Abstract
Psychological stress and its inevitable trajectory toward mental health deteriorations such as clinical and major depression has become an unprecedented global burden. The diagnostic procedures involved in the characterization of mental illnesses commonly follow qualitative and subjective measures of stress, often leading to greater socioeconomic burdens due to misdiagnosis and poor understanding of the severity of such illnesses, further fueled by the stigmatization surrounding mental health. In recent years, the application of cortisol and stress hormone measurements has given rise to an alternative, quantifiable approach for the psychological evaluation of stress and depression. This review comprehensively evaluates the current state-of-the-art technology for measuring cortisol and dehydroepiandrosterone (DHEA) and their applications within stress monitoring in humans. Recent advancements in these fields have shown the importance of measuring stress hormones for the characterization of stress manifestation within the human body, and its relevance in mental health decline. Preliminary results from studies considering multimodal approaches toward stress monitoring have showcased promising developments, emphasizing the need for further technological advancement in this field, which consider both neurochemical and physiological biomarkers of stress, for global benefit.
1. Introduction
Mental health and specifically, clinical depression can be monitored using various techniques, which can be categorized into physiological monitoring and biochemical signal analysis. Biochemical biomarkers monitoring can facilitate the understanding of underlying neurobiological processes involved in several mental illnesses (McGorry et al., Citation2014), such as clinical depression and bipolar disorder, as well as neurological diseases, for example, Alzheimer’s disease and dementia (Farah et al., Citation2018). The monitoring of specific biomarkers aids in the early detection and diagnosis of mental illnesses as well as simplifying the observation of illness progression (Farah et al., Citation2018). Clinical depression and major depressive disorder (MDD) are both highly associated with endocrine and metabolic dynamics (Schmidt et al., Citation2011). Intervention and deliberate influences on these factors often contribute to the treatment of this mental illness e.g. through use of antidepressants (Schmidt et al., Citation2011). Therefore, the observation of the endocrinology and metabolic markers is essential for the comprehension of psychological stress, its relationship to depression and the progression and treatment of the debilitating psychiatric disorder (Romero & Butler, Citation2007).
Psychological stress is often associated with fluctuations of stress hormones, such as cortisol and adrenaline (Tsigos & Chrousos, Citation2002). Cortisol is the primary stress hormone that is involved in governing the stress response from the moment of stress elicitation to recovery from stressful events. Alternatively, DHEA is a steroid hormone that has proven to express anti-glucocorticoid properties (Gallagher & Young, Citation2002). As two steroid hormones with inverse actions, the relationship between cortisol and DHEA is of great interest. The antagonistic relationship between cortisol and DHEA has been discussed at length in regard to their opposing actions on immune function, however, the relationship between cortisol and DHEA in stress management is seldom discussed (Buford & Willoughby, Citation2008). DHEA is primarily implicated in aging research, whereby an increase in cortisol/DHEA ratio can be a contributing factor of age-related declination in immune function (Buford & Willoughby, Citation2008). Evidently the biochemical interactions between cortisol and DHEA have proven to be of great importance in the determination of declining functions of biological systems. Therefore, observing the changes in these biomarkers with respect to stress and mental health monitoring could unveil vital details regarding stress management and mental health.
The key aims are to review the existing state-of-the-art technology comprehensively in the field of cortisol and dehydroepiandrosterone (DHEA) measurement and their applications within stress monitoring, whether it is in settings involving healthy participants or clinically depressed patients. Previous reviews within this field have focused primarily on the applications of cortisol monitoring for stress, albeit the importance of DHEA is seldom elaborated upon. Thereby, this review will consider the magnitude of DHEA in stress studies and discuss the strengths and shortcomings of measuring such stress biomarkers and whether it can impact the current perspective of mental health monitoring. The review will involve the evaluation of stress studies which focus on cortisol and DHEA measurements in various mediums, as well as its relationship with mental illnesses, primarily major depression. Stress studies include a broad set of studies involving either stress reactivity or chronic stress observations within adult populations. A broad set of stress studies were included to further comprehension regarding the cortisol/DHEA ratio and its implications in different modalities of stress.
2. Methods
The purpose of this review is to evaluate studies which have focused on the measurement of cortisol and DHEA, in different mediums e.g. saliva and plasma, simultaneously to comprehend the biochemical characteristics of psychological stress, and its trajectory to the manifestation of major depression. English-language articles were obtained from SCOPUS and PubMed databases. They were selected based on the search criteria of inclusion of specific words in their title, abstract or keywords. The searching criteria comprised of two fixed terms: ((“Cortisol”) AND (“DHEA”)), alongside two independent terms to obtain articles focusing on healthy subjects as well as depressed patients: (“Stress” OR “Depression”). Additional articles that were related were selected through reference lists and the “related articles” feature on SCOPUS and PubMed. After removal of duplicates, a total of 437 papers were obtained. Following the reading of paper abstracts, 98 papers were selected for further reading. The review focuses on the biochemical monitoring of psychological stress, therefore the most relevant papers that matched these criteria were selected. Ultimately, 31 papers were chosen for complete evaluation in this review. depicts a flow chart of the searching and selection process that was conducted for this review. Non-human studies were excluded from this review as the human stress response and stress hormones differ from other animals therefore the evaluation of animal studies would not be beneficial in the evaluation of stress biomarkers and the existing measurement techniques. Due to the implications of DHEA in aging function, the studies that were chosen for this review focus primarily on adults. Furthermore, papers involving participants with comorbid depression and other mental illnesses were excluded as these mental illnesses often have different and far more complex characteristics in stress hormone regulation which do not match the processes involved in acute or chronic psychological stress, nor major depression. The selection process was conducted by the primary author, upon shortlisting of articles a dual review was conducted with defined exclusion criteria to ensure minimization of bias and subjective errors. Exclusion criteria is noted in . Various studies were selected for review, including cross-sectional case control studies, randomized controlled trials and pilot studies. To ensure quality of the review was maintained, studies with lower sample sizes are explicitly mentioned in .
Figure 1. Flow chart of article selection process for comprehensive review of cortisol and DHEA monitoring for stress and depression.
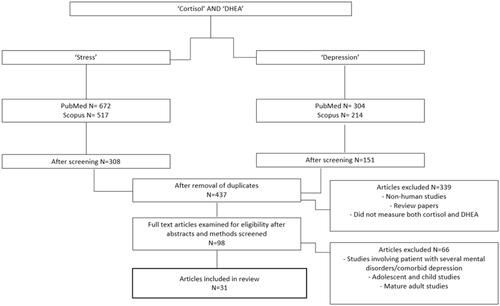
Table 1. Studies involving the measurement of cortisol and DHEA for stress and psychological evaluation in depressed and non-depressed groups.
3. Cortisol and DHEA as biomarkers for stress and depression monitoring
Stress is characterized by the biological or physiological action that occurs in response to a stressor i.e. environmental conditions, external stimuli, biological agents that interrupt homeostasis (Evans, Citation1950). Upon perception of a stressor, a response (mediation or modulation) is generated through a course of circuits and pathways which are activated depending on the classification of the stressor, i.e. physiological or psychological (Kovács et al., Citation2005; Shors & Horvath, Citation2001). Historically, stress has been described through the general adaptation syndrome (G.A.S.), which encompasses the emergency responses of the parasympathetic nervous system and the adrenocortical system (Evans, Citation1950; Mcewen, Citation2005).
The general adaptation syndrome comprises of three stages: the alarm reaction; the resistance and the exhaustion stage. Immediately upon the body’s perception of a stressor, the alarm reaction is triggered, i.e. the stress response or “fight or flight” response. The stress response is responsible for several physiological and biochemical changes in the body, to restore homeostasis (Mcewen, Citation2005). Once triggered, catecholamines (adrenaline and noradrenaline) are released into the bloodstream via the sympathetic-adrenal-medullary (SAM) axis for mobilization of energy required for the “fight or flight” responses (Juster et al., Citation2010; Romero & Butler, Citation2007). In parallel, corticotrophin-releasing hormone (CRH) is released from the hypothalamic-pituitary-adrenal (HPA) axis, which subsequently leads to the synthesis and release of glucocorticoids, such as cortisol, into the bloodstream (Sapolsky et al., Citation2000; Schmidt et al., Citation2011). As a result of the biochemical responses, there are several physiological changes that occurs, such as an increase in heart rate and blood glucose levels, muscular tension and perspiration (Charmandari et al., Citation2005; Evans, Citation1950). Furthermore, there are adaptive redirections of behavior such as increased arousal and alertness and, focused attention (Charmandari et al., Citation2005). These characteristics and responses contribute to the restoration of homeostasis interrupted by a short-term stressor.
In the resistance/maintenance stage, the body continues to activate afferent pathways in a series of allostatic mechanisms, leading to further physiological changes with increased metabolic activity, to restore balance in the organism from the perceived stress (Juster et al., Citation2010; Mcewen, Citation2005). In cases of persistent stressors, i.e. chronic stress, the exhaustion stage is reached wherein the energy and resources for prolonged adaptive responses to the stressor are depleted thus, efforts are ceased (Evans, Citation1950; Mcewen, Citation2005; Romero & Butler, Citation2007). Deterioration and “wear and tear” of the body, known as allostatic overload, can lead to immunosuppression, development of metabolic diseases e.g. diabetes and, progression of clinical depression or major depressive disorder (MDD) (Jefferies, Citation1991; Juster et al., Citation2010; Mcewen, Citation2005).
3.1. Cortisol
Cortisol is often considered the key logical indicator of stress and, in many cases, depression is characterized by the stable and sustained elevation of cortisol levels (Monroe, Citation2008; Schmidt et al., Citation2011). Hypercortisolaemia and reduction of the cortisol awakening response are characteristics of depression. These qualities are often monitored in the assessment of biomarkers of HPA axis activity and for neuroendocrinal analysis of depression and major depressive disorder.
Additionally, in studies where cortisol levels are monitored during treatment of first-episode psychosis, it was found that a decline in cortisol and cortisol/dehydroepiandrosterone sulfate (DHEAS) ratio directly correlated to an improvement in depressive and psychotic symptoms (Garner et al., Citation2011). As the most common HPA biomarker associated with depression, cortisol and its relationship with dexamethasone suppression is considered a promising neuroendocrine marker for analysis of treatment response, albeit not robust for clinical applications (Strawbridge et al., Citation2017).
The implication of cortisol level dynamics on the depressive symptoms of psychosis and other mental illnesses showcases that cortisol is a critical factor in the development of several mental illnesses, especially those involving depressive and negative symptoms (Knight et al., Citation2010). Thus, cortisol can be considered as the ideal biomarker for analysis and clinical staging in psychiatry (McGorry et al., Citation2014). The dexamethasone suppression test is categorized as the most promising neuroendocrine marker for treatment response in depression, although is it not considered robust for clinical applications (Strawbridge et al., Citation2017). In the dexamethasone test, post administration of dexamethasone resulting in non-suppression of cortisol translates to a lower likelihood of remission of the illness, post-treatment. Dysfunction in neuroendocrine hormone function is regularly associated with depression, specifically the dynamics of the hypothalamic pituitary adrenal (HPA) axis, is considered the primary contributing factor to the development of depression via endocrinal means (Stephens et al., Citation2014). Thus, the necessitation of further analysis of cortisol and a plethora of other potential biomarkers can facilitate greater comprehension of major depressive disorder and associated psychiatric illnesses (Strawbridge et al., Citation2017).
HPA activity leads to the release of neurohormones, such as CRH into general circulation (Schmidt et al., Citation2011). This triggers a hormonal cascade in which ACTH is released, which induces glucocorticoid synthesis and secretion of glucocorticoids into circulation. The central nervous system (CNS) and the endocrine system are tightly interconnected to coordinate glucocorticoid activity (Stephens et al., Citation2014). After a stressful event activates the HPA axis, the increase of cortisol and other glucocorticoids facilitate the body’s recovery from the stressor (Gjerstad et al., Citation2018). Cortisol regulates its secretion through a negative feedback mechanism involving the activation of the glucocorticoid receptor in the anterior pituitary gland. This mechanism is necessary to eliminate the HPA axis response to stress, i.e. to aid the body’s recovery from the stressor, as well as the maintenance of optimal levels of cortisol secretion in basal conditions (Gjerstad et al., Citation2018).
Cortisol increases blood pressure as well as blood glucose levels due to induction of insulin resistance (Kamba et al., Citation2016). Furthermore, excessively high cortisol levels in the body often result in suppression of the immune system (Jefferies, Citation1991). HPA axis dysfunctionality and dysregulation of the biological stress response system has been linked with risk of depression (Dienes et al., Citation2013). As the primary coordinator of the stress response, cortisol secretion patterns can indicate HPA axis dysfunction, in response to laboratory stressors and other interventions. Cortisol reactivity studies have shown that depressed individuals often have higher levels of cortisol during the recovery period post-stressor (Burke et al., Citation2005).
Commonly known as the “hormonal endpoint” of the HPA axis, cortisol is primarily responsible for the body’s reactions to stressors (Tsigos & Chrousos, Citation2002). Cortisol follows a circadian rhythm which is linked to the sleep/wake pattern in humans; therefore, basal levels of cortisol vary between the daytime and the evening (Tsigos & Chrousos, Citation2002). The rate of its secretion is dependent on the level of circulating corticotrophin, under extreme stimulating conditions, the level of cortisol in the human body can exceed 250 mg a day, approximately an 125% of its typical level of 20 mg (Garrod, Citation1958). In healthy and normal individuals, there are very low/undetectable levels of cortisol at midnight. This gradually builds up and peaks in the morning, known as the cortisol awakening response. After this peak, cortisol levels decline throughout the rest of the day (Chan & Debono, Citation2010). Comparatively, disturbances in this circadian rhythm are highly prevalent in individuals with depression (Germain & Kupfer, Citation2008). In normal individuals, there is a decline in mood in the evening, compared with the morning whereas in depressed individuals, there are mood improvements in the evening, which is associated with increased dorsal neural network activity. Sustained activity in the brainstem and hypothalamus involved with the sleep/wake cycle and, increased brain glucose metabolism is also observed throughout the day, which is reversed in healthy individuals (Germain & Kupfer, Citation2008). Due to these disturbances in the cortisol circadian rhythm in depressed individuals, irregularities in sleeping patterns and the sleep/wake cycle are commonly observed. 90% of depressed patients have complained about difficulties with sleeping, staying asleep and early morning awakening, compared to only 6% with complaints about hypersomnia (Almeida & Pfaff, Citation2005; Roberts et al., Citation2000). Thus, cortisol monitoring can simplify the comprehension of the circadian rhythms and chemical balances of depressed individuals compared to healthy subjects.
3.2. DHEA
Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) are steroid hormones that are regulated by the ACTH and possess anti-glucocorticoid properties (Gallagher & Young, Citation2002). DHEA is produced by the zona reticularis area in response to the ACTH. It has a regenerative role in the body, often associated with aging (Dutheil et al., Citation2021; Rutkowski et al., Citation2014). The primary function of DHEA is its involvement as a metabolic intermediate in sex hormone biosynthesis, i.e. to produce androgen and oestrogen (Mo et al., Citation2006). DHEA is known to improve physical well-being through reduction of total cholesterol, and prevention of bone mineral density. The steroid has an antagonistic relationship with cortisol, the primary stress hormone in humans (Gallagher & Young, Citation2002). This relationship can translate to reduced stress and improved psychological well-being. The cortisol-to-DHEA ratio has been considered as a precise method of assessing HPA axis functionality (Gallagher & Young, Citation2002). Several studies have shown an association between DHEA levels and stress intensity, as well as focusing on the cortisol/DHEA ratio. Although the magnitude of fluctuations in DHEA levels caused by stress is known to decrease with age (Dutheil et al., Citation2021). As a well-established biomarker of acute stress, the metabolism of DHEA and its release patterns in the human body are of great interest in stress studies. Several studies have noted that DHEA levels often appear as a peak at the end of a stressful period, and progressively return to baseline levels after recovery from stress (Dutheil et al., Citation2021). However, these factors are sex and age dependent therefore, it is imperative to delve deeper into the functionality of DHEA in the human body and the roles it plays within the stress response.
There are clear gender differences in circulating DHEAS levels, higher levels are found in men than in women, with peak levels around ages 25–30 (Dutheil et al., Citation2021). After this, there is an age-dependent decline in levels, which can also be influenced by drastic developmental changes. The reactivity to developmental changes in unique to DHEA secretion and not commonly observed for other steroid hormones. DHEA is a naturally occurring C-19 adrenal steroid synthesized by the adrenal cortex from cholesterol. The adrenal cortex secretes 75–90% if the body’s DHEA with the remainder produced by the sex organs, i.e. testes and ovaries (Webb et al., Citation2006). Clinical studies have shown that DHEA secretion has several effects on the human body such as, reduced inflammation, improved sexual function, cognitive function and memory enhancement (Traish et al., Citation2011). Furthermore, studies have shown that low DHEA and DHEAS levels are associated ischemic heart disease, endothelial dysfunction, atherosclerosis as well as psychological distress (Dutheil et al., Citation2021; Lennartsson et al., Citation2013; Traish et al., Citation2011).
4. Current state of the art monitoring techniques for cortisol and DHEA
The manifestation of stress and its effects on the human body are considerable for the comprehensive and quantitative evaluation of depression, and severe mental illnesses such as major depressive disorder. The relationship between stress hormones and clinical depression have been highlighted in a plethora of studies. Such studies have showcased the importance of cortisol monitoring for a better understanding of the neurobiological processes and their dysfunctionalities, which often lead to the development of depression. Evidently, Ter Horst et al. demonstrated the differences which exist between patients suffering from recurrent depression and healthy participants in their 2019 study (Ter Horst et al., Citation2019). This study highlighted HPA axis irregularities and subsequent hormonal imbalances which exist in depressed patients. Higher cortisol awakening responses were observed alongside lower cortisol/DHEA ratios, which have been noted as key characteristics commonly found in depressed patients.
The monitoring of stress-related biomarkers is well established in the field of stress studies, in cases of healthy participants as well as patients suffering from major depressive disorder. It is evident that the techniques used to measure cortisol, DHEA and other stress-related biomarkers often require the utilization of highly complex and expensive equipment, and highly skilled specialists to comprehend and evaluate the quantification of the biomarkers and its translation into psychological and neurobiological changes in the human body (Assies et al., Citation2004). Common techniques for the measurement of cortisol and DHEA include enzyme-linked immunoassays (ELISA) and liquid chromatography tandem mass spectrometry (Laudenslager et al., Citation2013; Lennartsson et al., Citation2022; Ota et al., Citation2015; Shors & Horvath, Citation2001).
The cortisol/DHEA ratio was first coined in 2001 by Goodyer et al. in a selective review which related the three aspects of behavioral endocrinology i.e. the developmental changes of cortisol and DHEA and their roles in psychopathology and neurobiological mechanisms (Goodyer et al., Citation2001). Goodyer observed that there exist medial changes in brain sensitivity following an excess in cortisol exposure, which often leads to dysfunctionalities and impairments in mental and behavioral function. Furthermore, it was noted that steroid hormones, therefore, contribute drastically to the shaping of behavioral and mental functions during early development and such dysfunctionalities act as risk factors for psychopathology. The cortisol/DHEA ratio is a measure of the relative activity of both steroids and can be indicative of psychopathological issues (Goodyer et al., Citation2001). Decreased ratios are often associated with dysfunctionality of the HPA axis. The two hormonal profiles that are common in depressed patients are either higher cortisol levels with normal levels of DHEA: or normal cortisol levels with lower levels of DHEA. Both of which lead to a lower cortisol/DHEA ratio. The cortisol/DHEA ratio has been a successful indicator of predicting recurrent major depressive disorder in adolescents (Goodyer et al., Citation1998). Such studies have shown the clinical relevance of the cortisol/DHEA ratio and its impact in the prediction and evaluation of major depression. Several studies have demonstrated the efficacy of monitoring cortisol and DHEA for the assessment of stress and major depressive disorder. Comparatively, some studies have showcased the shortcomings in monitoring the stress and gonadal hormones and its correlations with psychological changes in the body, therefore it is imperative to critically discuss the benefits and limitations of studies highlighting such methodologies for more robust application of biomarker monitoring technologies for stress evaluation. The complete evaluation of these studies can be seen in .
5. Discussion
Several studies have demonstrated the benefits of utilizing the cortisol/DHEA ratio as an objective and quantitative measure of stress in depressed and healthy participants (Asadikaram et al., Citation2019; Bae et al., Citation2019; Cutshall et al., Citation2016; Ge et al., Citation2016; Jeckel et al., Citation2010; Kim et al., Citation2010; Lennartsson et al., Citation2013, Citation2022). Moreover, some studies have noted the decline in cortisol levels, i.e. hypercortisolaemia and in DHEA levels were characteristic in patients suffering from depression, compared to controls (Osran et al., Citation1993; Ter Horst et al., Citation2019). Michael et al., Citation2000 study observed that a negative correlation relationship existed between DHEA awakening response levels and the severity of depression in a study consisting of 44 MDD patients and 35 partially depressed participants (Michael et al., Citation2000). They went on to suggest that lower DHEA levels are an additional state of abnormality in adult depression, alongside hypercortisolaemia, i.e. blunted cortisol response (Osran et al., Citation1993). Additionally, the results of Jozuka et al., Citation2003 study further justify this argument (Jozuka et al., Citation2003). In this study the cortisol and DHEA levels were observed to be radically lower in MDD patients compared to healthy controls, albeit in a smaller sample size of 17 MDD patients and 10 healthy participants. These studies suggest that the abnormalities found in secretion patterns of cortisol and DHEA may be indicative of the dysfunctionalities of the HPA axis, as well as irregularities in the antagonistic relationship between cortisol and DHEA, which would evidently be reflected in the cortisol/DHEA ratio.
In studies that compared the hormonal differences between depressed patients and healthy participants, such as Assies et al. (Citation2004) study on salivary cortisol and DHEAS, it was observed that DHEAS levels were elevated in MDD patients, whereas no noteworthy differences existed in salivary cortisol levels amongst MDD and healthy cohorts (Assies et al., Citation2004). Although this led to indications that MDD patients had greater cortisol/DHEA ratios, which correlate with existing literature, the behavior of salivary cortisol and DHEA and its fluctuations did not corroborate with the hormonal patterns expected from MDD patients. Comparatively, Boudarene et al. (Citation2002) study on the roles of cortisol and DHEA during the stress response showcased high levels of anxiety and stress were linked with higher cortisol levels and close correlations between DHEA and cortisol (Boudarene et al., Citation2002). As this study was conducted on healthy participants, it brings forward the question of whether the cortisol fluctuations in the Assies et al.’s study were a result of blunted cortisol responses, commonly observed in MDD patients (Assies et al., Citation2004; Mcewen, Citation2005; McEwen, Citation2000; Tsigos & Chrousos, Citation2002). Moreover, parallel increases in plasma cortisol and DHEA levels of depressed patients were found in a 1998 study conducted by Heuser et al. whereby it was found that the mental disorder led to large increases in diurnal minimal and mean plasma DHEA concentrations in a comparative study between depressed patients and healthy control participants (Heuser et al., Citation1998).
Evidently, stress studies which have been conducted on healthy participants have demonstrated results which further support this theory. In healthy human studies, such as those conducted by Izawa et al. and Irshad et al. the analysis of salivary biomarkers revealed the increase in salivary cortisol levels after stressful events (Irshad et al., Citation2020; Izawa et al., Citation2008). In both cases the presence of a stress-inducing event led to the increase in cortisol levels whereas, in Irshad et al.’s study there were no substantial changes in DHEA levels (Irshad et al., Citation2020). However, in Izawa et al.’s study, a peak in DHEA concentration was observed 10 minutes prior to peak cortisol concentration (Izawa et al., Citation2008). Both studies showcased increases in cortisol/DHEA ratios as a result of stress induction, albeit with different hormonal profiles. Although Izawa et al.’s study reveals the antagonistic nature of the relationship between salivary cortisol and DHEA, the results of Irshad et al.’s study suggests that further evaluation is required to fully assess the manner in which the fluctuations of cortisol and DHEA influence each other within the human body (Irshad et al., Citation2020; Izawa et al., Citation2008).
Thus, an argument can be derived that is dependent on the behavior of the stress-related biomarkers in the body and its roles in mental/psychological changes in humans. The cortisol/DHEA ratio can be considered as an objective indicator of mental stress in healthy humans. This is because the fluctuations of cortisol and DHEA are expected to follow a known pattern in response to stressors, i.e. stressor leads to an increase in cortisol levels, which results in a higher cortisol/DHEA ratio, as seen in several studies. Whereas, in studies involving depressed patients, a lower cortisol/DHEA ratio can be expected due to declines in cortisol or DHEA, which coincides with existing literature regarding blunted cortisol responses in patients suffering from MDD due to reaching the “exhaustive” stage of the stress or “fight or flight” response (Mcewen, Citation2005; McEwen, Citation2000; Tsigos & Chrousos, Citation2002).
It is therefore imperative to consider the methods in which cortisol and DHEA are measured from serum and saliva samples in stress studies. As previously mentioned, the primary methods of monitoring cortisol and DHEA involve immunoassay-based techniques or high-performance chromatography-based methods (Jeckel et al., Citation2010; Lennartsson et al., Citation2012). Although such methods yield highly accurate results regarding cortisol and DHEA concentrations, they require laboratory-based protocols and expensive equipment that are not easily accessible. Furthermore, in cases with high-risk individuals, such as those at risk of suicide, the time taken to obtain results is of utmost importance. The standard time taken to generate results from the gold standard salivary cortisol evaluation technique, i.e. ELISA testing is minimally 24–48 hours. Ideally, in high-risk cases, the existence of a rapid and continuous cortisol and DHEA monitor is compulsory for the betterment and improvement to quality of life for depressed patients.
Additionally, only one study considered the physiological changes which arise during stress, as well as the hormonal fluctuations which are implicated by the cortisol/DHEA ratio. Mazgelyte et al. investigated the associations between salivary steroid hormone fluctuations and time domain heart rate variability (HRV) indices in healthy individuals (Mazgelyte et al., Citation2021). Participants were asked to provide saliva samples during three collection sessions, which also involved a sociodemographic and lifestyle questionnaire, state trait anxiety inventory and the perceived stress scale. Salivary samples were analyzed for cortisol and DHEA concentrations via high performance liquid chromatography whilst HRV measures were taken issuing a high frequency infrared earlobe sensor. The results of this study demonstrated statistically significant associations between HRV measures and salivary cortisol and DHEA levels (Mazgelyte et al., Citation2021). The results coincided with previously mentioned studies whereby an increase in stress on the perceived stress scale correlated with an increased cortisol/DHEA ratio. Other studies involving multimodal measurements of stress include Ahrens et al.’s study on stress responses in recurrent MDD patients versus healthy participants through HRV, as well as serum and, salivary cortisol measurements (Ahrens et al., Citation2008). However, such studies did not consider the presence of DHEA in response to stress elicitation, therefore it was not considered within the scope of this review. The importance of physiological biomarkers of psychological stress in tandem with the evaluation of biochemical measurements is evidently essential for the comprehension of the stress response, as well as the relationship between steroid hormones and mental health deterioration in humans.
5.1. Considerations
The implication of monitoring stress-related biomarkers such as cortisol and DHEA has been highlighted throughout this comprehensive review. Evidently, the quantification of the relationship that exists between cortisol and DHEA is an important characteristic for the objective measurement of stress, and its manifestations in the human body. Various studies have shown promising findings which corroborate with the existing literature (Mazgelyte et al., Citation2021). Whereby, spikes in cortisol concentration leads to increased cortisol/DHEA ratios in healthy subjects, whilst blunted cortisol responses and irregularities in DHEA secretion led to subsequent declines in cortisol/DHEA ratios in depressed individuals (Osran et al., Citation1993; Ter Horst et al., Citation2019). Alternatively, several studies have demonstrated the insufficiencies in measuring only two steroid biomarkers for the understanding of the stress response. Evidently, the lack of standardized stress testing in stress studies involving healthy participants has led to diminished legitimacy in the results as there is no clear boundaries of stress elicitation in these individuals. The utilization of standardized stress tests such as the TSST validifies that individuals were subjected to stress and genuine stress response were elicited. For example, in Bae et al.’s. 2019 study, the TSST was used to investigate the stress biomarkers which had the highest discriminatory power between healthy cohorts undergoing stress tests versus controls (Bae et al., Citation2019). It was observed that salivary cortisone and salivary cortisol had substantial correlations with the subjective and autonomic stress measures, which were monitored via questionnaires and heart rate, respectively. Therefore, it is imperative to consider standardized stress tests such as the TSST when conducting stress studies as it is a reliable method for stress response elicitation.
5.2. Multimodal approaches
Undertaking a multimodal approach for monitoring of stress and depressive symptoms may lead to great advances for the complete quantification of psychophysiological stress evaluation (Ahmed et al., May Citation2022). Particularly, Mazgelyte et al.’s study showcases promising results in the use of chemical biomarker monitoring alongside physiological monitoring techniques such as HRV measures in the discrimination between stress responses versus resting state responses in healthy adults (Mazgelyte et al., Citation2021). Notably, the application of HRV measures in parallel with the cortisol/DHEA ratio established statistically significant associations between the physiological stress measures and biochemical stress biomarkers, which could be a promising characteristic to incite further investigations in stress measurement. In a recent review of studies involving the measurement of cortisol in tandem with physiological measurements of stress, promising results were obtained which further bridges the gap in knowledge in the quantification of psychological stress (Ahmed et al., May Citation2022). Therefore, efforts in the investigation of DHEA and the cortisol/DHEA ratio in similar studies would ideally lead to a greater understanding of the fluctuations of stress hormones during the stress response, and the physiological markers that are expressed during an episode.
6. Conclusion
To conclude, the prominence of cortisol and DHEA monitoring for the evaluation of psychological stress and its discriminatory power between patients of major depressive disorder and healthy individuals is inevitable. Further investigations with an emphasis on multimodal techniques could lead to a greater understanding regarding the relationship between the cortisol/DHEA ratio and physiological measures of stress such as HRV and electrodermal activity. Thus, leading to the robust quantification of stress. With this understanding and the aide of standardized stress testing, it could direct efforts away from existing subjective stress monitoring practices and drive research toward the complete quantification of psychological stress in the human body, for the improvement of quality of life for stressed individuals as well as those suffering from chronic stress or, clinical depression.
Author contributions
Tashfia Ahmed: Conceptualization, Investigation, Writing – Original Draft.
Meha Qassem: Supervision, Writing – Review & Editing.
Panayiotis Kyriacou: Supervision, Writing – Review & Editing.
Disclosure statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Additional information
Funding
References
- Ahmed, T., Qassem, M., & Kyriacou, P. A. (May 2022). Physiological monitoring of stress and major depression: A review of the current monitoring techniques and considerations for the future. Biomedical Signal Processing and Control, 75, 103591. https://doi.org/10.1016/j.bspc.2022.103591
- Ahrens, T., Deuschle, M., Krumm, B., van der Pompe, G., den Boer, J. A., & Lederbogen, F. (2008). Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosomatic Medicine, 70(4), 461–467. https://doi.org/10.1097/PSY.0b013e31816b1aaa
- Almeida, O. P., & Pfaff, J. J. (2005). Sleep complaints among older general practice patients: Association with depression. British Journal of General Practice, 55(520), 864–866.
- Asadikaram, G., Khaleghi, E., Sayadi, A., Foulady, S., Ghasemi, M. S., Abolhassani, M., Garrusi, B., & Nematollahi, M. H. (2019). Assessment of hormonal alterations in major depressive disorder: A clinical study. PsyCh Journal, 8(4), 423–430. https://doi.org/10.1002/pchj.290
- Assies, J., Visser, I., Nicolson, N. A., Eggelte, T. A., Wekking, E. M., Huyser, J., Lieverse, R., & Schene, A. H. (2004). Elevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: Preliminary findings. Psychiatry Research, 128(2), 117–122. https://doi.org/10.1016/j.psychres.2004.05.016
- Bae, Y. J., Reinelt, J., Netto, J., Uhlig, M., Willenberg, A., Ceglarek, U., Villringer, A., Thiery, J., Gaebler, M., & Kratzsch, J. (2019). Salivary cortisone, as a biomarker for psychosocial stress, is associated with state anxiety and heart rate. Psychoneuroendocrinology, 101, 35–41. https://doi.org/10.1016/j.psyneuen.2018.10.015
- Boudarene, M., Legros, J. J., & Timsit-Berthier, M. (2002). Study of the stress response: Role of anxiety, cortisol and DHEAs. L'Encephale, 28(2), 139–146.
- Buford, T. W., & Willoughby, D. S. (2008). Impact of DHEA(S) and cortisol on immune function in aging: A brief review. Applied Physiology, Nutrition, and Metabolism = Physiologie appliquee, nutrition et metabolisme, 33(3), 429–433. https://doi.org/10.1139/H08-013
- Burke, H. M., Davis, M. C., Otte, C., & Mohr, D. C. (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. https://doi.org/10.1016/j.psyneuen.2005.02.010
- Chan, S., & Debono, M. (2010). Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Therapeutic Advances in Endocrinology and Metabolism, 1(3), 129–138. https://doi.org/10.1177/2042018810380214
- Charmandari, E., Tsigos, C., & Chrousos, G. (2005). ENDOCRINOLOGY OF THE STRESS RESPONSE. Annual Review of Physiology, 67(1), 259–284. https://doi.org/10.1146/annurev.physiol.67.040403.120816
- Cutshall, S. M., Bergstrom, L. R., & Kalish, D. J. (2016). Evaluation of a functional medicine approach to treating fatigue, stress, and digestive issues in women. Complementary Therapies in Clinical Practice, 23, 75–81. https://doi.org/10.1016/j.ctcp.2016.03.005
- Dienes, K. A., Hazel, N. A., & Hammen, C. L. (2013). Cortisol secretion in depressed and at-risk adults. Psychoneuroendocrinology, 38(6), 927–940. https://doi.org/10.1016/j.psyneuen.2012.09.019
- Du, C.-L., Lin, M. C., Lu, L., & Tai, J. (2011). Correlation of occupational stress index with 24-hour urine cortisol and serum DHEA sulfate among city bus drivers: A cross-sectional study. Safety and Health at Work, 2(2), 169–175. https://doi.org/10.5491/SHAW.2011.2.2.169
- Dutheil, F., de Saint Vincent, S., Pereira, B., Schmidt, J., Moustafa, F., Charkhabi, M., Bouillon-Minois, J. B., & Clinchamps, M. (2021). DHEA as a biomarker of stress: A systematic review and meta-analysis. Frontiers in Psychiatry, 12, 688367. https://doi.org/10.3389/FPSYT.2021.688367/FULL
- Ebrahimpour, Z., Shakibaee, A., Farzanegi, P., & Azarbayjani, M. A. (2011). Changes of Dehydroepiandrosterone (DHEA) and Cortisol in response to competition in female volleyball players. Journal of Military Medicine, 13(1), 43–48.
- Evans, G. S. W. (1950). Stress and the general adaptation syndrome. British Medical Journal, 2(4670), 105–106. https://doi.org/10.1136/bmj105
- F., Ge, P. R., Pietromonaco, C. J., DeBuse, S. I., Powers., & D. A., Granger. (2016). Concurrent and prospective associations between HPA axis activity and depression symptoms in newlywed women. Psychoneuroendocrinology, 73, 125–132. https://doi.org/10.1016/j.psyneuen.2016.07.217
- Farah, R., Haraty, H., Salame, Z., Fares, Y., Ojcius, D. M., & Said Sadier, N. (2018). Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomedical Journal, 41(2), 63–87. https://doi.org/10.1016/j.bj.2018.03.004
- Gallagher, P., & Young, A. (2002). Cortisol/DHEA ratios in depression. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 26(3), 410. https://doi.org/10.1016/S0893-133X(01)00362-1
- Garner, B., Phassouliotis, C., Phillips, L. J., Markulev, C., Butselaar, F., Bendall, S., Yun, Y., & McGorry, P. D. (2011). Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. Journal of Psychiatric Research, 45(2), 249–255. https://doi.org/10.1016/j.jpsychires.2010.06.008
- Garrod, O. (1958). The pharmacology of cortisone, cortisol (hydrocortisone) and their new analogues. Postgraduate Medical Journal, 34(392), 300–304. https://doi.org/10.1136/pgmj.34.392.300
- Germain, A., & Kupfer, D. J. (2008). Circadian rhythm disturbances in depression. Human Psychopharmacology, 23(7), 571–585. https://doi.org/10.1002/hup.964
- Ghiciuc, C. M., Cozma-Dima, C. L., Pasquali, V., Renzi, P., Simeoni, S., Lupusoru, C. E., & Patacchioli, F. R. (2011). Awakening responses and diurnal fluctuations of salivary cortisol, DHEA-S and α-amylase in healthy male subjects. Neuro Endocrinology Letters, 32(4), 475–480.
- Gjerstad, J. K., Lightman, S. L., & Spiga, F. (2018). Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress (Amsterdam, Netherlands), 21(5), 403–416. https://doi.org/10.1080/10253890.2018.1470238
- Goodyer, I. M., Herbert, J., & Altham, P. M. E. (1998). Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychological Medicine, 28(2), 265–273. https://doi.org/10.1017/S0033291797006314
- Goodyer, I. M., Park, R. J., & Herbert, J. (2001). Psychosocial and endocrine features of chronic first-episode major depression in 8-16 year olds. Biological Psychiatry, 50(5), 351–357. https://doi.org/10.1016/S0006-3223(01)01120-9
- Goodyer, I. M., Park, R. J., Netherton, C. M., & Herbert, J. (2001). Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. The British Journal of Psychiatry : The Journal of Mental Science, 179(3), 243–249. https://doi.org/10.1192/BJP.179.3.243
- Grillon, C., Pine, D. S., Baas, J. M. P., Lawley, M., Ellis, V., & Charney, D. S. (2006). Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacology, 186(3), 434–441. https://doi.org/10.1007/s00213-005-0124-2
- Heuser, I., Deuschle, M., Luppa, P., Schweiger, U., Standhardt, H., & Weber, B. (1998). Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. The Journal of Clinical Endocrinology and Metabolism, 83(9), 3130–3133. https://doi.org/10.1210/jcem.83.9.5081
- Irshad, L., Faustini, S., Evans, L., Drayson, M. T., Campbell, J. P., & Heaney, J. L. (2020). Salivary free light chains as a new biomarker to measure psychological stress: The impact of a university exam period on salivary immunoglobulins, cortisol, DHEA and symptoms of infection. Psychoneuroendocrinology, 122, 104912. https://doi.org/10.1016/j.psyneuen.2020.104912
- Izawa, S., Saito, K., Shirotsuki, K., Sugaya, N., & Nomura, S. (2012). Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: A study of a two-week teaching practice. Psychoneuroendocrinology, 37(6), 852–858. https://doi.org/10.1016/j.psyneuen.2011.10.001
- Izawa, S., Sugaya, N., Shirotsuki, K., Yamada, K. C., Ogawa, N., Ouchi, Y., Nagano, Y., Suzuki, K., & Nomura, S. (2008). Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biological Psychology, 79(3), 294–298. https://doi.org/10.1016/j.biopsycho.2008.07.003
- Jeckel, C. M. M., Lopes, R. P., Berleze, M. C., Luz, C., Feix, L., Argimon, I. I. D. L., Stein, L. M., & Bauer, M. E. (2010). Neuroendocrine and immunological correlates of chronic stress in ‘strictly healthy’ populations. Neuroimmunomodulation, 17(1), 9–18. https://doi.org/10.1159/000243080
- Jefferies, W. M. K. (1991). Cortisol and immunity. Medical Hypotheses, 34(3), 198–208. https://doi.org/10.1016/0306-9877(91)90212-H
- Jozuka, H., Jozuka, E., Takeuchi, S., & Nishikaze, O. (2003). Comparison of immunological and endocrinological markers associated with major depression. The Journal of International Medical Research, 31(1), 36–41. https://doi.org/10.1177/147323000303100106
- Juster, R. P., McEwen, B. S., & Lupien, S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. https://doi.org/10.1016/j.neubiorev.2009.10.002
- Kamba, A., Daimon, M., Murakami, H., Otaka, H., Matsuki, K., Sato, E., Tanabe, J., Takayasu, S., Matsuhashi, Y., Yanagimachi, M., Terui, K., Kageyama, K., Tokuda, I., Takahashi, I., & Nakaji, S. (2016). Association between higher serum cortisol levels and decreased insulin secretion in a general population. PLoS One. 11(11), e0166077. https://doi.org/10.1371/journal.pone.0166077
- Kim, M.-S., Lee, Y.-J., & Ahn, R.-S. (2010). Day-to-day differences in cortisol levels and molar cortisol-to-DHEA ratios among working individuals. Yonsei Medical Journal, 51(2), 212–218. https://doi.org/10.3349/ymj.2010.51.2.212
- Knight, J. M., Avery, E. F., Janssen, I., & Powell, L. H. (2010). Cortisol and depressive symptoms in a population-based cohort of midlife women. Psychosomatic Medicine, 72(9), 855–861. https://doi.org/10.1097/PSY.0b013e3181f4ab87
- Kovács, K. J., Miklós, I. H., & Bali, B. (2005). Chapter 6.1: Psychological and physiological stressors. In Techniques in the behavioral and neural sciences (Vol. 15, Part 1, pp. 775–792). Academic Press. https://doi.org/10.1016/S0921-0709(05)80041-0
- Lac, G., Dutheil, F., Brousse, G., Triboulet-Kelly, C., & Chamoux, A. (2012). Saliva DHEAS changes in patients suffering from psychopathological disorders arising from bullying at work. Brain and Cognition, 80(2), 277–281. https://doi.org/10.1016/j.bandc.2012.07.007
- Laudenslager, M. L., Calderone, J., Philips, S., Natvig, C., & Carlson, N. E. (2013). Diurnal patterns of salivary cortisol and DHEA using a novel collection device: Electronic monitoring confirms accurate recording of collection time using this device. Psychoneuroendocrinology, 38(9), 1596–1606. https://doi.org/10.1016/j.psyneuen.2013.01.006
- Lennartsson, A.-K., Arvidson, E., Börjesson, M., & Jonsdottir, I. H. (2022). DHEA-S production capacity in relation to perceived prolonged stress. Stress (Amsterdam, Netherlands), 25(1), 105–112. https://doi.org/10.1080/10253890.2021.2024803
- Lennartsson, A.-K., Kushnir, M. M., Bergquist, J., & Jonsdottir, I. H. (2012). DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biological Psychology, 90(2), 143–149. https://doi.org/10.1016/j.biopsycho.2012.03.003
- Lennartsson, A.-K., Theorell, T., Kushnir, M. M., Bergquist, J., & Jonsdottir, I. H. (2013). Perceived stress at work is associated with attenuated DHEA-S response during acute psychosocial stress. Psychoneuroendocrinology, 38(9), 1650–1657. https://doi.org/10.1016/j.psyneuen.2013.01.010
- Mazgelyte, E., Chomentauskas, G., Dereškevičiūtė, E., Rekienė, V., Jakaitienė, A., Petrėnas, T., Songailienė, J., Utkus, A., Aušrelė, K. Z., Karčiauskaitė, D. (2021). Association of salivary steroid hormones and their ratios with time-domain heart rate variability indices in healthy individuals. Journal of Medical Biochemistry, 40(2), 173–180. https://doi.org/10.5937/jomb0-26045
- McEwen, B. S. (2000). The neurobiology of stress: From serendipity to clinical relevance. Brain Research, 886(1–2), 172–189. https://doi.org/10.1016/S0006-8993(00)02950-4
- McEwen, B. S. (2005). Stressed or stressed out: What is the difference? Journal of Psychiatry & Neuroscience, 30(5), 315–318.
- McGorry, P., Keshavan, M., Goldstone, S., Amminger, P., Allott, K., Berk, M., Lavoie, S., Pantelis, C., Yung, A., Wood, S., & Hickie, I. (2014). Biomarkers and clinical staging in psychiatry. World Psychiatry : Official Journal of the World Psychiatric Association (WPA), 13(3), 211–223. https://doi.org/10.1002/wps.20144
- Michael, A., Jenaway, A., Paykel, E. S. S., & Herbert, J. (2000). Altered salivary dehydroepiandrosterone levels in major depression in adults. Biological Psychiatry, 48(10), 989–995. https://doi.org/10.1016/S0006-3223(00)00955-0
- Mo, Q., Fang Lu, S., & Simon, N. G. (2006). Dehydroepiandrosterone and its metabolites: Differential effects on androgen receptor trafficking and transcriptional activity. The Journal of Steroid Biochemistry and Molecular Biology, 99(1), 50–58. https://doi.org/10.1016/J.JSBMB.2005.11.011
- Mocking, R. J. T., Pellikaan, C. M., Lok, A., Assies, J., Ruhé, H. G., Koeter, M. W., Visser, I., Bockting, C. L., Olff, M., & Schene, A. H. (2015). DHEAS and cortisol/DHEAS-ratio in recurrent depression: State, or trait predicting 10-year recurrence? Psychoneuroendocrinology, 59, 91–101. https://doi.org/10.1016/j.psyneuen.2015.05.006
- Monroe, S. M. (2008). Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology, 4(1), 33–52. https://doi.org/10.1146/annurev.clinpsy.4.022007.141207
- Noser, E., Fischer, S., Ruppen, J., & Ehlert, U. (2018). Psychobiological stress in vital exhaustion. Findings from the Men Stress 40 + study. Journal of Psychosomatic Research, 105, 14–20. https://doi.org/10.1016/j.jpsychores.2017.11.019
- Osran, H., Reist, C., Chen, C. C., Lifrak, E. T., Chicz-DeMet, A., & Parker, L. N. (1993). Adrenal androgens and cortisol in major depression. The American Journal of Psychiatry, 150(5), 806–809. https://doi.org/10.1176/ajp.150.5.806
- Ota, A., Yatsuya, H., Mase, J., & Ono, Y. (2015). Psychological job strain, social support at work and daytime secretion of dehydroepiandrosterone (DHEA) in healthy female employees: Cross-sectional analyses. Scientific Reports, 5, 15844. https://doi.org/10.1038/srep15844
- Pérez-Valdecantos, D., Caballero-García, A., Del Castillo-Sanz, T., Bello, H. J., Roche, E., & Córdova, A. (2021). Stress salivary biomarkers variation during the work day in emergencies in healthcare professionals. International Journal of Environmental Research and Public Health, 18(8), 3937. https://doi.org/10.3390/ijerph18083937
- Persson, R., Ørbaek, P., Kecklund, G., & Akerstedt, T. (2006). Impact of an 84-hour workweek on biomarkers for stress, metabolic processes and diurnal rhythm. Scandinavian Journal of Work, Environment & Health, 32(5), 349–358. https://doi.org/10.5271/sjweh.1030
- Roberts, R. E., Shema, S. J., Kaplan, G. A., & Strawbridge, W. J. (2000). Sleep complaints and depression in an aging cohort: A prospective perspective. The American Journal of Psychiatry, 157(1), 81–88. https://doi.org/10.1176/ajp.157.1.81
- Romero, L. M., & Butler, L. K. (2007). Endocrinology of stress. The International Journal of Comparative Psychology, 20(2), 89–95. https://doi.org/10.1016/j.neubiorev.2005.03.022
- Rutkowski, K., Sowa, P., Rutkowska-Talipska, J., Kuryliszyn-Moskal, A., & Rutkowski, R. (2014). Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs, 74(11), 1195–1207. https://doi.org/10.1007/S40265-014-0259-8
- Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. doi: 10.1210/er.21.1.55.
- Schmidt, H. D., Shelton, R. C., & Duman, R. S. (2011). Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 36(12), 2375–2394. https://doi.org/10.1038/npp.2011.151
- Shors, T. J., & Horvath, B. (2001). Stress, neural basis of. In International encyclopedia of the social and behavioral sciences (pp. 15194–15198). Pergamon. https://doi.org/10.1016/B0-08-043076-7/03534-8
- Stephens, M. A. C., McCaul, M. E., & Wand, G. S. (2014). The potential role of glucocorticoids and the HPA axis in alcohol dependence. In Neurobiology of alcohol dependence (pp. 429–450). Elsevier. https://doi.org/10.1016/B978-0-12-405941-2.00021-3
- Strawbridge, R., Young, A. H., & Cleare, A. J. (2017). Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatric Disease and Treatment, 13, 1245–1262. https://doi.org/10.2147/NDT.S114542
- Ter Horst, D. M., Schene, A. H., Figueroa, C. A., Assies, J., Lok, A., Bockting, C. L. H., Ruhé, H. G., & Mocking, R. J. T. (2019). Cortisol, dehydroepiandrosterone sulfate, fatty acids, and their relation in recurrent depression. Psychoneuroendocrinology, 100, 203–212. https://doi.org/10.1016/j.psyneuen.2018.10.012
- Traish, A. M., Kang, H. P., Saad, F., & Guay, A. T. (2011). Dehydroepiandrosterone (DHEA)—a precursor steroid or an active hormone in human physiology (CME). The Journal of Sexual Medicine, 8(11), 2960–2982; quiz 2983. https://doi.org/10.1111/J.1743-6109.2011.02523.X
- Tsigos, C., & Chrousos, G. P. (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. https://doi.org/10.1016/S0022-3999(02)00429-4
- Webb, S. J., Geoghegan, T. E., Prough, R. A., & Miller, K. K. M. (2006). The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metabolism Reviews, 38(1–2), 89–116. https://doi.org/10.1080/03602530600569877

