations with stress. Biological Psychology, 85(1), 179–181. https://doi.org/10.1016/j.biopsycho.2010.04.005
- Wolf, J. M., Nicholls, E., & Chen, E. (2008). Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biological Psychology, 78(1), 20–28. https://doi.org/10.1016/j.biopsycho.2007.12.004
- Yorbik, O., Mutlu, C., Ozturk, O., Altinay, D. K., Tanju, I. A., & Kurt, I. (2016). Salivary alpha amylase levels in youths with anxiety disorders. Psychiatry Research, 235, 148–153. https://doi.org/10.1016/j.psychres.2015.11.021
