Abstract
To date, only a few studies have examined whether and when stressed individuals are still prosocially motivated and willing to help others, which is in contrast to the relevance and importance that helping others has for our society. The present study investigates the impact of affective and biopsychological acute stress responses on prosociality (prosocial motivation, helping behavior) under controlled laboratory conditions. In addition, it was examined whether this relationship is affected by individuals’ current life stress and the cognitive ability to keep stress-related thoughts at bay. To induce acute stress responses (heart rate, negative affect, salivary alpha-amylase, cortisol), 55 individuals (28 women, M = 24 years old, SD = 4.53) were exposed to the Trier Social Stress Test (TSST). Current life stress (cortisol) was assessed over two days of participants’ everyday lives. Thought control ability was assessed with the think/no-think paradigm and was additionally manipulated after the acute stress intervention (TSST) via instructions. The results showed that acute stress was positively associated with prosociality. Specifically, negative affect was positively related to prosocial motivation and salivary alpha-amylase was positively associated with helping behavior. Current life stress moderated the relationship between salivary cortisol and helping behavior: the association was positive at low levels of current life stress. The instruction to control one’s thoughts but not participants’ general ability to do so reduced stress responses (negative affect). In sum, the findings suggest that prosociality increases following acute stress and that this effect depends on the level of current life stress. Additionally, adopting the strategy of controlling stress-related thoughts was found to be promising for attenuating individuals’ stress responses.
Introduction
Increasing numbers of individuals are reporting enhanced stress levels at work and in their private lives (American Psychological Association, Citation2018). Although researchers have begun to emphasize stress as an important antecedent of prosociality, only a few studies have investigated whether stressed individuals are still prosocially motivated and willing to help others (e.g., Passarelli & Buchanan, Citation2020; Sollberger et al., Citation2016; von Dawans et al., Citation2019). The present study addresses this issue by investigating whether and when acute stress responses result in lower motivation and willingness to help others, as being prosocial benefits individuals, organizations, and society (e.g., Podsakoff et al., Citation2009).
Traditionally, individuals respond to acute stressors with the fight-or-flight pattern (Everly & Lating, Citation2019). Here, the body prepares its muscles to respond to perceived threats (discharge of the sympathetic nervous system, e.g., increased heart rate; Everly & Lating, Citation2019), suggesting a potential negative relationship between acute stress responses and prosociality (flight reaction). Research has found that acute stress induced by the Trier Social Stress Test (TSST; Kirschbaum et al., Citation1993) diminishes charitable giving (prosocial behavior; Vinkers et al., Citation2013). Sollberger et al. (Citation2016) even found that acute stress increased donation frequency, but the amount of money donated decreased with higher levels of stress. Field studies captioning stress exposure mostly substantiate the negative associations between stress and prosociality (e.g., Larson & Moses, Citation2017) and argue that this is due to individuals’ limited resources in stressful situations (Hobfoll, Citation1989). However, some studies have also found positive associations between acute stress and prosocial behaviors (e.g., sharing patterns; Tomova et al., Citation2017; von Dawans et al., Citation2019), which is explained by the tend-and-befriend pattern (Taylor et al., Citation2000). The tend-and-befriend pattern states that individuals affiliate themselves in times of stress which states and therefore adopt to stressful situations by associating with others which in turn promotes prosociality. Initially, the tend-and-befriend pattern has been applied to women’s stress responses only, but was later extended to men’s stress responses as well (Taylor, Citation2006; Taylor et al., Citation2000; von Dawans et al., Citation2019). Additionally, researchers have already shown that there are differences in stress reactivity between women and men (Kudielka & Kirschbaum, Citation2005), for example, men having greater cortisol secretion after acute psychosocial stress than women. Hence, there are differences in stress reactivity for men and women that should be respected.
We follow the majority of research, and propose that acute stress responses are negatively related to prosociality. We induced stress with the protocol of the TSST (Kirschbaum et al., Citation1993). The TSST is a psychosocial stressor, i.e. a stimulus that uses public speaking and cognitive tasks to trigger stress responses (Kirschbaum et al., Citation1993). The difference to stress overall is, that the TSST artificially triggers acute stress responses, however, induced stress in the laboratory is expected to reflect the way individuals react to stress in natural settings (Cohen & Hamrick, Citation2003). We also take into account the variability of the findings by examining two moderators (current life stress, thought control) which might shape the negative impact of acute stress on prosociality. First, we expand existing research by examining individuals’ current life stress. Laboratory settings only capture temporary stress responses, but fail to account for individuals’ current life stress, which could potentially impact the extent of acute stress responses (Kidd et al., Citation2014). Second, a growing body of literature has examined cognitive factors that mitigate individual stress responses, such as thought control (Ma et al., Citation2017; Webb et al., Citation2012), but has not yet proven potential positive consequences for individuals’ prosociality. Thought control is a cognitive control process that helps to regulate emotions and to maintain a positive self-image (Anderson & Hanslmayr, Citation2014; Engen & Anderson, Citation2018). By controlling one’s own thoughts valuable resources can be protected which can lead to better stress recovery and prosociality. As both the ability to control one’s thoughts and the adoption of a thought control strategy (distracting oneself by thinking of something positive unrelated to the stress situation; Webb et al., Citation2012) can be beneficial for stress experiences, we investigate both the ability and the adoption of a strategy to cope with acute stress. Both should mitigate a) stress reactions and b) enable individuals to invest in prosociality.
In sum, this study examines the potential corrosive association between acute stress responses and two aspects of prosociality (prosocial motivation, helping behavior; Hypothesis 1). It was hypothesized that high levels of current life stress (Hypothesis 2) would intensify the potential negative relationship, whereas thought control (Hypotheses 3 and 4) would attenuate the proposed association. Our model and hypotheses are shown in .Footnote1
Figure 1. Conceptual model. Note. HR: heart rate; NA: negative affect; TNT: think/no-think task. H1: Acute stress responses [(a) HR, (b) NA, (c) alpha-amylase, (d) cortisol] are negatively associated with prosociality [prosocial motivation, helping behavior]. H2: Current life stress moderates the relationship between acute stress responses [(a) HR, (b) NA, (c) alpha-amylase, (d) cortisol] and prosociality [prosocial motivation, helping behavior]. The negative relationship between acute stress responses and prosociality is stronger for individuals with high current life stress. H3: The thought control strategy moderates the effect of acute stress responses [(a) HR, (b) NA] on stress recovery [(a) HR, (b) NA] and prosociality [prosocial motivation, helping behavior] such that individuals with the thought control strategy should be less stressed and more prosocial. H4: Thought control ability and the thought control strategy moderate the effect of acute stress responses [(a) HR, (b) NA] on stress recovery [(a) HR, (b) NA] and prosociality [prosocial motivation, helping behavior] such that individuals with the ability to control thoughts and the thought control strategy should be less stressed and more prosocial.
![Figure 1. Conceptual model. Note. HR: heart rate; NA: negative affect; TNT: think/no-think task. H1: Acute stress responses [(a) HR, (b) NA, (c) alpha-amylase, (d) cortisol] are negatively associated with prosociality [prosocial motivation, helping behavior]. H2: Current life stress moderates the relationship between acute stress responses [(a) HR, (b) NA, (c) alpha-amylase, (d) cortisol] and prosociality [prosocial motivation, helping behavior]. The negative relationship between acute stress responses and prosociality is stronger for individuals with high current life stress. H3: The thought control strategy moderates the effect of acute stress responses [(a) HR, (b) NA] on stress recovery [(a) HR, (b) NA] and prosociality [prosocial motivation, helping behavior] such that individuals with the thought control strategy should be less stressed and more prosocial. H4: Thought control ability and the thought control strategy moderate the effect of acute stress responses [(a) HR, (b) NA] on stress recovery [(a) HR, (b) NA] and prosociality [prosocial motivation, helping behavior] such that individuals with the ability to control thoughts and the thought control strategy should be less stressed and more prosocial.](/cms/asset/fc4f4db3-7457-4acf-91c5-389430478e65/ists_a_2054697_f0001_b.jpg)
Materials and method
Participants
Fifty-six participants were recruited via social media platforms and flyers. We excluded participants who met one of the following criteria: presence of a chronic cardiovascular, endocrine, or psychiatric disease; currently taking psychoactive medication, glucocorticoids or beta-blockers; and a body mass index (BMI) under 18 or above 30 kg/m2. We further included only female participants who did not use hormonal contraceptives, as free cortisol reactivity is blunted in this group (Kirschbaum et al., Citation1999). Additionally, only individuals with no prior experience with the Trier Social Stress Test (TSST; Kirschbaum et al., Citation1993) or similar paradigms were permitted to participate. Based on these criteria, one person was excluded from all analyses as he or she had already participated in a TSST test; three persons from the cortisol analysis due to elevated baseline cortisol levels (z-scores or 10+ nmol/L), and one person was excluded from the heart rate analysis due to an invalid response by the heart rate monitor. In the final sample (N = 55), participants’ average age was 24 years old (SD = 4.53; BMI = 23.56 CG/m2, SD = 3.27). Twenty-eight participants were female (50.91%), and participants were predominantly students (85.45%), 9.09% were employed, 3.64% were unemployed and one person did not answer (1.81%). All participants gave written consent before participation. The study was preregistered (AsPredicted #45244) and approved by the local ethics committee in accordance with the Declaration of Helsinki.
Procedure
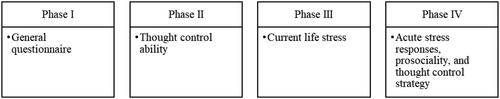
The study was organized in four phases: (1) completing the general questionnaire, (2) conducting the think/no-think task (TNT; Anderson & Green, Citation2001) in the laboratory to measure thought control ability, (3) collecting three salivary cortisol samples a day for two consecutive days to measure current life stress, (4) completing the TSST (Kirschbaum et al., Citation1993; ).
General questionnaire: Individuals were asked to complete a general online questionnaire, which included demographic variables and variables which were used to determine the inclusion criteria.
TNT task: One week later, participants completed the TNT (Anderson & Green, Citation2001) in the lab to assess their thought control ability. We selected 58 slightly linked, neutrally valenced word pairs (nouns; Melinger & Weber, Citation2006). Ten word pairs were used for training purposes. The remaining 48 word pairs were divided into three groups (16 words pe
r group: baseline, think, no-think) and rotated across subjects and experimental conditions (three versions).
The TNT comprises four p
hases: the learning phase, first recall phase, think/no-think phase, and second recall phase (Anderson & Green, Citation2001). In the learning phase, all 58 word pairs (Melinger & Weber, Citation2006) were presented on a screen for 6000 ms each in randomized order and were studied by participants. In the first recall phase, cue words were presented in a pseudo-randomized order for 4000 ms each. Participants were instructed to vocally express the corresponding response word.
In the think/no-think phase, cue words were presented for 3000 ms each. Green-colored think cues signaled that participants should silently think of the response word in order to retain the association in memory. Red-colored no-think cues asked participants to focus on the cue word only, and suppress all thoughts of the response word, without otherwise distracting themselves. The 16 think and 16 no-think cues were presented 12 times in randomized order. The 16 baseline words were not presented. Lastly, in the second recall phase, all 48 cue words were again presented in black text in pseudo-randomized order. Participants were instructed to vocally express the previously studied response word associated with each cue, without regard for the instructions in the think/no-think phase.
3. Current life stress: Then, as they went about their daily lives, participants were instructed to collect three saliva samples a day for two consecutive days to measure their current life stress. After completing the TNT, participants received a study package that included precise instructions on saliva collection, a diary booklet, and six labeled salivettes (Sarstedt, Nümbrecht, Germany). Current life stress was measured by means of cortisol levels. The procedure and saliva sampling method was thoroughly explained in-person while participants were still in the lab. Each salivette had to be inserted into and moved back and forth in the mouth for at least 1 min. Participants were asked to provide saliva samples (to measure cortisol) on two consecutive days immediately after waking up, 30 min after waking up, and at bedtime. The two consecutive days had to be from Monday to Friday. Participants were also instructed to place the samples in their freezer immediately afterwards.
4. TSST protocol: Acute stress responses were induced with the TSST (Kirschbaum et al., Citation1993). It has been repeatedly shown that the TSST reliably elicits physiological and subjective stress responses in individuals (Kirschbaum, Citation2010). The measurement points within this phase are displayed in .
Figure 3. Measurement points in Phase 4. Note. Base: baseline; ASR: acute stress response; Rec: later stress response; Strategy: thought control strategy; HR: heart rate; NA: negative affect; AA: alpha amylase; C: cortisol. NA, A, and C were measured at s0, s1, s2, s3, s4. HR was measured continuously.
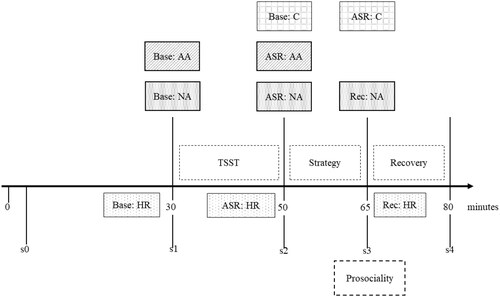
Three to five days before the appointment, participants received an e-mail instructing them to refrain from alcohol, caffeine and smoking for the 24 hours prior to their laboratory appointment. Additionally, they were asked to ensure that they got at least 7 hours of sleep and to get up before 9 a.m. on the target day. To control for diurnal variations, all appointments took place between 13:00 and 21:00 hours. After arriving at the laboratory, participants were seated in a separate room and were asked to read and sign the informed consent form. Participants were then provided with a heart rate monitor (V800, Polar Electro, (need city/country), Giles et al., Citation2016) and asked to take the first salivary sample (s0; ); simultaneous to the diary report, participants had to insert the salivette into their mouths keep it in for at least 1 min while moving it back and forth. 20 min after the first sample, the baseline salivary sample (s1) was taken. Immediately after the baseline sample, participants were led to another room, where the TSST (Kirschbaum et al., Citation1993) took place. In the present study, the TSST consisted of the following tasks: a 3-min preparation period followed by a 5-min self-presentation speech task (mock job interview) and a 5-min arithmetic task. Both tasks were conducted in front of an allegedly expert audience of two neutrally dressed professionals in lab coats and were videorecorded. Immediately afterwards, participants were asked to return to their separate room and take another saliva sample (s2). After the TSST, participants’ thought control strategy was manipulated and prosociality (prosocial motivation, helping behavior) was measured. Applying a double-blind setting, participants either received the experimental (EG) or the control group (CG) instructions regarding the thought control strategy (for details, see measures section). 15 min after s2, another saliva sample was taken (s3). Subsequently, prosocial motivation and helping behavior were measured. Another 15 min after s3, the final saliva sample (s4) was taken. Participants were then debriefed about the stress setting and the purpose of the study.
Measures
Acute stress responses
We assessed acute stress responses using heart rate, negative affect, salivary alpha-amylase, and salivary cortisol in the fourth phase of the study. Heart rate responses were captured continuously. We exported and analyzed heart rate time intervals using Kubios version 2.2. (Tarvainen et al., Citation2014) applying medium-level artifact correction. We assessed negative affect with ten items from the Positive and Negative Affect Schedule by Watson et al. (Citation1988) at s0, s1, s2, s3, and s4 (see ). A sample item is “Please indicate to what extent you feel distressed at the present moment”. Participants indicated their responses on a 5-point Likert-type scale ranging from 1 = not at all to 5 = extremely. Cronbach’s alpha at the five measurement points was 0.73, 0.76, 0.91, 0.92, and 0.88 (in order of measurement). Salivary alpha-amylase and salivary cortisol were also assessed at s0, s1, s2, s3, and s4. All saliva samples were deposited in a freezer at −30 °C immediately after the laboratory appointment. Before analyzing salivary cortisol and alpha-amylase, the salivettes were defrosted and centrifuged at 2000 g and 20 °C for 10 min. Cortisol concentrations were determined by chemiluminescence immunoassay (CLIA, IBL, Hamburg, Germany) in duplicates. To measure alpha-amylase, we used reagents with a proprietary enzyme kinetics assay from DiaSys Diagnostic Systems GmbH (Holzheim, Germany). Saliva was diluted with ultrapure water (1:625), and then incubated for 3 min at 37 °C with substrate reagent (α-amylase CC FS; DiaSys Diagnostic Systems). An absorbance reading was conducted immediately afterwards with a Tecan Infinite 200 PRO reader (Tecan, Crailsheim, Germany). A second reading was performed after 5 min’ incubation at 37 °C. The increase in absorbance was transformed into alpha-amylase concentrations (U/mL) using a standard curve solution (Roche Diagnostics). For both salivary cortisol and alpha-amylase, intra- and inter-assay variation coefficients were below 10%.
As acute stress response indicators, we used negative affect and salivary alpha-amylase data from s2 and cortisol data from s3, as cortisol changes in response to stressors are delayed. For heart rate, we used the respective time interval (TSST; ) and calculated average values to generate mean heart rate. We controlled for baseline values. To measure the stress response outcomes, we took negative affect data from s3 and the average heart rate during the first 5 min of the recovery phase ().
Prosociality
Prosocial motivation
We measured prosocial motivation in Phase 4 of the study, after s3 (see ), with four items adapted from Grant (2008). A sample item is “I want to have a positive impact on others” (5-point Likert-type scale ranging from 1 = strongly disagree to 5 = strongly agree). After removing one item, Cronbach’s alpha was 0.69 instead of 0.44 (value for the complete scale).
Helping behavior
We measured helping behavior in Phase 4 right after s3 by recording participants’ willingness to complete a further questionnaire-based survey might take 10–12 min after the actual laboratory meeting to help a student with his/her thesis. Participants were informed that this task would not be financially compensated.
Thought control
We assessed thought control as an individual ability, and manipulated thought control with a specific strategy. We assessed thought control ability with the TNT (Anderson & Green, Citation2001, see above). The participant’s recall rate for the baseline items minus his/her recall rate for the no-think items served as an indicator of thought control. Higher values indicate higher thought control ability. To manipulate the adoption of a thought control strategy, we asked individuals in the EG to think about a hobby or another leisure activity that they enjoy doing and with which they associate positive feelings (see Sonnentag & Niessen, Citation2020), and to take notes regarding their feelings and thoughts. In the CG, participants were instructed to follow their stream of consciousness and write down everything that entered their mind. At the end, we asked to what extent participants in both groups thought about their favorite hobby or leisure activity (manipulation check). Response anchors ranged on a 5-point Likert-type scale from 1 = strongly disagree to 5 = strongly agree.
Analytical procedure
To analyze our data and test our hypotheses, we conducted hierarchical (logistic) regression analyses using SPSS version 26 (IBM Corporation, New York, NY). Prior to hypotheses testing, we analyzed the TNT data, whether stress responses had been successfully induced, and whether participants successfully applied the thought control strategy (see preliminary analyses). For all analyses, we considered absolute values for salivary cortisol and alpha-amylase and means for negative affect and heart rate. However, to assess current life stress and capture overall salivary cortisol secretion, we followed Pruessner et al. (Citation2003) and calculated the area under the curve with respect to ground. In general, higher values indicate a stronger stress reaction.
For hypotheses testing, we first included sex and baseline stress values (mean of the 5 min prior to s2 for heart rate; s1 for negative affect and salivary alpha-amylase; s2 for salivary cortisol; see ) as control variables. Second, we added all study variables into the analysis (Hypothesis 1), followed by the respective interaction terms. The interaction terms for Hypotheses 2, 3, and 4 were included in the model one-by-one and not tested simultaneously due to statistical power issues.
To evaluate stress recovery, we used the average heart rate value in the first 5 min after s3 and negative affect at s3. Salivary alpha-amylase and salivary cortisol were only used as stress response indicators when examining the direct relationship with prosociality and its moderation by current life stress.
Results
Preliminary analyses
The preliminary analyses included (1) the manipulations check of the TSST, (2) the manipulation check of the thought control strategy, and (3) the analysis of the TNT data.
To test if stress had been successfully induced by the TSST (Kirschbaum et al., 1933), we conducted repeated-measure ANOVAs with the factor time for negative affect, heart rate, salivary alpha-amylase, and salivary cortisol. All repeated-measure ANOVAs (negative affect, heart rate, alpha-amylase, cortisol) revealed a significant effect of measurement point (negative affect: F(1.837,99.192) = 32.275, p < 0.000, partial η2 = 0.374; heart rate: F(1.327,70.332) = 209.998, p < 0.000, partial η2 = 0.798; alpha-amylase F(2.822,152.370) = 16.080, p < 0.000, partial η2 = 0.229; cortisol F(1.493,76.649) = 20.366, p < 0.000, partial η2 = 0.289), indicating a successful stress induction.
In order to monitor whether the thought control strategy had been successfully applied, we asked all participants to what extent they had thought about their favorite hobby or leisure activity (manipulation check). An independent sample t-test revealed significant differences between participants in the EG and CG (t(53) = -17.21, p < .000), EG: M = 4.59 (SD = 0.64), CG: M = 1.50 (SD = 0.69), indicating that attentional deployment had been successfully applied.
To analyze the TNT data (Anderson & Green, Citation2001), we calculated a mixed ANOVA with experimental condition (baseline, think, no-think) and version (1, 2, 3) as within and between factors and applied contrast analyses to determine whether the paradigm worked. The mixed ANOVA revealed no differences between versions of the TNT (F(2,52) = 0.930, p = 0.400, η2 = 0.030). Contrast analyses revealed that no-think items were recalled at a worse rate than baseline items (F(1,52) = 5.070, p = 0.030, η2 = 0.090), providing evidence that thought control ability had been successfully measured. Following Anderson et al. (Citation2004), we z-normalized all values for each test version to ensure that the results are independent of differences in memorability between different word pairs.
Hypotheses testing
shows means, standard deviations, and correlations between all study variables. Hypothesis 1 proposed that acute stress responses should reduce participants’ prosocial motivation. Regression analyses indicated that negative affect at s2 (after the TSST intervention) was positively associated with prosocial motivation (F(6,54) = 2.959, p = 0.015, r2 = 0.270; ) and that salivary alpha-amylase at s2 was positively associated with helping behavior (χ2(6) = 11.469, p = .075, Nagelkerke r2 = 0.258; ). Heart rate and salivary cortisol as indicators for acute stress response had no impact on prosociality ( and ). Thus, Hypothesis 1 was not supported.
Table 1. Means standard deviations, and correlations for all study variables.
Table 2. Regression analyses predicting prosocial motivation.
Table 3. Logistic regression analyses predicting helping behavior.
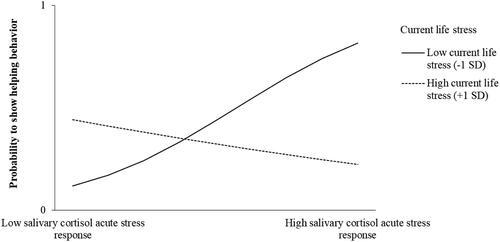
Regarding Hypothesis 2, we found that current life stress moderates the association between salivary cortisol and helping behavior (χ2(7) = 11.916, p = .103, Nagelkerke r2 = 0.278). Contrary to our expectations, the interaction plot showed that at low levels of current life stress, salivary cortisol and helping behavior had a positive association that was significantly different from zero (low levels of current life stress, −1 SD: simple slope = 0.364, SE = .168, p = .030, , ), thus indicating that participants with an acute stress response helped others only when their general stress level was low. Salivary cortisol and helping behavior were not significantly associated at moderate or high levels of current life stress (moderate levels of current life stress: simple slope =.216, SE = .134, p = .107, high levels of current life stress, +1 SD: simple slope = −0.094, SE = .144, p = .514). Neither were heart rate, negative affect, or salivary alpha-amylase associated with helping behavior or prosocial motivation ( and ).
Figure 4. The relationships between cortisol acute stress responses (low level: −1 SD, high level: +1SD) and helping behavior at low to high values of current life stress.
Regarding Hypothesis 3, we found that the thought control strategy manipulation moderates the relationship between negative affect at s2 and negative affect at s3 (F(7,47) = 20.651, p < .001, r2 = 0.755, , ). The interaction plot showed that individuals in the EG who experienced high levels of negative affect at s2 had lower negative affect at s3 than individuals in the CG (EG: simple slope = .707, SE = .073, p < .001; CG: simple slope = 0.297, SE = .091, p = .002), demonstrating that individuals in the EG were less stressed after applying the thought control strategy. This is in line with Hypothesis 3. Yet, there were no other findings concerning this hypothesis, as the thought control strategy did not attenuate heart rate stress responses and did not moderate the association of negative affect or heart rate with prosociality ().
Figure 5. The relationships between negative affective acute stress responses at s2 (low level: −1 SD, high level: +1SD) and negative affective stress recovery at s3 in the EG and CG condition.
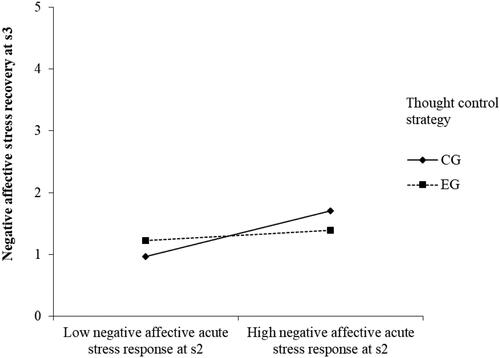
Table 4. Regression analyses predicting stress recovery at s3.
Hypothesis 4 investigated moderation by the thought control strategy and thought control ability. We found no results supporting this hypothesis ().
Discussion
This study examined whether and when acute stress responses impact prosociality. We considered a broad range of responses to stressors in the laboratory and in daily life, such as subjective stress responses (negative affect), heart rate and alpha-amylase as indicators of an activated sympathetic nervous system, and cortisol as indicator of hypothalamic-pituitary-adrenal axis activation. The sympathetic nervous system is the initial response to stress and rapidly promotes physiological changes, some time later, the activation of the hypothalamic-pituitary-adrenal axis follows in response to signals of the sympathetic nervous system (Everly & Lating, Citation2019). Contrary to our hypothesis, acute stress responses (e.g., negative affect, alpha-amylase, cortisol) were positively rather than negatively related to prosociality, but in the case of salivary cortisol, only when individuals’ general stress level was low. Additionally, our research indicated that applying a thought control strategy resulted in weaker stress responses (negative affect) compared to participants in the control group. Thought control ability had no impact on acute stress responses and prosociality.
We argued that acute stress responses should impair prosociality. However, three of the four acute stress responses we examined (all except heart rate) were positively associated with prosociality. Negative affect increased prosocial motivation, while salivary alpha-amylase and salivary cortisol (when current life stress was low) were positively associated with helping behavior. These findings are in line with some studies finding acute stress to increase prosocial behavior (e.g., Tomova et al., Citation2017; von Dawans et al., Citation2019), and support the tend-and-befriend hypothesis, which posits that individuals respond to stressful situations in order to protecting themselves by adapting to these circumstances by caring for and befriending others, thus facilitating prosociality (Taylor et al., Citation2000). Thus, acute stress induced by a social stressor in the TSST paradigm might drain energy resources, and stimulate a flight reaction, but might also threaten individuals` self-esteem (Dickerson & Kemeny, Citation2004; Het et al., Citation2009). One way to protect self-esteem is by helping others when the opportunity arises (Fu et al., Citation2017; Padilla-Walker et al., Citation2020). Future research should focus more on the processes triggered by the TSST that are responsible for prosocial behavior in order to predict when acute stress leads to an increase or decrease in prosociality. The results also indicate that stressed individuals need energy resources to engage in helping behavior. Individuals who are already drained in their daily life (current life stress, e.g., Kidd et al., 2014) and then exposed to acute stressors might view helping others as too energy-consuming and therefore not the appropriate strategy to protect or repair their self-esteem. However, this interaction was only found for salivary cortisol, not for the other indicators, which limits this interpretation. It is likely that acute stressors outweigh current life stress, with current life stress therefore having little impact on acute stress responses.
In line with previous research, the study showed that inducing a thought control strategy (shifting attention from the stressful situation to positive thoughts and feelings; Webb et al., Citation2012) fosters recovery from negative affective stress responses (e.g., Sonnentag & Niessen, Citation2020; Webb et al., Citation2012). Unexpectedly, we did not find this pattern for heart rate. As a sensitive indicator of acute stress response (Kudielka et al., Citation2004), the heart rate responds instantaneously to psychosocial stressors and also quickly returns to baseline level after recovery (Kudielka et al., Citation2004). It could be that both the thought control strategy and the instructions in the CG condition, which directed participants’ attention to their stream of consciousness, decreased heart rate over time and thus led to the null findings for heart rate. There is already evidence that mindfulness (being attentive in the present; Bell, Citation2015) decreases heart rate (Bell, Citation2015; Breines et al., Citation2015). It might be that our CG instructions induced mindfulness and thus produced a similar effect in the CG.
In addition, we did not find our thought control strategy to moderate the stress-prosociality relationship. Although negative affect was mitigated by attentional deployment, this strategy might not be strong enough to also engender more prosocial motivation and helping behavior. Again, it might be that the TSST threatened self-esteem (Dickerson & Kemeny, Citation2004; Het et al., Citation2009), which cannot be restored by shifting one’s attention to something positive.
Although prior research highlights the importance of thought control ability in making individuals more resilient to stressors (e.g., Engen & Anderson, Citation2018; Streb et al., Citation2016), we found thought control ability to neither attenuate individual stress responses nor moderate the association between acute stress responses and prosociality. The literature suggests that executive control capacities, which are highly related to thought control ability (Anderson & Hanslmayr, Citation2014; Engen & Anderson, Citation2018), are likely exhausted in acute stress situations, impairing the exertion of thought control (Qin et al., Citation2009; Shields et al., Citation2016). Hence, individuals may not be able to use their thought control ability in and after stressful situations.
To investigate our hypotheses, we applied a multifaceted study design comprising several parts: assessment of thought control ability, current life stress, and a broad range of acute stress responses followed by prosociality. This is particularly advantageous, as it allowed us to capture whether an individual’s current life stress impacts their acute stress responses and the corresponding associations. We also prevent common method bias by including different measurement points. However, the present study does have a few limitations. The study’s external validity is restricted, as only current life stress was assessed outside of the laboratory. Additionally, one might argue that we lacked a stressor control group condition, as all participants underwent the TSST procedure (Kirschbaum et al., Citation1993) to induce acute stress. However, because we hypothesized that the relationship between acute stress responses and prosociality will be moderated by several factors, our primary goal was to resolve and explain these associations with the moderators.
Despite the aforementioned limitations, the study showed that acute stress responses are not necessarily negatively associated with prosociality. Contrary to our hypotheses but consistent with the tend-and-befriend hypothesis (Taylor et al., Citation2000), we actually found positive relationships. However, this seems to be true only for specific stress responses and depends on individuals’ level of daily stress.
Supplemental Material
Download MS Word (51 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Additional information
Funding
Notes on contributors
Lisa Hensel
Lisa Hensel, MSc Psychology graduated from Ulm University in Germany in 2018. She is currently working on her PhD under the supervision of Cornelia Niessen at the Friedrich-Alexander-University of Erlangen-Nürnberg on the relationship between stress and prosociality.
Nicolas Rohleder
Nicolas Rohleder, Ph.D. graduated from University of Trier in Germany, in 2003. He is a psychologist with a focus on biological and health psychology. His main research interest is on the pathways between Central Nervous System (CNS) states such as stress, depression, and trauma and pathophysiological changes in the organism. He currently directs the chair of Health Psychology at Friedrich-Alexander University Erlangen-Nürnberg.
Cornelia Niessen
Cornelia Niessen is a full professor of Work and Organizational Psychology at the Friedrich-Alexander University of Erlangen‐Nürnberg, Germany. She received her Ph.D 2002 from the Technical University of Berlin. Her research focuses on human adaptivity in the context of work, occupational health psychology, and cognitive functioning.
Notes
1 The study was preregistered (AsPredicted #45244). In the preregistered version, empathy was included in the study model. However, analyses revealed that the corresponding scale (Koller & Lamm, Citation2014) was not reliable. Therefore, and due to space limitations, we decided to report these analyses in the supplement. Additionally, we removed donation frequency as indicator of prosociality, as we assumed that it would be biased due to financial needs caused by the COVID-19 pandemic.
References
- American Psychological Association. (2018). 2018 work and well-being survey. American Psychological Association.
- Anderson, M. C., & Green, C. (2001). Suppressing unwanted memories by executive control. Nature, 410(6826), 366–369. https://doi.org/10.1038/35066572
- Anderson, M. C., & Hanslmayr, S. (2014). Neural mechanisms of motivated forgetting. Trends in Cognitive Sciences, 18(6), 279–292. https://doi.org/10.1016/j.tics.2014.03.002
- Anderson, M. C., Ochsner, K. N., Kuhl, B., Cooper, J., Robertson, E., Gabrieli, S. W., Glover, G. H., & Gabrieli, J. D. E. (2004). Neural systems underlying the suppression of unwanted memories. Science (New York, N.Y.), 303(5655), 232–235. https://doi.org/10.1126/science.1089504
- Bell, T. P. (2015). Meditative practice cultivates mindfulness and reduces anxiety, depression, blood pressure, and heart rate in a diverse sample. Journal of Cognitive Psychotherapy, 29(4), 343–355. https://doi.org/10.1891/0889-8391.29.4.343
- Breines, J. G., McInnis, C. M., Kuras, Y. I., Thoma, M. V., Gianferante, D., Hanlin, L., Chen, X., & Rohleder, N. (2015). Self-compassionate young adults show lower salivary alpha-amylase responses to repeated psychosocial stress. Self and Identity : The Journal of the International Society for Self and Identity, 14(4), 390–402. https://doi.org/10.1080/15298868.2015.1005659
- Cohen, S., & Hamrick, N. (2003). Stable individual differences in physiological response to stressors: Implications for stress-elicited changes in immune related health. Brain, Behavior, and Immunity, 17(6), 407–414. https://doi.org/10.1016/S0889-1591(03)00110-7
- Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. https://doi.org/10.1037/0033-2909.130.3.355
- Engen, H. G., & Anderson, M. C. (2018). Memory control: a fundamental mechanism of emotion regulation. Trends in Cognitive Sciences, 22(11), 982–995. https://doi.org/10.1016/j.tics.2018.07.015
- Everly, G. S., & Lating, J. M. (2019). The anatomy and physiology of the human stress response. In A clinical guide to the treatment of the human stress response (pp. 19–56). Springer.
- Fu, X., Padilla-Walker, L. M., & Brown, M. N. (2017). Longitudinal relations between adolescents’ self-esteem and prosocial behavior toward strangers, friends and family. Journal of Adolescence, 57, 90–98. https://doi.org/10.1016/j.adolescence.2017.04.002
- Giles, D., Draper, N., & Neil, W. (2016). Validity of the Polar V800 heart rate monitor to measure RR intervals at rest. European Journal of Applied Physiology, 116(3), 563–571. https://doi.org/10.1007/s00421-015-3303-9
- Het, S., Rohleder, N., Schoofs, D., Kirschbaum, C., & Wolf, O. T. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology, 34(7), 1075–1086. https://doi.org/10.1016/j.psyneuen.2009.02.008
- Hobfoll, S. E. (1989). Conservation of resources: A new attempt at conceptualizing stress. American Psychologist, 44(3), 513–524. https://doi.org/10.1037/0003-066X.44.3.513
- Kidd, T., Carvalho, L. A., & Steptoe, A. (2014). The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biological Psychology, 99, 34–40. https://doi.org/10.1016/j.biopsycho.2014.02.010
- Kirschbaum, C. (2010). Trier social stress test. In I. Stolerman (Ed.), Encyclopedia of psychopharmacology (p. 1346). Springer.
- Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C., & Hellhammer, D. H. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162. https://doi.org/10.1097/00006842-199903000-00006
- Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1-2), 76–81. https://doi.org/10.1159/000119004
- Koller, I., & Lamm, C. (2014). Item response model investigation of the (German) interpersonal reactivity index empathy questionnaire: Implications for analyses of group differences. European Journal of Psychological Assessment, 31(3), 211–221. https://doi.org/10.1027/1015-5759/a000227
- Kudielka, B. M., & Kirschbaum, C. (2005). Sex differences in HPA axis responses to stress: a review. Biological Psychology, 69(1), 113–132. https://doi.org/10.1016/j.biopsycho.2004.11.009
- Kudielka, B. M., Schommer, N. C., Hellhammer, D. H., & Kirschbaum, C. (2004). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology, 29(8), 983–992. https://doi.org/10.1016/j.psyneuen.2003.08.009
- Larson, A., & Moses, T. (2017). Examining the link between stress events and prosocial behavior in adolescents: More ordinary magic? Youth & Society, 49(6), 779–804. https://doi.org/10.1177/0044118X14563049
- Ma, A., Landau, M. J., Narayanan, J., & Kay, A. C. (2017). Thought-control difficulty motivates structure seeking. Journal of Experimental Psychology. General, 146(8), 1067–1072. https://doi.org/10.1037/xge0000282
- Melinger, A., & Weber, A. (2006). Database of noun associations for German. http://www.coli.uni-saarland.de/projects/nag/
- Padilla-Walker, L. M., Millett, M. A., & Memmott-Elison, M. K. (2020). Can helping others strengthen teens? Character strengths as mediators between prosocial behavior and adolescents' internalizing symptoms. Journal of Adolescence, 79, 70–80. https://doi.org/10.1016/j.adolescence.2020.01.001
- Passarelli, T. O., & Buchanan, T. W. (2020). How do stress and social closeness impact prosocial behavior? Experimental Psychology, 67(2), 123–131. https://doi.org/10.1027/1618-3169/a000482
- Podsakoff, N. P., Whiting, S. W., Podsakoff, P. M., & Blume, B. D. (2009). Individual-and organizational-level consequences of organizational citizenship behaviors: A meta-analysis. The Journal of Applied Psychology, 94(1), 122–141. https://doi.org/10.1037/a0013079
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/10.1016/S0306-4530(02)00108-7
- Qin, S., Hermans, E. J., van Marle, H. J., Luo, J., & Fernández, G. (2009). Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry, 66(1), 25–32. https://doi.org/10.1016/j.biopsych.2009.03.006
- Shields, G. S., Sazma, M. A., & Yonelinas, A. P. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668. https://doi.org/10.1016/j.neubiorev.2016.06.038
- Sollberger, S., Bernauer, T., & Ehlert, U. (2016). Stress influences environmental donation behavior in men. Psychoneuroendocrinology, 63, 311–319. https://doi.org/10.1016/j.psyneuen.2015.10.017
- Sonnentag, S., & Niessen, C. (2020). To detach or not to detach? Two experimental studies on the affective consequences of detaching from work during non-work time. Frontiers in Psychology, 11, 560156 https://doi.org/10.3389/fpsyg.2020.560156
- Streb, M., Mecklinger, A., Anderson, M. C., Lass-Hennemann, J., & Michael, T. (2016). Memory control ability modulates intrusive memories after analogue trauma. Journal of Affective Disorders, 192, 134–142. https://doi.org/10.1016/j.jad.2015.12.032
- Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-Aho, P. O., & Karjalainen, P. A. (2014). Kubios HRV–heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220. https://doi.org/10.1016/j.cmpb.2013.07.024
- Taylor, S. E. (2006). Tend and befriend. Current Directions in Psychological Science, 15(6), 273–277. https://doi.org/10.1111/j.1467-8721.2006.00451.x
- Taylor, S. E., Klein, L. C., Lewis, B. P., Gruenewald, T. L., Gurung, R. A., & Updegraff, J. A. (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. https://doi.org/10.1037/0033-295x.107.3.411
- Tomova, L., Majdandžić, J., Hummer, A., Windischberger, C., Heinrichs, M., & Lamm, C. (2017). Increased neural responses to empathy for pain might explain how acute stress increases prosociality. Social Cognitive and Affective Neuroscience, 12(3), 401–408. https://doi.org/10.1093/scan/nsw146
- Vinkers, C. H., Zorn, J. V., Cornelisse, S., Koot, S., Houtepen, L. C., Olivier, B., Verster, J. C., Kahn, R. S., Boks, M. P. M., Kalenscher, T., & Joëls, M. (2013). Time-dependent changes in altruistic punishment following stress. Psychoneuroendocrinology, 38(9), 1467–1475. https://doi.org/10.1016/j.psyneuen.2012.12.012
- von Dawans, B., Ditzen, B., Trueg, A., Fischbacher, U., & Heinrichs, M. (2019). Effects of acute stress on social behavior in women. Psychoneuroendocrinology, 99, 137–144. https://doi.org/10.1016/j.psyneuen.2018.08.031
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. https://doi.org/10.1037/0022-3514.54.6.1063
- Webb, T. L., Miles, E., & Sheeran, P. (2012). Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138(4), 775–808. https://doi.org/10.1037/a0027600