Abstract
Stress-related exhaustion is associated with cognitive deficits, measured subjectively using questionnaires targeting everyday slips and failures or more objectively as performance on cognitive tests. Yet, only weak associations between subjective and objective cognitive measures in this group has been presented, theorized to reflect recruitment of compensational resources during cognitive testing. This explorative study investigated how subjectively reported symptoms of cognitive functioning and burnout levels relate to performance as well as neural activation during a response inhibition task. To this end, 56 patients diagnosed with stress-related exhaustion disorder (ED; ICD-10 code F43.8A) completed functional magnetic resonance imaging (fMRI) using a Flanker paradigm. In order to investigate associations between neural activity and subjective cognitive complaints (SCCs) and burnout, respectively, scores on the Prospective and Retrospective Memory Questionnaire (PRMQ) and the Shirom-Melamed Burnout Questionnaire (SMBQ) were added as covariates of interest to a general linear model at the whole-brain level. In agreement with previous research, the results showed that SCCs and burnout levels were largely unrelated to task performance. Moreover, we did not see any correlations between these self-report measures and altered neural activity in frontal brain regions. Instead, we observed an association between the PRMQ and increased neural activity in an occipitally situated cluster. We propose that this finding may reflect compensational processes at the level of basic visual attention which could go unnoticed in cognitive testing but still be reflected in the experience of deficits in everyday cognitive functioning.
Neural activation and performance on the Flanker task in stress-related exhaustion
Exhaustion disorder (ED; ICD-10-SE code F43.8A) is a diagnose adapted into Swedish healthcare practice to operationalize stress-related exhaustion, or clinical burnout. This novel construct defines a response to long-term psychosocial stress characterized by psychological and physical symptoms of exhaustion, including cognitive deficits (Grossi et al., Citation2015). Accordingly, a recent meta-analysis supported that patients with clinical burnout perform worse than healthy controls in multiple cognitive areas, such as episodic and working memory, attention and processing speed, fluency and executive functions, albeit with modest between-group effect sizes for all cognitive domains (Gavelin et al., Citation2022). These findings broadly indicate deficits in cognitive control which accord with neuro-imaging studies of people with clinical burnout, showing structural and functional aberrations in primarily prefrontal and striatal brain areas, suggesting a biological link to impaired cognitive functioning in clinical burnout (Blix et al., Citation2013; Gavelin et al., Citation2017, Citation2020; Golkar et al., Citation2014; Jovanovic et al., Citation2011; Sandström et al., Citation2012; Savic, Citation2015). Clinical burnout is also associated with high levels of subjective cognitive complaints (SCCs) as measured with questionnaires targeting everyday cognitive slips and failures (Ellbin et al., Citation2018; Jonsdottir et al., Citation2013; Krabbe et al., Citation2017; Nelson et al., Citation2021; Oosterholt et al., Citation2014; Österberg et al., Citation2009). However, previous studies by our group and others have highlighted limited associations between cognitive test performance and the magnitude of self-reported burnout and SCCs (Ellbin et al., Citation2018; Gavelin et al., Citation2017; Jonsdottir et al., Citation2013; Nelson et al., Citation2021; Österberg et al., Citation2009). This could reflect an inability of traditional neuropsychological tests to adequately capture the experienced cognitive impairment and it has been proposed that patients with clinical burnout may take a “high-effort approach” toward cognitive tasks, compensating for slight neural deficits by increasing their mental effort, a strategy which may help to uphold test performance but in the long run exacerbate the core symptom of exhaustion (Krabbe et al., Citation2017; Oosterholt et al., Citation2014).
Compensational processes have also been implicated at the neural level where structural magnetic resonance imaging has shown a link between smaller striatal volumes and elevated levels of mental fatigue, in turn associated with stronger working memory performance (Gavelin et al., Citation2020). Using functional magnetic resonance imagining (fMRI), altered neural activation during cognitive tasks has been shown. For example, patients with clinical burnout displayed less activation than healthy controls in prefrontal cortical areas during the n-back task, tapping working memory updating (Sandström et al., Citation2012), and the Stroop-Simon task, measuring response inhibition (Skau et al., Citation2021), while performing as accurately but with slower response times (RT). Contrastingly, however, a study by our group found burnout levels in patients with ED to be uncorrelated with task performance, but instead associated with more neural activation in the prefrontal cortex (PFC), the posterior parietal cortex and striatum when performing the n-back task, suggesting that hyper-recruitment of these regions may be a neural correlate to a compensational effort put in by ED patients during working memory updating (Gavelin et al., Citation2017).
The concept of compensational neural processes has been repeatedly studied in aging populations where similar discrepancies between subjective and objective cognitive measures have long been noted. Specifically, resting-state fMRI studies of persons with SCCs have revealed altered functional connectivity in structures associated with the visual network and the default mode network (Hafkemeijer et al., Citation2013; Kawagoe et al., Citation2019). Further, task-fMRI studies have found individuals with subjective cognitive decline (i.e. self-reported cognitive impairment in the absence of lessened cognitive test performance) to show altered neural activity during cognitive tasks when compared to controls, suggesting that SCCs may be an indicator of slight neural changes undetectable in behavioral performance. For instance, Rodda et al. observed an association between subjective cognitive decline and increased activation in the left prefrontal cortex (PFC) during an episodic memory encoding task (Rodda et al., Citation2009) and in the left medial temporal lobe, bilateral thalamus, posterior cingulate and caudate during a divided attention task (Rodda et al., Citation2011). Erk et al. (Citation2011) similarly saw increased activity in the right dorsolateral PFC relating to subjective cognitive decline during an episodic memory recall task, and that this increase was concurrent with a decrease in hippocampal activity, suggesting a compensational mechanism counteracting early neural deterioration.
In sum, research from the aging field suggests that even when SCCs are uncorrelated with cognitive performance, they may be associated with alterations at the neural level, possibly indicating compensational processes as a response to neurodegeneration (Sun et al., Citation2015). The overall aim of this explorative study was to examine if similar associations could be seen in the clinical burnout population using task fMRI. To this end, we examined fMRI data of the Flanker task from 56 patients diagnosed with ED. Using an event-related design, we specifically investigated if SCCs and burnout levels were associated with (a) behavioral performance on the Flanker task, measured as accuracy and response time inhibition costs and (b) functional brain response during task performance. Drawing from previous research we expected that SCCs and burnout, respectively, would be uncorrelated with task performance but positively associated with a stronger BOLD response when contrasting incongruent and congruent trials, possibly indicating compensational neural activity during response inhibition in this clinical group.
Methods
Participants
This study was a part of the research project Rehabilitation for improved cognition (RECO) conducted at the Stress Rehabilitation Clinic at the University Hospital in Umeå, Sweden (ClinicalTrials.gov: NCT03073772). This three-armed randomized trial primarily examined the effects of cognitive and aerobic training as additional interventions to a 24-week multimodal stress rehabilitation (MMR) programme for patients diagnosed with Exhaustion disorder (ED), according to the diagnostic criteria coded F43.8A in the Swedish version of the International Classification of Diseases and Related Health problems (ICD-10). The methods and outcomes of the RECO project has previously been described in detail (for a review of the overall trial, see Gavelin et al., Citation2018; for a presentation of the n-back fMRI study, see Gavelin et al., Citation2017). The study was conducted in agreement with the Declaration of Helsinki and approved by the Umeå Regional Ethical Review Board (Dnr 2010-53-31). All participants provided written informed consent prior to the start of the study. The patients were screened for eligibility and recruited from the Stress rehabilitation clinic and the Social insurance agency in Umeå from April 2010 until June 2013. Inclusion criteria for the patients were (1) ED diagnosis, confirmed by a physician and a psychologist; (2) 18–60 years old; (3) currently employed; (4) considered by a physician and a psychologist to be suitable for group-based stress rehabilitation; (5) no known abuse of alcohol or drugs; (6) not in need of more urgent treatment; and (7) not participating in other interventional studies. Patients with other diagnoses in addition to ED that required special care (e.g. neurological or chronic psychiatric diagnoses) were excluded from the group-based rehabilitation programme, and thus also from this study. All patients were on partial or fulltime sick-leave when recruited to the study. A total of 161 patients were consecutively recruited to the randomized trial. At baseline (after 12 weeks of MMR rehabilitation, before randomization to the experimental arms), a subsample of 60 patients were examined with additional fMRI. Of these, two participants were excluded due to technical reasons, one to reporting poor eyesight during the imaging procedure, and one due to response time on the Flanker task being more than three standard deviations from the mean. Thus, data from 56 patients were included in the present study. This subsample did not significantly differ from the excluded participants with respect to age, sex, education level or burnout level (analyses are presented in Table S1 in the Supplemental Material). Figure S1 in the Supplemental Material shows the flow and attrition of participants graphically. Demographic and clinical characteristics of the sample, as well as self-reported occupation, are displayed in .
Table 1. Demographic and clinical characteristics.
Measures and procedure
Questionnaires
SCCs were operationalized using the Prospective and Retrospective Memory Questionnaire (PRMQ), a validated survey comprised of 16 items describing memory failures in everyday situations. The questionnaire is constructed to describe situations dependent on prospective, retrospective, short-term, long-term, self-cued or environmentally cued memory functioning. In this study the results were analyzed as the summed total score of all items (Cronbach’s alpha: 0.89) expressing a general memory failures factor, with a possible range between 16 and 80. Answers were given on a Likert scale ranging from never (1) to very often (5). Two sample items are “Do you fail to recall things that have happened to you in the last few days” and “Do you mislay something that you have just put down, like a magazine or glasses?.” Detailed descriptions of the scale structure, items, and properties of the Swedish adaptation can be found in Crawford et al. (Citation2003) and Rönnlund et al. (Citation2008), respectively. Burnout was measured using the Shirom-Melamed Burnout Questionnaire (SMBQ), a validated instrument consisting of 22 items targeting physical fatigue, experiences of tension, listlessness, and cognitive weariness (Lundgren-Nilsson et al., Citation2012; Melamed et al., Citation1992). Answers were rated on a seven-point Likert scale (1 = almost never, 7 = almost always) and results were analyzed as the mean score of all items (Cronbach’s alpha: 0.91). Two sample items are “I feel physically exhausted” and “I feel an intense inner tension.” Depression and anxiety levels were assessed with the Hospital anxiety and depression scale (HADS), a questionnaire comprising items rated on a four-point Likert scale (0–3), targeting both anxiety (seven items. Cronbach’s alpha: 0.79) and depression (seven items, Cronbach’s alpha: 0.87) (Zigmond & Snaith, Citation1983). The total score on each scale (possible range: 0–21) was used as the outcome measure. For all questionnaires, higher scores indicate more symptoms.
In-scanner task
The Flanker task was conducted at the same experiment session as the n-back task, previously presented in Gavelin et al., Citation2017. Flanker was the first of the two tasks being administered and was followed by a period of rest. The rationale behind including these two tasks in the RECO trial were two-fold. First, to map executive functioning and their neural correlates in this clinical group. Second, to evaluate physical exercise and computerized cognitive training as add-on interventions to standard clinical rehabilitation. The Flanker task was an adaptation of the Eriksen Flanker paradigm (Eriksen & Eriksen, Citation1974) and consisted of trials in which five horizontally aligned arrows appeared on the computer screen. The participants were instructed to indicate as fast and accurately as possible if the middle arrow pointed to the left (<) or right (>) using their right-hand index and middle finger. The four adjacent arrows (i.e. the flankers) were either pointing in the same or opposite direction as the targeted middle arrow, thus forming a congruent (⋘≪ or ⋙≫) or incongruent (≪>≪ or ≫<≫) task condition. Each trial consisted of a fixation cross (+) presented for 2 s, immediately followed by a 2 s display of the principal stimulus (i.e. the arrows). The design was event-related. The congruent and incongruent trials appeared in a random order and the interval between each trial was jittered according to the reaction time of each participant. The trials were presented in four blocks with a total of 68 trials. Between each block, a message was presented saying: “Part [“one,” “two” or “three”] is now over. The next part will soon begin”. There were 34 congruent trials and 34 incongruent trials; the number of correct answers were therefore 34 per condition. Accuracy was defined as correct answers given within the 2 s time limit. If a person failed to respond within this limit, the event was coded as “No answer.”
Data analysis
Behavioral data
Since incongruent trials normally require increased levels of inhibitory control, and therefore longer RTs, inhibition cost variables were calculated by (1) subtracting the mean RT of correct answers given in the incongruent trials from those of the congruent trials and (2) by subtracting the mean accuracy of the incongruent trials from that of the congruent trials, respectively. As none of these variables were normally distributed (see ), differences were evaluated using the Wilcoxon paired ranks test. In order to assess the relationships between test performance, SCCs and burnout, Kendall’s tau correlation analysis was conducted. Bayes factors (BF) were estimated to draw inferences about the correlation coefficients, investigating the likelihood of the alternative hypothesis (i.e. a non-zero correlation) in favor of the null hypothesis. These analyses included the PRMQ and SMBQ total scores, the RT and accuracy variables for the congruent and incongruent conditions, as well as the inhibition cost measures. The statistical analyses of behavioral data were completed using IBM SPSS, versions 27 and 28 with the exception of the correlation analysis which was conducted using JASP, version 0.16. Following Wagenmakers et al. (Citation2011), we interpret a BF (BF10) of: 1–3 as anecdotal evidence for the alternative hypothesis (H1), 3–10 as substantial evidence, 10–30 as strong evidence for H1, 30–100 as very strong evidence, and >100 as extreme evidence for H1.
Table 2. Performance on the flanker task.
Image acquisition
Functional and structural neuroimaging was obtained using a 32-channel head coil 3 T scanner (GE Medical Systems). The parameters of the sequence were: echo time (TE) = 30 ms, repetition time (TR) = 2000 ms, flip angle = 80°, field of view (FOV) = 25 × 25 cm, matrix size = 96 × 96, slice thickness = 3.4 mm, number of slices = 37. A total of 10 dummy scans were collected before image acquisition in order to calibrate the fMRI signal. Structural T1-weighted images were attained for each subject using the following parameters: 180 slices, 1 mm thickness, TE = 3.2 ms; TR = 8.2 ms, flip angle 12°, FOV = 25 × 25 cm. During image acquisition, stimuli were presented on a computer screen through a mirror mounted on the head coil, using E-prime version 2.0 (Psychology Software Tools, Inc., USA). Responses during the Flanker task were given using the index finger (“yes”/“<”) and middle finger (“no”/“>”) of the right hand using a fiber optic response pad (Current Designs, Philadelphia, PA, USA).
Pre-processing
Pre-processing and statistical analysis of the neuroimaging data were performed with a standard general linear model approach using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), with help of an inhouse program (DataZ). All functional images were corrected for slice timing and realigned and unwarped to correct for head movements. The anatomic images were segmented into grey and white matter maps and used for creating a sample-specific group template with DARTEL (Ashburner, Citation2007). The functional images were co-registered to the anatomic images, normalized to MNI space with DARTEL-created transformation maps and smoothed using an 8 mm Gaussian filter. The voxel size was set to 2 × 2 × 2 mm.
Statistical analyses of neuroimaging data
A general linear model was designed with event regressors for the incongruent and congruent conditions, and for a baseline block consisting of the last 60 s of rest. The regressors were convolved with the hemodynamic response function. The six movement parameters from the realignment procedure were added as regressors of no interest to the model. A high-pass filter of 128 s/0.0078 Hz was applied. A first-level analysis contrasted [Congruent–Rest], [Incongruent-Rest], and [Incongruent–Congruent] for each participant. In order to assess the relationship between the inhibition cost and neural activity, an [Incongruent–Congruent] contrast for all subjects were included in a t-test at the group level, using a FWE corrected p < 0.05 threshold, minimum cluster size 10 voxels. To investigate the relationship between functional brain response and level of burnout and subjective cognitive complaints, respectively, we added the total scores of the SMBQ and the PRMQ as covariates of interest to the model at the group level using an uncorrected threshold of p < 0.001, minimum cluster size 10 voxels. An explicit mask defined by thresholding the grey-matter part of the DARTEL template in MNI space (probability level 0.2) was applied on all group analyses.
Results
Behavioral data
Performance on the Flanker task
The Wilcoxon paired ranks test revealed that performance was significantly worse in the incongruent compared to the congruent condition, indicated by both slower response times (RT) (Z = 6.57, p= <0.001) and less accurate responses (Z = 4.78, p= <0.001). Mean RT and number of correct answers for the congruent and incongruent conditions as well as for the inhibition cost measures are presented in .
SCCs and burnout level
As shown by the correlation matrix (), the Bayes factors revealed that there is moderate to substantial evidence in favor of a non-zero correlation between PRMQ and SMBQ levels (Bayes Factor 10 = 3.58), indicating that more burnout symptoms are associated with higher levels of SCCs. Substantial evidence was found for zero correlations between PRMQ levels and the other test performance levels, as well as between SMBQ levels and Incongruent accuracy, Inhibition cost RT and -accuracy. Only anecdotal evidence was found for a correlation between SMBQ and congruent RT, as well as for zero-correlations between SMBQ and congruent accuracy and incongruent RT. Figure S2 in the Supplemental Material shows the distribution of the values visually.
Table 3. Associations between PRMQ and SMBQ levels and flanker task performance.
Neuroimaging
Functional brain response during task performance
Contrasting the incongruent and congruent conditions revealed a significant association with BOLD response in eight clusters, primarily located in occipital and parietal areas, and the supplementary motor area and insula (see for statistics, location, coordinates in MNI-space, and cluster extent), suggesting differentiated patterns of neural activation for the incongruent and congruent trials, and thus a link between inhibition cost and neural activity.
Table 4. Regions showing significant activation in the incongruent relative congruent interference contrast.
SCCs and burnout level
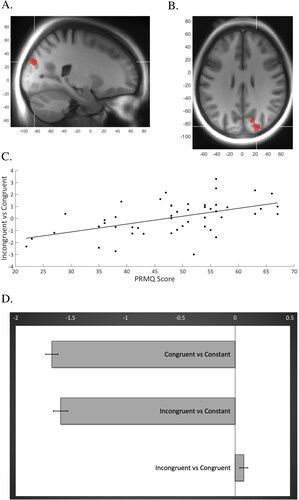
When adding the scores of the PRMQ and SMBQ as covariates to the model, PRMQ score was positively correlated with activity to the [Incongruent – congruent] contrast in a cluster comprising 245 voxels, with peak activations in the right superior occipital gyrus (x = 22, y= −84, z = 28, t = 4.22; and x = 22, y= −82, z = 18, t = 3.42) and the cuneus, (x = 16, y= −72, z = 28; t = 3.69). This finding, shown visually in , suggests an association between higher level of SCCs and more activity in this brain area as demands of cognitive control increases. As an additional analysis, we plotted the beta values of the same cluster during the congruent and incongruent trials, respectively, to the mean activity of the entire session. also displays how the mean BOLD response of the session was stronger compared to the task conditions, implying a deactivation of this area during task response. No significant correlation was revealed between burnout level and neural activity.
Figure 1. Cluster showing a positive correlation between beta weights and PRMQ-score when contrasting incongruent and congruent Flanker trials. (A,B) Sagittal and axial views of the cluster (x = 22, y = −84, z = 28; t = 4.22) comprising the right superior occipital gyrus and cuneus. (C) The regression analysis revealing a significant positive association between beta weights of this cluster for the incongruent vs congruent contrast (y-axis) and PRMQ score (x-axis), R = 0.27, p =.0001. (D) The additional analysis showing differences in beta values of the same cluster (x-axis) when contrasting the two task conditions (i.e. the incongruent and congruent trials, respectively) to the session constant, indicating lower neural activity during task compared to mean session activity. The bar furthest down in the chart displays the difference in beta values when contrasting incongruent vs congruent trials.
Discussion
This exploratory study investigated if SCCs and burnout levels were associated with response inhibition performance or altered brain activity. The results revealed (anecdotal to substantial) evidence for zero-correlations between SCCs or burnout levels and the behavioral Flanker measures, agreeing with previous studies showing no associations between measures of self-reported cognitive functioning and objective cognitive test performance in stress-related exhaustion (e.g. Nelson et al., Citation2021; Österberg et al., Citation2009). A sole exception was anecdotal evidence that burnout level correlated positively with response time during the congruent condition. That is, individuals who reported more burnout symptoms tended to perform more slowly during the less executively demanding task condition, which accord with studies displaying generally slower responses in patients with clinical burnout (Sandström et al., Citation2012; Skau et al., Citation2021). On a similar note, we did not see any correlation between SCCs or burnout to the magnitude of neural activation in the frontal brain areas predominantly associated with cognitive control. Instead, the results showed that level of SCCs was positively associated with activity in a right-sided cluster containing the superior occipital gyrus and the cuneus. We thus observed that during the more challenging task condition, patients reporting more SCCs tended to recruit these posteriorly located brain regions to a higher degree. Previous ED research has primarily highlighted the role of frontally located neural networks, with the overarching idea that stress-related exhaustion may elicit additional executive resources to more demanding cognitive tasks (Gavelin et al., Citation2017; Oosterholt et al., Citation2014). Yet, for response inhibition in particular, involvement of occipital brain regions has been repeatedly reported with smaller cuneus volume being associated with poorer inhibition performance (Haldane et al., Citation2008) and amplified cuneus activation being noted during inhibitions tasks, including Flanker (e.g. Imburgio et al., Citation2020; Siemann et al., Citation2018; Ware et al., Citation2015; Wittfoth et al., Citation2006; Zhu et al., Citation2010). The cunei, which are functionally and anatomically connected to the precuneus through the dorsal attention network, have predominantly been associated with fundamental spatial and visual processing, such as visual attention and imagery, orientation and motion (Palejwala et al., Citation2021; Parks & Madden, Citation2013) and are in the explicit context of response inhibition theorized to contribute more to attentional processes and motor response than to error monitoring (Haldane et al., Citation2008; Wittfoth et al., Citation2006). Thus, a possible interpretation of our results is that the SCCs indicate a slight compensational response operative at the level of basic visual attention, perception or motor control, rather than executive functioning per se. Such conception would harmonize with resting-state fMRI studies in aging proposing associations between SCCs and altered functional brain activity in the cuneus and adjacent structures. Specifically, Kawagoe et al. (Citation2019) found that while SCCs were uncorrelated with objective cognitive functioning in elderly subjects, persons who reported higher levels of SCCs showed stronger functional connectivity within occipital regions seeded at the cuneus. The authors’ interpretation was that these results may reflect a compensatory response to pathological processes affecting the occipital cortex at an early stage of neural degeneration. Similarly, Hafkemeijer et al. (Citation2013) compared functional connectivity in eight predefined resting-state networks between elderly subjects with subjective memory complaints and healthy controls. The SCCs group displayed increased functional connectivity of structures within the visual resting state network and the default mode network, including the cuneus which was defined as an integral region of both networks.
In the present study, despite the discrepancy in occipital response between the incongruent versus the congruent condition, we observed a deactivation during task performance when plotting the response versus the mean activity of the entire session. Notably, the mean of session included a 1 min rest condition at the end of the paradigm. Considering the aforementioned link between the cuneus and the default mode network, we propose that future studies should explicitly address whether SCCs in stress-related exhaustion reflect changes in functional connectivity of task-positive and task-negative networks during response inhibition and resting-state alike. Specifically, such studies should investigate if the integrity of these networks interact with task difficulty and behavioral measures of attention to address neural alterations involving the interchange between the default mode and task-positive networks when task difficulty increases, possibly reflecting difficulties in switching between different attentional states for this patient group. If empirically supported, such interpretation would harmonize with observations that the neural response to the [Incongruent vs Congruent] contrast in the Flanker task is correlated with the functional integrity of resting-state attentional networks, an effect primarily driven by the incongruent trials in healthy individuals (Mennes et al., Citation2010).
The lack of neural correlates between burnout levels and task-evoked response during the Flanker task is interesting in that we did see such an association in the same subjects during n-back performance (Gavelin et al., Citation2017). There are several feasible explanations for this discrepancy. Given that the n-back task primarily taps working memory updating, rather than response inhibition, one possibility is that the compensational neural response suggested in the previous study is domain specific. Similarly, it should be noted that the correlation between burnout level and activity in the fronto-striatal network was primarily seen in the two-back condition, and to a lesser extent in the three-back ditto, indicating that recruitment of these processes may be dependent on cognitive load. That is, compensational employment of fronto-parietal, top down control processes possibly only occurs when task difficulty is at an optimal level. Moreover, Flanker was the first of the two tasks to be administered in the scanner which could have influenced the dissimilar neural findings of the two tests as patients with stress-related exhaustion may be particularly prone to fatigue during elongated cognitive test procedure (e.g. Oosterholt et al., Citation2014).
There are limitations to this study that need to be considered. Firstly, the explorative approach of this study entailed investigation of neural activity at the whole-brain level by using a relatively liberal statistical threshold for the covariate analyses. Given the modest variability in task performance, it is also possible that other, or additional, more cognitively demanding paradigms would have been better apt to investigate the relationship to SCCs and to find the comparatively small neural effects previously noted in this clinical group. We suggest that future studies systematically manipulate the difficulty of cognitive tasks and also perceived task-associated effort and fatigue, thereby providing more detailed information on potential effort-related compensational mechanisms. Furthermore, the lack of a healthy control group prohibits conclusions to be drawn whether the results are specific for clinical burnout. While we consider the well-defined patient group – assessed using standardized diagnostic criteria – a strength of this study, it should be noted that our sample is heterogeneous with regard to both demographic and clinical characteristics, which holds true for the clinical group in general (Lindsäter et al., Citation2022). Hence, we recommend future research on this population to include not only healthy controls but also additional clinical control participants such as patients with clinical depression, which may provide more in-depth information on how to best interpret potential cognitive and neural findings in ED. Future studies should seek to replicate and expand our results by investigating the compensational approach in response inhibition by targeting the cuneus and the superior occipital gyrus as specific regions of interest. We recommend such studies to include multiple inhibition tasks permitting separation of possible task-specific effects, and also to include resting-state measurement of functional connectivity in order to directly test a hypothesis that SCCs are associated with altered functionality of the default mode network and to use inhibition paradigms that allow more elaborate analyses of the behavioral strategy and cognitive processing employed by the participants, such as diffusion modeling.
In conclusion, this exploratory study found that in a group of patients with stress-related exhaustion disorder, SCCs and burnout symptoms were essentially uncorrelated with performance on the Flanker task. Moreover, the fMRI analyses did not reveal any link between neural activity in the frontally located areas usually expected during cognitive control performance and self-reported measures of SCCs and burnout symptoms. Overall, these findings add to a general notion that the cognitive dysfunction experienced by this group is difficult to capture using the neuroimaging paradigm of the current study. Nevertheless, we did observe an association between level of SCCs and less attenuated occipital activity during the more executively demanding task condition, possibly due to deviation in the interchange between task-positive and task-negative neural networks, applying to basic visual attention. We thus propose that SCCs may be an indicator of subtle compensatory processes and attentional changes that are indistinguishable in standardized tests of response inhibition but still reflected in everyday slips and failures. Given that patients with clinical burnout have been attributed a high-effort, arduous approach toward cognitive tasks, such concept could inform development of more efficient assessment of cognitive functioning as well as the core symptom of exhaustion, and should be further investigated and empirically validated by future research.
Supplemental Material
Download MS Word (343.4 KB)Additional information
Funding
Notes on contributors
Andreas Nelson
Andreas Nelson is a licenced psychologist and PhD-student in psychology with a research interest in clinical neuropsychology and cognitive neuroscience.
Hanna Malmberg Gavelin
Hanna Malmberg Gavelin is a licensed psychologist and PhD in psychology with expertise in clinical neuropsychology and stress-related illness.
Micael Andersson
Micael Andersson is a research engineer at the Umeå Center for Functional Brain Imaging with expertise in brain imaging and data analysis.
Maria Josefsson
Maria Josefsson is an associate professor of statistics with expertise in developing methods for studying cognitive aging and risk factors.
Therese Eskilsson
Therese Eskilsson is a licensed physiotherapist and associate professor of physiotherapy with expertise in preventive health care and rehabilitation in stress-related illness.
Lisbeth Slunga Järvholm
Lisbeth Slunga Järvholm is a licenced senior consultant physician and professor with expertise in work-related health and stress-related illness.
Anna Stigsdotter Neely
Anna Stigsdotter Neely is a professor of psychology with expertise in clinical neuropsychology and cognitive neuroscience.
Carl-Johan Boraxbekk
Carl Johan Boraxbekk is a professor of neurology with expertise in cognitive neuroscience and brain imaging. All authors are associated with the RECO project, studying methods to improve cognitive functioning in stress-related exhaustion. The authors reported no potential conflict of interest.
References
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007
- Blix, E., Perski, A., Berglund, H., & Savic, I. (2013). Long-term occupational stress is associated with regional reductions in brain tissue volumes. PLoS One, 8(6), e64065. https://doi.org/10.1371/journal.pone.0064065
- Crawford, J., Smith, G., Maylor, E., Della Sala, S., & Logie, R. (2003). The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory, 11(3), 261–275. https://doi.org/10.1080/09658210244000027
- Ellbin, S., Engen, N., Jonsdottir, I. H., & Nordlund, A. I. (2018). Assessment of cognitive function in patients with stress-related exhaustion using the Cognitive Assessment Battery (CAB). Journal of Clinical and Experimental Neuropsychology, 40(6), 567–575. https://doi.org/10.1080/13803395.2017.1388359
- Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. https://doi.org/10.3758/BF03203267
- Erk, S., Spottke, A., Meisen, A., Wagner, M., Walter, H., & Jessen, F. (2011). Evidence of neuronal compensation during episodic memory in subjective memory Impairment. Archives of General Psychiatry, 68(8), 845–852. https://doi.org/10.1001/archgenpsychiatry.2011.80
- Gavelin, H. M., Domellöf, M. E., Åström, E., Nelson, A., Launder, N. H., Neely, A. S., & Lampit, A. (2022). Cognitive function in clinical burnout: A systematic review and meta-analysis. Work & Stress, 36(1), 86–104. https://doi.org/10.1080/02678373.2021.2002972
- Gavelin, H. M., Eskilsson, T., Boraxbekk, C. J., Josefsson, M., Neely, A. S., & Järvholm, L. S. (2018). Rehabilitation for improved cognition in patients with stress-related exhaustion disorder: RECO – a randomized clinical trial. Stress, 21(4), 279–291. https://doi.org/10.1080/10253890.2018.1461833
- Gavelin, H. M., Neely, A. S., Andersson, M., Eskilsson, T., Järvholm, L. S., & Boraxbekk, C.-J. (2017). Neural activation in stress-related exhaustion: Cross-sectional observations and interventional effects. Psychiatry Research. Neuroimaging, 269, 17–25. https://doi.org/10.1016/j.pscychresns.2017.08.008
- Gavelin, H. M., Neely, A. S., Dunås, T., Eskilsson, T., Järvholm, L. S., & Boraxbekk, C.-J. (2020). Mental fatigue in stress-related exhaustion disorder: Structural brain correlates, clinical characteristics and relations with cognitive functioning. NeuroImage: Clinical, 27, 102337. https://doi.org/10.1016/j.nicl.2020.102337
- Golkar, A., Emilia, J., Maki, K., Walter, O., Perski, A., & Savic, I. (2014). The influence of work-related chronic stress on the regulation of emotion and on functional connectivity in the brain. PLoS One. 9((9), e104550. https://doi.org/10.1371/journal.pone.0104550
- Grossi, G., Perski, A., Osika, W., & Savic, I. (2015). Stress-related exhaustion disorder – Clinical manifestation of burnout? A review of assessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scandinavian Journal of Psychology, 56(6), 626–636. https://doi.org/10.1111/sjop.12251
- Hafkemeijer, A., Altmann-Schneider, I., Oleksik, A. M., van de Wiel, L., Middelkoop, H. A. M., van Buchem, M. A., van der Grond, J., & Rombouts, S. A. R. B. (2013). Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connectivity, 3(4), 353–362. https://doi.org/10.1089/brain.2013.0144
- Haldane, M., Cunningham, G., Androutsos, C., & Frangou, S. (2008). Structural brain correlates of response inhibition in bipolar disorder I. Journal of Psychopharmacology, 22(2), 138–143. https://doi.org/10.1177/0269881107082955
- Imburgio, M. J., Banica, I., Hill, K. E., Weinberg, A., Foti, D., & MacNamara, A. (2020). Establishing norms for error-related brain activity during the arrow Flanker task among young adults. NeuroImage, 213, 116694. https://doi.org/10.1016/j.neuroimage.2020.116694
- Jonsdottir, I., Nordlund, A., Ellbin, S., Ljung, T., Glise, K., Währborg, P., & Wallin, A. (2013). Cognitive impairment in patients with stress-related exhaustion. Stress, 16(2), 181–190. https://doi.org/10.3109/10253890.2012.708950
- Jovanovic, H., Perski, A., Berglund, H., & Savic, I. (2011). Chronic stress is linked to 5-HT1A receptor changes and functional disintegration of the limbic networks. NeuroImage, 55(3), 1178–1188. https://doi.org/10.1016/j.neuroimage.2010.12.060
- Kawagoe, T., Onoda, K., & Yamaguchi, S. (2019). Subjective memory complaints are associated with altered resting-state functional connectivity but not structural atrophy. NeuroImage. Clinical, 21, 101675. https://doi.org/10.1016/j.nicl.2019.101675
- Krabbe, D., Ellbin, S., Nilsson, M., Jonsdottir, I. H., & Samuelsson, H. (2017). Executive function and attention in patients with stress-related exhaustion: Perceived fatigue and effect of distraction. Stress, 20(4), 333–340. https://doi.org/10.1080/10253890.2017.1336533
- Lindsäter, E., Svärdman, F., Wallert, J., Ivanova, E., Söderholm, A., Fondberg, R., Nilsonne, G., Cervenka, S., Lekander, M., & Rück, C. (2022). Exhaustion disorder: scoping review of research on a recently introduced stress-related diagnosis. BJPsych Open, 8(5), e159. https://doi.org/10.1192/bjo.2022.559
- Lundgren-Nilsson, Å., Jonsdottir, I. H., Pallant, J., & Ahlborg, G. (2012). Internal construct validity of the Shirom-Melamed Burnout Questionnaire (SMBQ). BMC Public Health, 12(1), 1. https://doi.org/10.1186/1471-2458-12-1
- Melamed, S., Kushnir, T., & Shirom, A. (1992). Burnout and risk factors for cardiovascular diseases. Behavioral Medicine, 18(2), 53–60. https://doi.org/10.1080/08964289.1992.9935172
- Mennes, M., Kelly, C., Zuo, X.-N., Di Martino, A., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2010). Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. NeuroImage, 50(4), 1690–1701. https://doi.org/10.1016/j.neuroimage.2010.01.002
- Nelson, A., Gavelin, H. M., Boraxbekk, C.-J., Eskilsson, T., Josefsson, M., Slunga Järvholm, L., & Neely, A. S. (2021). Subjective cognitive complaints in patients with stress-related exhaustion disorder: A cross sectional study. BMC Psychology, 9(1), 84. https://doi.org/10.1186/s40359-021-00576-9
- Oosterholt, B. G., Maes, J. H., Van der Linden, D., Verbraak, M. J., & Kompier, M. A. (2014). Cognitive performance in both clinical and non-clinical burnout. Stress, 17(5), 400–409. https://doi.org/10.3109/10253890.2014.949668
- Österberg, K., Karlson, B., & Hansen, Å. (2009). Cognitive performance in patients with burnout, in relation to diurnal salivary cortisol: Original research report. Stress, 12(1), 70–81. https://doi.org/10.1080/10253890802049699
- Palejwala, A. H., Dadario, N. B., Young, I. M., O’Connor, K., Briggs, R. G., Conner, A. K., O’Donoghue, D. L., & Sughrue, M. E. (2021). Anatomy and white matter connections of the lingual gyrus and cuneus. World Neurosurgery, 151, e426–e437. https://doi.org/10.1016/j.wneu.2021.04.050
- Parks, E. L., & Madden, D. J. (2013). Brain connectivity and visual attention. Brain Connectivity, 3(4), 317–338. https://doi.org/10.1089/brain.2012.0139
- Rodda, J., Dannhauser, T., Cutinha, D. J., Shergill, S. S., & Walker, Z. (2011). Subjective cognitive impairment: Functional MRI during a divided attention task. European Psychiatry, 26(7), 457–462. https://doi.org/10.1016/j.eurpsy.2010.07.003
- Rodda, J. E., Dannhauser, T. M., Cutinha, D. J., Shergill, S. S., & Walker, Z. (2009). Subjective cognitive impairment: Increased prefrontal cortex activation compared to controls during an encoding task. International Journal of Geriatric Psychiatry, 24(8), 865–874. https://doi.org/10.1002/gps.2207
- Rönnlund, M., Mäntylä, T., & Nilsson, L.-G. (2008). The Prospective and Retrospective Memory Questionnaire (PRMQ): Factorial structure, relations to global subjective memory ratings, and Swedish norms. Scandinavian Journal of Psychology, 49(1), 11–18. https://doi.org/10.1111/j.1467-9450.2007.00600.x
- Sandström, A., Säll, R., Peterson, J., Salami, A., Larsson, A., Olsson, T., & Nyberg, L. (2012). Brain activation patterns in major depressive disorder and work stress-related long-term sick leave among Swedish females. Stress, 15(5), 503–513. https://doi.org/10.3109/10253890.2011.646347
- Savic, I. (2015). Structural changes of the brain in relation to occupational stress. Cerebral Cortex, 25(6), 1554–1564. https://doi.org/10.1093/cercor/bht348
- Siemann, J., Herrmann, M., & Galashan, D. (2018). The effect of feature-based attention on flanker interference processing: An fMRI-constrained source analysis. Scientific Reports, 8(1), 1580. https://doi.org/10.1038/s41598-018-20049-1
- Skau, S., Jonsdottir, I. H., Sjörs Dahlman, A., Johansson, B., & Kuhn, H. G. (2021). Exhaustion disorder and altered brain activity in frontal cortex detected with fNIRS. Stress, 24(1), 64–75. https://doi.org/10.1080/10253890.2020.1777972
- Sun, Y., Yang, F. C., Lin, C. P., & Han, Y. (2015). Biochemical and neuroimaging studies in subjective cognitive decline: Progress and perspectives. CNS Neuroscience & Therapeutics, 21(10), 768–775. https://doi.org/10.1111/cns.12395
- Wagenmakers, E.-J., Wetzels, R., Borsboom, D., & Van Der Maas, H. L. (2011). Why psychologists must change the way they analyze their data: the case of psi: comment on Bem (2011). Journal of Personality & Social Psychology, 100(3), 426–432. https://doi.org/10.1037/a0022790.
- Ware, A. L., Infante, M. A., O’Brien, J. W., Tapert, S. F., Jones, K. L., Riley, E. P., & Mattson, S. N. (2015). An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behavioural Brain Research, 278, 137–146. https://doi.org/10.1016/j.bbr.2014.09.037
- Wittfoth, M., Buck, D., Fahle, M., & Herrmann, M. (2006). Comparison of two Simon tasks: Neuronal correlates of conflict resolution based on coherent motion perception. NeuroImage, 32(2), 921–929. https://doi.org/10.1016/j.neuroimage.2006.03.034
- Zhu, D. C., Zacks, R. T., & Slade, J. M. (2010). Brain activation during interference resolution in young and older adults: An fMRI study. NeuroImage, 50(2), 810–817. https://doi.org/10.1016/j.neuroimage.2009.12.087
- Zigmond, A. S., & Snaith, R. P. (1983). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x

