Abstract
Childhood adversity might impair corticolimbic brain regions, which play a crucial role in emotion processing and the acute stress response. The dimensional model of childhood adversity proposed that deprivation and threat dimensions might associated with individuals’ development through different mechanisms. However, few studies have explored the relationship between different dimensions of childhood stress, emotion processing, and acute stress reactivity despite the overlapping brain regions of the last two. With the aid of the event-related potentials technique, we explore whether negative emotion processing, which might be particularly relevant for adaptive stress responding among individuals with adverse childhood experience, mediates the relationship between dimensional childhood stress and acute stress response. Fifty-one young adults completed a free-viewing task to evaluate neural response to negative stimuli measured by late positive potential (LPP) of ERPs (Event-related potentials). On a separate day, heart rate and salivary cortisol were collected during a social-evaluative stress challenge (i.e. TSST, Trier Social Stress Test). After the TSST, the childhood trauma questionnaire was measured to indicate the level of abuse (as a proxy of threat) and neglect (as a proxy of deprivation) dimensions. Multiple linear regression and mediation analysis were used to explore the relationship among childhood stress, emotion processing, and acute stress response. Higher level of childhood abuse (but not neglect) was distinctly related to smaller LPP amplitudes to negative stimuli, as well as smaller heart rate reactivity to acute stress. For these participants, smaller LPP amplitudes were linked with smaller heart rate reactivity to acute stress. Furthermore, decreased LPP amplitudes to negative stimuli mediated the relationship between higher level of childhood abuse and blunted heart rate reactivity to stress. Consistent with the dimensional model of childhood stress, our study showed that childhood abuse is distinctly associated with neural as well as physiological response to threat. Furthermore, the blunted neural response to negative stimuli might be the underlying mechanism in which childhood abuse leads to the blunted acute stress response. Considering that all the participants are healthy in the present study, the blunted processing of negative stimuli might rather reflect adaptation instead of vulnerability, in order to prevent stress overshooting in the face of early-life threatening experiences.
Introduction
Exposure to different types of childhood stress, ranging from physical, emotional, sexual abuse, and emotional and physical neglect, is widespread with a prevalence of 30%–53% across the globe (Stoltenborgh et al., Citation2015). Numerous studies have documented the long-term deleterious effects of childhood stress on psychological and physical health (for reviews, see Hughes et al., Citation2017). However, individuals vary greatly in responses to childhood stress, and many exhibit intact daily functioning and emotional health in adulthood (Teicher et al., Citation2016). To date, the mechanisms underlying the long-lasting influence of childhood stress on health remain unclear.
Literature show that childhood stress might disrupt neural networks involved in emotion processing, particularly for threatening stimuli (Kraaijenvanger et al., Citation2020; Moreno-López et al., Citation2020). Consistent with this, a lower threshold for threat detection, attention bias toward negative information or increased attention to threat has been found in maltreated children (McCrory et al., Citation2011; Pollak et al., Citation2009). For example, event-related potential (ERP) studies showed that children with physical maltreatment had larger P3b amplitudes, thus increased attention to angry faces (or voices) compared to normal controls (Pollak et al., Citation2001; Shackman et al., Citation2007). Notably, among physically-abused children, increased attention to threat predicted more anxiety symptoms (Shackman et al., Citation2007), while attention bias away from threat was associated with diagnoses of posttraumatic stress disorder (Pine et al., Citation2005). Furthermore, the disrupted emotion processing among maltreated children might also persist into adults. Gibb et al. (Citation2009) found that young adults with a history of moderate to severe childhood abuse exhibited attention bias as well as interpretation bias to angry expressions. Another study showed that adults with childhood interpersonal trauma exposure failed to differentiate between non-threat and threat-related cues (Chu et al., Citation2016).
The amygdala plays a central role in the processing of emotional information, including threat detection and appraisal, and facilitated attention to salient stimuli (Cunningham & Kirkland, Citation2014; Sergerie et al., Citation2008). Meanwhile, corticolimbic brain regions such as the medial prefrontal cortex, hippocampus, and amygdala play an important role in the activation and regulation of stress responses (Ulrich-Lai & Herman, Citation2009). An empirical study found that reduced emotion regulation ability(indicated by decreased prefrontal cortex activation)predicted enhanced cortisol and α-amylase responses to acute social stress (Kaldewaij et al., Citation2019). The link between stress and corticolimbic functioning is bidirectional, such that crucial corticolimbic brain regions (e.g. prefrontal cortex, hippocampus, and amygdala) not only are impaired by chronic stress but also play a crucial role in neuroendocrine and autonomic regulation and thus the acute stress response (Herman & Cullinan, Citation1997; Lupien et al., Citation2009).
Altogether, these above studies suggest a possible link between childhood stress, emotion processing, and acute stress response. However, only a limited number of studies have examined these associations. For example, Kircanski et al. (Citation2019) found that in early puberty (mean age 11.37 years), a higher level of childhood stress was related to a decreased cortisol level during a psychosocial stress task, while decreased cortisol was related to increased white matter integrity in the frontolimbic tracts. However, there was no direct relationship between childhood stress and white matter integrity in the frontolimbic tracts. Results from Kaiser et al. (Citation2018) showed that among unmedicated young women (mean age 26.41) with variable psychiatric diagnoses, higher severity of threat-related childhood stress was associated with altered resting-state functional connectivity within corticolimbic circuits, which in turn was related to blunted cortisol response to acute stress. Although these two studies provide evidence of a possible relationship among childhood adversity, emotion processing, and physiological acute stress response, they did not measure the emotional process directly. Furthermore, they only focused on the cortisol response to acute stress.
Therefore, our current study used a free viewing paradigm to measure emotion processing with the ERP method. The late positive potential (LPP), an ERP component characterized by a prolonged positive deflection peaking approximately 300–400 ms after the onset of an emotional stimulus, is an important marker of emotion processing (Cuthbert et al., Citation2000). The LPP is sensitive to intrinsically salient stimuli and reflects attention toward affective stimuli (for a review, see Hajcak & Foti, Citation2020). Furthermore, source analysis and simultaneous EEG-fMRI recording revealed that the neural substrates of the LPP recorded at the parietal region involve both cortical and subcortical brain regions associated with visual and emotion processing including the prefrontal, temporal cortex, and amygdala (De Rover et al., Citation2012; Liu et al., Citation2012; Sabatinelli et al., Citation2007). Test-retest stability among different population suggests the LPP following emotional stimuli is a stable and reliable marker of emotion processing (Bondy et al., Citation2018). Furthermore, we measured cortisol (represents HPA axis) and heart rate (represents ANS) in the acute response to stress in healthy young participants.
According to a recent theoretical model, there are two dimensions underlying diverse childhood stressors, i.e. threat and deprivation, and the two dimensions would have distinct influence on brain and biological systems (McLaughlin et al., Citation2014). Specifically, threat, referring to interpersonal violence exposures of actual or threatening harm (e.g. physical, emotional, and sexual abuse), influences the development of cortico-limbic circuits that underlie fear learning and salience processing; deprivation, referring to the lack of expected cognitive and social inputs as in the case of physical and emotional neglect, influences the development of the association cortex, which in turn might produce difficulties in multiple domains in executive functioning (Teicher et al., Citation2016; Teicher & Samson, Citation2016). Interestingly, there is some empirical evidence suggesting that childhood experiences of threat but not deprivation are associated with blunted sympathetic and cortisol responses to acute stress (Busso et al., Citation2017), as well as with blunted salience processing in terms of self-inflicted error and fear (Machlin et al., Citation2019; Wu et al., Citation2021).
To summarize, in the current study, we aimed to investigate whether emotional processing would mediate the relationship between childhood stress and acute stress response. Combined with the view of the two dimensions model of childhood adversity, we hypothesized that emotion processing would play a mediating role in the relationship between childhood threat and acute stress response. Specifically, we predicted that neural processing of negative stimuli (indicated by LPP amplitudes) would mediate the relationship between childhood threat and acute stress response.
Method
Participants
As part of a larger project addressing the stress reactivity (Chen et al., Citation2018; Xin et al., Citation2017, Citation2020; Zhang et al., Citation2019), this study sought to specifically investigate the impact from the perspective of two dimensions of childhood stress (i.e. threat and deprivation) on stress reactivity. Considering potential influences on stress responses, all participants were prescreened and excluded according to the following criteria: major chronic physiological disease or endocrine disorder; history of psychiatric or neurological disorders; symptoms of chronic anxiety, depression, or insomnia; chronic use of psychiatric, neurological, or endocrine medicine; chronic overnight work or irregular day/night patterns; any medication use within three days before participating in the study; current periodontitis; excessive consumption of alcohol (more than two alcoholic drinks a day) or nicotine (more than five cigarettes a day). For females, we included those who did not take oral contraceptives during recruitment and invited them to participate in the experiment before or after their ovulation period (which is defined as the 12th–16th days prior to the first day of the next menstrual cycle), in order to control the potential influence of sex hormone on stress responses (Kudielka & Kirschbaum, Citation2005). Fifty-two healthy young adults were recruited from the student population of local universities. One male participant was excluded due to the abuse score over 3 standard deviations. Among the remaining 51 participants, there were 19 females and 32 males. The mean age was 22.55 (SD: 1.63), and the mean education year was 15.84 (SD: 1.35). The utilizing of this specific sample was to exclude the possible influence of psychiatric condition and developmental stage (Bunea et al., Citation2017). For example, adolescents with mild/moderate depression combined with a history of maltreatment showed higher cortisol reactivity, but those with moderate/severe depression exhibited blunted cortisol reactivity regardless of childhood maltreatment history (Harkness et al., Citation2011). A meta-analysis found that adults who experienced childhood adversity had a more blunted cortisol response to stress than children (Bunea et al., Citation2017).
All participants gave written informed consent at the beginning of the experiment and got monetary compensation for their participation. This experiment was approved by the Ethics Committee of Human Experimentation at the Institute of Psychology, Chinese Academy of Sciences.
General procedure
Participants completed two experimental sessions within two weeks (the interval between the first and second session was within 6 d, except for one participant with a 13-d delay). Participants completed the passive viewing task for the first session with their EEG continuously recorded. For the second session, participants returned to our lab to complete the TSST while their acute stress responses were monitored. The TSST was implemented between 1:00 pm and 5:00 pm to avoid the circadian fluctuation of cortisol levels (Kudielka et al., Citation2004) and heart rates (e.g. Vandewalle et al., Citation2007). Upon arrival, participants were instructed to rest in a quiet room for 30 min, during which they filled in demographic information. After the rest period, participants provided the first salivary sample for a baseline measurement. Thereafter, participants completed the TSST task for stress induction, during which heart rate was continuously recorded. Salivary samples were provided at 0 min (post-TSST 1), 20 min (post-TSST 2), 45 min (post-TSST 3), and 60 min (post-TSST 4) after the end of the TSST task. To avoid the negative recalling effect on the stress response, we administered the trait anxiety and CTQ questionnaires after the TSST task.
Passive viewing task
A total of 60 pictures were selected from the International Affective Picture System (IAPS; Lang et al., Citation1999), of which 30 were negative, depicting unpleasant scenes (e.g. threat and mutilation), and 30 were neutral, depicting neutral scenes (e.g. household objects, leaves, trees). The negative and neutral pictures differed significantly on normative ratings of valence (negative: M = 2.48, SD = 0.57; neutral: M = 5.03, SD = 0.34; t(58) = −21.112, p < .001), arousal (negative: M = 5.66, SD = 0.54; neutral: M = 2.92, SD = 0.49; t(58) = 20.479, p < .001), and dominance (negative: M = 3.77, SD = 0.60; neutral: M = 6.02, SD = 0.36; t(58) = −17.455, p < .001).
After an initial practice block, three experimental blocks were completed with 1–2 min breaks between the blocks. The 60 pictures were presented only once in a random order in each experimental block. Each picture was displayed for 1000 ms in full screen on a 17-in. (43.18-cm) monitor, occupying about 27.3° of horizontal visual angle and about 21.8°of vertical visual angle with a viewing distance of approximately 70 cm. The inter-trial interval varied randomly between 1200 and 1800 ms, during which a white cross was presented in the center of a black background (Hot et al., Citation2006). Participants were instructed to watch the pictures attentively.
EEG recording and processing
During the free-viewing task, the EEG was recorded from 64 scalp sites using Ag/AgCl electrodes, which were placed according to the international 10–20 system and mounted in an elastic cap (Neuroscan Inc., Charlotte, North Carolina, USA). The EEG data were processed using Scan 4.3 software (Neuroscan, USA). Eye-movement artifacts were corrected from the EEG data using a regression procedure implemented in the Neuroscan software (Semlitsch et al., Citation1986). Data were digitally low-pass filtered with 30 Hz and were epoched into periods of 1200 ms (including 200 ms pre-stimulus time as baseline) time-locked to the onset of the emotional pictures. Trials with artifacts exceeding ±100 μV were rejected from the analysis.
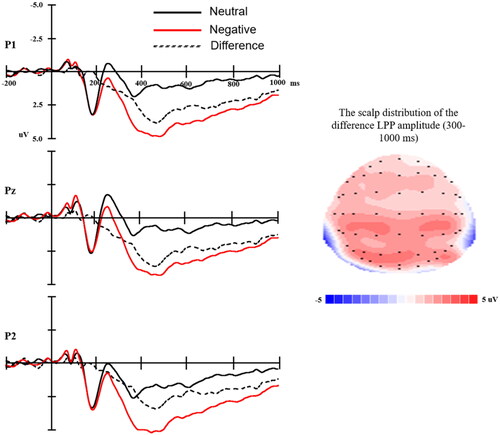
For each participant, ERP waveforms were averaged for the negative and neutral pictures separately. The LPP was defined as the mean amplitude in the time window of 300–1000 ms at the parietal region (P1, Pz, and P2). The electrodes and time window were chosen according to previous literature (Hajcak & Foti, Citation2020; Vallet et al., Citation2020) and our own data, which indicated the location of the maximum LPP amplitude.
Acute stress induction
The TSST task has been shown to be effective in eliciting stress responses (Buchanan et al., Citation2009, Citation2012). The task consisted of a 5-min preparation, a 5-min speech, and a 5-min mental arithmetic. In the preparation period, participants were seated in laboratory room A and instructed to prepare a speech for an imagined scenario in which they were accused of shoplifting, and they had to defend themselves in front of the store managers. They were also informed that their performance would be videotaped and evaluated. After preparation, participants were escorted to laboratory room B, where three experimenters (two females) in white coats pretending to be managers were presented. After the speech, participants were asked to complete a mental arithmetic task, which was to subtract serially the number 13 starting at 1022 as fast and accurate as possible. Once an error was made, participants had to restart at 1022. Throughout the speech and arithmetic task, participants spoke into a microphone and a video camera in front of the three experimenters. The experimenters communicated with the participant in a neutral manner with a neutral expression and provided no facial or verbal feedback.
Measurement of the acute stress response
A wireless chest heart rate transmitter and a wrist monitor recorder (Polar RSC800CX, Polar Electro, Finland) were used for heart rate recording. The heart rate was recorded for 5 min at baseline, continuously recorded for 15 min throughout the TSST task, and recorded for 3 min each for the four post-stress measurements at 0 min, 20 min, 45 min, and 60 min after the end of the TSST (i.e. post-TSST 1-4). Averaged heart rate across each recording period was obtained from the Polar performance software and was defined as the number of beats per minutes (bpm). Heart rate values were entered into the analysis as bpm for the different measurement time points.
Salivette collection tubes (Sarstedt, Rommelsdorf, Germany) were used to collect saliva samples for salivary cortisol levels at baseline and at 0 min (post-TSST 1), 20 min (post-TSST 2), 45 min (post-TSST 3), and 60 min (post-TSST 4) after the end of the TSST. Saliva samples were frozen at −22 °C immediately after collection until analysis and were thawed and centrifuged at 3000 rpm for 5 min before analysis. Cortisol concentration was determined by using a commercial electrochemiluminescence immunoassay (Cobas e 601, Roche Diagnostics, Numbrecht, Germany) with a lower sensitivity of 0.5 nmol/l. Three cortisol values were missing due to insufficient saliva and imputed by combining the group mean and standard deviation for the missing cortisol sample at that time point, and the mean of the available cortisol samples of the participant (Booij et al., Citation2013). Cortisol values were entered into the analysis as nmol/l concentrations for the different measurement time points.
Questionnaires
Childhood stress was assessed by a 28-item version CTQ on a 5-point Likert scale (1, never true; 5, very often true) (Bernstein & Fink, Citation1998). The CTQ is a self-reported questionnaire, which is used as a quantitative measure of the severity of childhood adversity within different populations with or without psychopathology (Viola et al., Citation2016). The exemplary item is “when I was growing up, I got hit so hard by someone in my family that I had to see a doctor or go to the hospital”. There are five subscales in the CTQ including emotional, physical, and sexual abuse, and emotional and physical neglect. According to the dimensional model of childhood stress (McLaughlin et al., Citation2014; Teicher et al., Citation2016; Teicher & Samson, Citation2016). We calculated the abuse total score (as a proxy of threat) by summing up scores from emotional, physical, and sexual abuse subscales, and the neglect total score (as a proxy of deprivation) by summing up scores from emotional and physical neglect subscales.
Trait anxiety was measured with the Chinese version of the trait subscale of the State-Trait Anxiety Inventory, which showed good reliability and validity (STAI-T; Spielberger, Citation1983; Zhang et al., Citation2012). The higher scores indicate higher trait anxiety levels.
Data preparation
For emotion processing, the mean LPP amplitude was calculated by averaging the LPP amplitude across the P1, Pz, and P2 electrodes where they have the maximum amplitude and in line with previous research (Garrison et al., Citation2017; van Dongen, Citation2018). To validate the valence effect on the LPP amplitude, a one-way repeated-measures ANOVA with valence (negative vs. neutral) as a within-subjects factor was performed. Then, the LPP difference waves (ΔLPP) were calculated by subtracting neutral from negative LPPs to serve as an ERP index of emotional processing.
For the stress reactivity, to evaluate physiological stress reactivity to the TSST, repeated-measures ANOVAs were first performed on salivary cortisol levels and HR with the measurement time point as the within-subject variable. The Greenhouse-Geisser correction was employed when the sphericity assumption in the ANOVA for repeated measures was violated. Bonferroni correction was used in posthoc multiple comparisons. Then, heart rate increase (ΔHR) was calculated by subtracting the baseline from the average HR of the TSST. The area under the curve with respect to ground (AUCg) of cortisol was computed to indicate the HPA response to stress (Pruessner et al., Citation2003).
Data analysis
Multiple linear regression with bootstrap (n = 1000 times of resampling with replacement) was used to investigate the differential influence of the two dimensions of childhood stress (i.e. threat and deprivation) on emotion processing and acute stress reactivity in our study.
Firstly, the primary independent variable were abuse and neglect total scores for the relationship between childhood stress and emotion processing (path 1). Sex and trait anxiety were covariate variables. The dependent variable was the emotion processing index (i.e. ΔLPP amplitude).
Secondly, for the relationship between childhood stress and acute stress response (path 2), the primary independent variable and covariate variables were consistent with path 1. The dependent variables were acute stress reactivity index (i.e. ΔHR and cortisol AUCg).
Furthermore, in order to test the hypothesis that abuse and neglect are distinctly associated with emotion processing and acute stress reactivity, we used a conservative method by examining the overlap of CIs from abuse and neglect. In the event that the CIs overlapped by less than 50% of one CI arm, the beta weights would be considered statistically significantly different from each other (p < .05, Cumming, Citation2009, Citation2014).
Thirdly, to explore the relationship between emotion processing and acute stress reactivity (path 3), hierarchical regression analyses with bootstrap (n = 1000 times of resampling with replacement) were then conducted to investigate the value of the ΔLPP amplitudes on the stress response (i.e. ΔHR and cortisol AUCg). Due to the sex difference in the response to acute stress, sex was treated as a control variable (dummy-coded male = 1, female = 2) (Childs et al., Citation2010). Previous researchers found that trait anxiety was associated with a negative recall bias and heighten psychophysiological stress reactivity (Boudarene et al., Citation2002; Reidy and Richards, Citation1997). Therefore, trait anxiety was also treated as a control variable in the current study.
Finally, we used the simple mediation model (Model 4) from the PROCESS tool for SPSS (Hayes, Citation2013) to determine whether emotion processing (ΔLPP amplitude) mediated the association between childhood stress (abuse or neglect) and physiological reactivity (ΔHR or cortisol AUCg) (path 4). Sex and trait anxiety were included as covariates in these models. To determine the significance of the indirect effect, PROCESS produces bootstrapped CIs (CIs that do not include zero indicate significant mediation; Hayes, Citation2013). The standard error of the model is estimated by HC3 (Davidson & MacKinnon, Citation1993).
Results
Subjective measurements
The mean score of trait anxiety was 40.20 (SD: 5.41, Range: 27.00–53.00). The mean score of CTQ total score was 33.45(SD: 6.39, Range: 25–50). The mean scores of CTQ five subscales were as following: emotional abuse 6.14 (SD:1.39, Range: 5–11), physical abuse 5.29 (SD:0.76, Range: 5–9), sexual abuse 5.24 (SD: 0.68, Range: 5–9), emotional neglect 9.27 (SD:3.49, Range: 5–18) and physical neglect 7.51 (SD:2.61, Range: 5–15). When the CTQ was subdivided into dimensions of abuse and neglect, the average abuse dimension score was 16.67 (SD: 1.89, Range: 15–22) and the average neglect dimension score was 16.78 (SD: 5.51, Range: 10–32).
ERP data
The ERP waveforms time-locked to the negative and neutral picture onset, the average accepted trials were 86, 87 in the negative and neutral conditions, respectively. The difference wave (negative minus neutral) are shown in . Negative pictures elicited larger LPP amplitudes as compared to neutral pictures (F(1,50) = 112.35, p < .001). The mean amplitude (± SD) of ΔLPP was 2.36 (± 1.59) μV.
Stress response
The means and standard deviation of HR and salivary cortisol levels measured before, during, and after the TSST are depicted in .
Figure 2. Mean values and standard deviation (SD.) of heart rate (left) and salivary cortisol level (right) measured before, during, and after the TSST task. Baseline: measured after 30-min rest but before TSST; TSST: measured during the TSST task (the mean value of preparation, speech, and mental arithmetic); Post-TSST 1–4: measured at 0 min, 20 min, 45 min, and 60 min after the end of the TSST task.
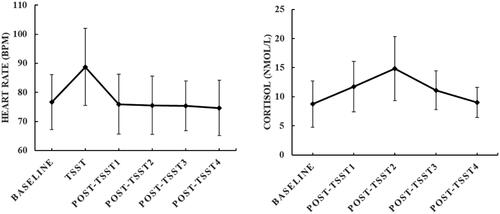
For the HR, the repeated-measures ANOVA revealed a significant main effect of Time (F(5, 250) = 63.67, p < .01). Post hoc analysis indicated significantly higher HR during the TSST task compared to the baseline and post-TSST measures (ps < .01). The differences between the baseline HR and the three post-TSST (Post-TSST 1–3) measures were not significant (ps > .10). The HR at post-TSST4 was lower than at baseline (p < .05). The mean value (± SD) of the ΔHR was 12.07 (± 8.31).
For the cortisol response, the repeated-measures ANOVA also revealed a significant main effect of Time (F(4, 200) = 40.03, p < .01). Post hoc analysis showed that salivary cortisol levels measured at 0 min, 20 min, and 45 min after the end of the TSST were significantly higher than cortisol levels measured at baseline (ps < .01). There was no significant difference between cortisol levels at baseline and that measured at 60 min after the end of the TSST (p > .10). The cortisol level reached the peak at 20 min after the end of the TSST task, which was higher than cortisol at 0 min and 45 min after the end of the TSST task (ps < .01). The mean value (± SD) of AUCg was 19.58 (± 5.74).
The correlation between two dimensions of childhood stress, emotion processing, and acute stress response
Path 1: the relationship between childhood stress and emotion processing
For emotion processing, a multiple linear regression () was conducted with the independent variable of abuse and neglect and control variables (sex and trait anxiety). The model explained 12% of the variance in ΔLPP amplitudes (R2 = 0.12, F(4,50) = 1.57, p > .10), and only abuse score but not neglect was significantly negatively associated with ΔLPP amplitudes (). To evaluate the hypothesis more precisely, standardized beta coefficients and CIs were compared between CTQ abuse and CTQ neglect. As shown in Left, CIs of ΔLPP mean amplitudes for abuse were clearly distinguished from neglect (less than 50%), indicating that the relationships for ΔLPP amplitudes were significantly different between CTQ-abuse and CTQ-neglect.
Figure 3. Left: Pooled standardized beta coefficients (with 95% CIs), representing the magnitude of ΔLPP amplitudes, by CTQ-abuse and CTQ-neglect. Middle: Pooled standardized beta coefficients (with 95% CIs), representing ΔHR, by CTQ-abuse and CTQ-neglect. Right: Pooled standardized beta coefficients (with 95% CIs), representing cortisol AUCg, by CTQ-abuse and CTQ-neglect.
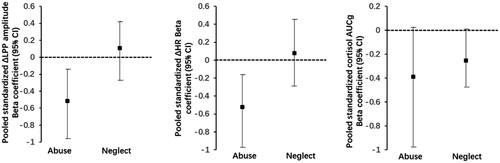
Table 1. Bootstrapping regression of CTQ’s two dimensions on ΔLPP amplitudes.
Path 2: the relationship between childhood stress and acute stress response
For the heart rate reactivity to TSST, the multiple regression model explained 16.5% of the variance of ΔHR (R2 = 0.165, F(4,50) = 2.27, p = .08), and only abuse score but not neglect was significantly negatively associated with ΔHR (). Furthermore, as shown in Right, CIs of ΔHR for abuse were distinguished from neglect (less than 50%), demonstrating that the regression coefficient for CTQ-abuse was considered significantly different from the CTQ-neglect regression coefficient.
Table 2. Bootstrapping regression of CTQ’s two dimensions on ΔHR.
For the cortisol reactivity to TSST, the regression model was significant and explained 20.9% of the variance of cortisol AUCg (R2 = 0.209, F(4,50) = 3.04, p = .03). The 95% CIs showed that neglect was marginally negatively associated with cortisol reactivity (see ). However, the overlapping CIs analysis showed that CIs of cortisol AUCg for neglect were not significantly distinguished from abuse (more than 50%), indicating that the relationships for cortisol AUCg were not different between CTQ-abuse and CTQ-neglect.
Table 3. Bootstrapping regression of CTQ’s two dimensions on cortisol AUCg.
Path 3: the relationship between emotion processing and acute stress response
shows the results of the regression analysis with bootstrap for the HR response to stress. The model explained 17% of the variance (R2 = 0.17, F(3, 50) = 3.20, p < .05) in the HR response to the acute psychosocial stressor (ΔHR), and ΔLPP amplitude was significantly positively associated with ΔHR (see ).
Table 4. Bootstrapping regression of emotion processing (indicated by ΔLPP amplitude) on ΔHR.
In contrast, the hierarchical regression model for the cortisol response to stress (AUCg) was not significant (R2 = 0.03, F(3, 50) = 0.52, p > .10), and the amplitude of ΔLPP could not be associated with the AUCg (95% CI: [−0.26, 0.26], p > .10).
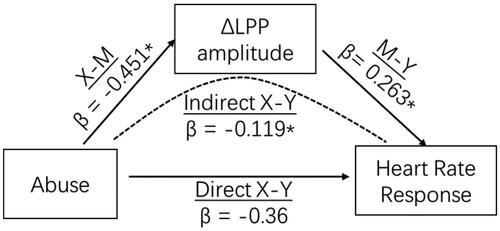
Path 4: Mediation analysis
We first found that only the level of childhood abuse is significantly associated with blunted emotion processing (ΔLPP amplitudes) and blunted heart rate response to stress (ΔHR). Then, we ran a mediation model to test whether the emotion process mediated the association of childhood abuse and HR reactivity to the TSST. Adjusting for sex and trait anxiety, a significant indirect effect of abuse through blunted emotion processing to HR reactivity was observed (95%CI: −0.325, −0.002) (). The indirect effect of childhood abuse on heart rate response to acute social stress through emotion processing (indicated by ΔLPP amplitudes) accounted for 33.1% (−0.119/−0.36) of the total effect. After including ΔLPP amplitude as a mediator, the direct effect of abuse on heart rate response became non-significant. Therefore, after controlling sex and trait anxiety, a higher level of childhood abuse was associated with a smaller heart rate response to acute stress and this relationship was fully mediated by blunted processing of negative stimuli.
Discussion
In this study, we found that a higher level of childhood abuse (as a proxy of threat) but not neglect (as a proxy of deprivation) was distinctly associated with blunted neural reactivity to negative stimuli (as indicated by smaller LPP amplitudes) on the one hand, and with blunted HR response to acute psychosocial stress on the other hand. Smaller LPP amplitudes were also related to blunted HR acute stress response. Furthermore, the mediation model showed that childhood abuse was associated with blunted HR response through blunted neural reactivity to negative stimuli. These results even hold after controlling sex and trait anxiety in a sample of healthy young adults.
First of all, we found that enhanced LPP amplitudes to negative compared to neutral pictures were associated with higher HR responses to acute psychosocial stress. The LPP is a reliable marker reflecting elaborated attention toward affective stimuli Bondy et al., Citation2018), which involves cortical and subcortical emotion-processing brain regions, including the prefrontal cortex and amygdala (De Rover et al., Citation2012; Liu et al., Citation2012; Sabatinelli et al., Citation2007). As an important part of the limbic system, the amygdala is well positioned to control basic autonomic arousal processes through the hypothalamus and brainstem circuits (LeDoux, Citation2000), and generates visceral signs of emotional arousal—e.g. changes in heart rate (Critchley et al., Citation2005; Kuniecki et al., Citation2002). Prior studies using simultaneous fMRI and EEG recording (Liu et al., Citation2012; Sabatinelli et al. Citation2007), genetic analysis, and pharmacological manipulation (De Rover et al., Citation2012) have suggested that the increased LPP amplitude reflects amygdala modulation, which plays an important role in autonomic stress response (e.g. Fortaleza et al., Citation2012). Consistently, we found that enhanced LPP amplitudes to negative compared to neutral pictures were associated with higher HR responses to acute psychosocial stress. It adds to the evidence that negative bias plays a causal role in stress vulnerability (e.g. Fox et al., Citation2011). For example, prior fMRI and ERP studies found that relatively exaggerated amygdala reactivity and increased LPP amplitudes toward negative stimuli before trauma exposure were predictive to posttraumatic symptoms in response to traumatic stressors (Admon et al., Citation2009; Swartz et al., Citation2015). Egloff et al. (Citation2002) found that attentional bias to negative stimuli measured by response times positively predicted HR and blood pressure responses to an evaluated speech task in females. Thus, a hypersensitive amygdala may explain larger LPP amplitudes toward negative stimuli and higher HR response to stress.
When adding the factor of childhood stress, our results showed that childhood abuse is associated with smaller LPP amplitudes to negative stimuli as well as smaller HR reactivity to acute stress. As the theoretical model has proposed, childhood threat in abusive conditions may differentiate in shaping neurobiological development, such that abuse exposure influences the development of cortico-limbic circuits that underlie salience processing (McLaughlin et al., Citation2014; Teicher et al., Citation2016; Teicher & Samson, Citation2016), in turn, modifying physiologic responses to stress (Busso et al., Citation2017). Consistent with the model, we found that childhood abuse but not neglect was distinctly related to blunted processing of negative stimuli on the one hand, and with blunted heart rate response to acute stress on the other hand. This effect, to some extent, was in line with previous studies that abused children tended to avoid negative information, shown as biased attention away from threat (Pine et al., Citation2005), and as decreased activation of the amygdala to negative stimuli (Puetz et al., Citation2016). Meanwhile, the current result also echoed with one previous study from our lab that childhood abuse rather than neglect was associated with the blunted motivational evaluation of self-inflicted errors among healthy young males (Wu et al., Citation2021). Furthermore, our mediation analysis showed that childhood abuse was associated with blunted HR response through blunted processing of negative stimuli. Considering the role of amygdala sensitivity in both emotion processing and autonomic stress response, as we discussed above, the blunted amygdala might be the mechanism underlying the association between childhood stress and decreased autonomic stress response. Specifically, individuals with a higher level of childhood abuse might show diminished amygdala response revealed as smaller LPP reactivity to negative stimuli, which further lead to a smaller autonomic response to the acute psychosocial stressor-the universal negative event.
Given the healthy characteristics of our participants, the results of blunted salience processing in the current study could be explained by the cortical and cognitive adaptation mechanism, i.e. following iterative exposure to threat, the response to negative events might become habituated or downregulated in order to prevent stress overshoot (Heim et al., Citation2013). Accordingly, a recent scoping review showed that resilient adults after childhood adversity (i.e. those reporting childhood maltreatment without psychopathology) demonstrated an improved ability to regulate emotions through medial prefrontal cortex-amygdala downregulation, lower hippocampal activation to emotional faces, and increased amygdala habituation to stress (Moreno-López et al., Citation2020). Similarly, a study found that higher trait resilience was related to blunted negative emotion processing indicated by smaller LPP amplitudes (Chen et al., Citation2018). In other words, our study suggested that dampened threat processing (indicated by smaller LPP reactivity to negative stimuli) might rather reflect resilience to childhood abuse in young adults, and this dampened threat processing further leads to diminished stress responses (indicated by smaller HR response to acute stress). Our findings may have some clinical implications. Previous studies proposed that emotion and its regulation could be used as an intervention target to enhance resilience to early life adversity (see reviews, see Leal & Silvers, Citation2021; Moreno-López et al., Citation2020). Therefore, targeting emotion processing (such as adaptive emotion regulation strategies) might be suitable and practical for interventions of relieving current acute stress response among individuals with early life adversity.
It’s also worthwhile to mention that we did not find similar results on the cortisol response to acute stress. Although contrary to our expectations, this result was consistent with a previous meta-analysis which failed to detect any significant relationship between early life stress and cortisol reactivity to an acute stressor (Fogelman & Canli, Citation2018). Furthermore, another meta-analysis also showed a weak association between childhood stress (measured by the CTQ) and salivary cortisol responses to the TSST (Lai et al., Citation2021). Both these two meta-analyses and our study focus on individuals without psychopathology, and this might eliminate the people who were severely influenced by childhood adversity (in terms of developing psychopathology later on). In other words, this healthy sample potentially limits the overlapping impact of childhood adversity and psychopathology on cortisol.
There are some limitations to the current study. First, the sample from the current study was rather small. According to a simulation study (Fritz & MacKinnon, Citation2007), the results from our study might be underpowered. Future studies should try to replicate this study by using a larger sample size. Furthermore, considering that the sample from the current study was consisted of young undergraduate students and that childhood maltreatment was at a mild level, the results might not be able to generalize to other samples. Second, we only used the retrospective self-reported questionnaire, which might not be a precise way to measure childhood stress. Participants with enhanced emotion processing might have a negative recall bias, in turn, report more severity of childhood stress. By controlling trait anxiety level, we might be able to, to some extent, partial out the effects of personality on recall bias and stress response. Furthermore, although the CTQ is a commonly used instrument for assessing childhood stress, it does not include information about the time the adversity occurred. Therefore, it limits our understanding of the specific developmental stage (s) of adversity exposure, which is a crucial factor on the effect of childhood stress on the brain and cognitive development (McLaughlin et al., Citation2014; Teicher et al., Citation2016). Future studies with the retrospective method should consider more sophisticated instruments like the “Maltreatment and Abuse Chronology of Exposure” (Teicher & Parigger, Citation2015).
In conclusion, building on the previous work, the current study explored the two dimensions of childhood stress on negative emotion processing and acute stress response among the same group of healthy young adults. The results revealed that the severity of childhood abuse is uniquely associated with blunted emotion processing and blunted acute stress response, and blunted emotion processing significantly mediates the relationship between childhood abuse and acute stress response. Considering that all the participants are healthy, the blunted processing of negative stimuli might reflect adaptive responses to prevent stress overshooting in the face of frequent early-life-threatening experiences.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Admon, R., Lubin, G., Stern, O., Rosenberg, K., Sela, L., Ben-Ami, H., & Hendler, T. (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences, 106(33), 14120–14125. https://doi.org/10.1073/pnas.0903183106
- Bernstein, D. P., & Fink, L. (1998). Childhood Trauma Questionnaire: A Retrospective Self-report: Manual. Psychological Corporation.
- Bondy, E., Stewart, J. G., Hajcak, G., Weinberg, A., Tarlow, N., Mittal, V. A., & Auerbach, R. P. (2018). Emotion processing in female youth: Testing the stability of the late positive potential. Psychophysiology, 55(2), e12977. https://doi.org/10.1111/psyp.12977
- Booij, S. H., Bouma, E. M., de Jonge, P., Ormel, J., & Oldehinkel, A. J. (2013). Chronicity of depressive problems and the cortisol response to psychosocial stress in adolescents: The TRAILS study. Psychoneuroendocrinology, 38(5), 659–666. https://doi.org/10.1016/j.psyneuen.2012.08.004
- Boudarene, M., Legros, J. J., & Timsit-Berthier, M. (2002). Study of the stress response: Role of anxiety, cortisol and DHEAs. Encephale, 28(2), 139–146.
- Buchanan, T. W., Bagley, S. L., Stansfield, R. B., & Preston, S. D. (2012). The empathic, physiological resonance of stress. Social Neuroscience, 7(2), 191–201. https://doi.org/10.1080/17470919.2011.588723
- Buchanan, T. W., Tranel, D., & Kirschbaum, C. (2009). Hippocampal damage abolishes the cortisol response to psychosocial stress in humans. Hormones and Behavior, 56(1), 44–50. https://doi.org/10.1016/j.yhbeh.2009.02.011
- Bunea, I. M., Szentágotai-Tătar, A., & Miu, A. C. (2017). Early-life adversity and cortisol response to social stress: A meta-analysis. Translational Psychiatry, 7(12), 1–8.
- Busso, D. S., McLaughlin, K. A., & Sheridan, M. A. (2017). Dimensions of adversity, physiological reactivity, and externalizing psychopathology in adolescence: Deprivation and threat. Psychosomatic Medicine, 79(2), 162. https://doi.org/10.1097/PSY.0000000000000369
- Chen, D., Wu, J., Yao, Z., Lei, K., Luo, Y., & Li, Z. (2018). Negative association between resilience and event-related potentials evoked by negative emotion. Scientific Reports, 8(1), 1–6.
- Childs, E., Dlugos, A., & De Wit, H. (2010). Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology, 47(3), 550–559. https://doi.org/10.1111/j.1469-8986.2009.00961.x
- Chu, D. A., Bryant, R. A., Gatt, J. M., & Harris, A. W. (2016). Failure to differentiate between threat-related and positive emotion cues in healthy adults with childhood interpersonal or adult trauma. Journal of Psychiatric Research, 78, 31–41. https://doi.org/10.1016/j.jpsychires.2016.03.006
- Critchley, H. D., Rotshtein, P., Nagai, Y., O'Doherty, J., Mathias, C. J., & Dolan, R. J. (2005). Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage, 24(3), 751–762. https://doi.org/10.1016/j.neuroimage.2004.10.013
- Cumming, G. (2009). Inference by eye: Reading the overlap of independent confidence intervals. Statistics in Medicine, 28(2), 205–220. https://doi.org/10.1002/sim.3471
- Cumming, G. (2014). The new statistics: Why and how. Psychological Science, 25(1), 7–29. https://doi.org/10.1177/0956797613504966
- Cunningham, W. A., & Kirkland, T. (2014). The joyful, yet balanced, amygdala: moderated responses to positive but not negative stimuli in trait happiness. Social Cognitive and Affective Neuroscience, 9(6), 760–766. https://doi.org/10.1093/scan/nst045
- Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., & Lang, P. J. (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. https://doi.org/10.1016/s0301-0511(99)00044-7
- Davidson, R., & MacKinnon, J. G. (1993). Estimation and inference in econometrics. OUP Catalogue.
- De Rover, M., Brown, S. B. R. E., Boot, N., Hajcak, G., Van Noorden, M. S., Van Der Wee, N. J. A., & Nieuwenhuis, S. (2012). Beta receptor-mediated modulation of the late positive potential in humans. Psychopharmacology, 219(4), 971–979. https://doi.org/10.1007/s00213-011-2426-x
- Egloff, B., Wilhelm, F. H., Neubauer, D. H., Mauss, I. B., & Gross, J. J. (2002). Implicit anxiety measure predicts cardiovascular reactivity to an evaluated speaking task. Emotion, 2(1), 3–11. https://doi.org/10.1037/1528-3542.2.1.3
- Fogelman, N., & Canli, T. (2018). Early life stress and cortisol: A meta-analysis. Hormones and Behavior, 98, 63–76. https://doi.org/10.1016/j.yhbeh.2017.12.014
- Fortaleza, E. A. T., Scopinho, A. A., & Corrêa, F. M. A. (2012). β-Adrenoceptors in the medial amygdaloid nucleus modulate the tachycardiac response to restraint stress in rats. Neuroscience, 227, 170–179. https://doi.org/10.1016/j.neuroscience.2012.09.048
- Fox, E., Zougkou, K., Ridgewell, A., & Garner, K. (2011). The serotonin transporter gene alters sensitivity to attention bias modification: Evidence for a plasticity gene. Biological Psychiatry, 70(11), 1049–1054. https://doi.org/10.1016/j.biopsych.2011.07.004
- Fritz, M. S., & MacKinnon, D. P. (2007). Required sample size to detect the mediated effect. Psychological Science, 18(3), 233–239. https://doi.org/10.1111/j.1467-9280.2007.01882.x
- Garrison, K. E., Crowell, A. L., Finley, A. J., & Schmeichel, B. J. (2017). Effects of prior mental effort on picture processing: An ERP investigation. Psychophysiology, 54(11), 1714–1725. https://doi.org/10.1111/psyp.12914
- Gibb, B. E., Schofield, C. A., & Coles, M. E. (2009). Reported history of childhood abuse and young adults’ information-processing biases for facial displays of emotion. Child Maltreatment, 14(2), 148–156. https://doi.org/10.1177/1077559508326358
- Hajcak, G., & Foti, D. (2020). Significance?… Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology, 57(7), e13570.
- Harkness, K. L., Stewart, J. G., & Wynne-Edwards, K. E. (2011). Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology, 36(2), 173–181. https://doi.org/10.1016/j.psyneuen.2010.07.006
- Hayes, A. F. (2013). Introduction to mediation, moderation, and conditional process analysis. Guilford Press.
- Heim, C. M., Mayberg, H. S., Mletzko, T., Nemeroff, C. B., & Pruessner, J. C. (2013). Decreased cortical representation of genital somatosensory field after childhood sexual abuse. The American Journal of Psychiatry, 170(6), 616–623. https://doi.org/10.1176/appi.ajp.2013.12070950
- Herman, J. P., & Cullinan, W. E. (1997). Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends in Neurosciences, 20(2), 78–84. https://doi.org/10.1016/S0166-2236(96)10069-2
- Hot, P., Saito, Y., Mandai, O., Kobayashi, T., & Sequeira, H. (2006). An ERP investigation of emotional processing in European and Japanese individuals. Brain Research, 1122(1), 171–178. https://doi.org/10.1016/j.brainres.2006.09.020
- Hughes, K., Bellis, M. A., Hardcastle, K. A., et al. (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health, 2(8), e356–66. https://doi.org/10.1016/S2468-2667(17)30118-4
- Kaiser, R. H., Clegg, R., Goer, F., Pechtel, P., Beltzer, M., Vitaliano, G., … Pizzagalli, D. A. (2018). Childhood stress, grown-up brain networks: Corticolimbic correlates of threat-related early life stress and adult stress response. Psychological Medicine, 48(7), 1157–1166. https://doi.org/10.1017/S0033291717002628
- Kaldewaij, R., Koch, S. B., Zhang, W., Hashemi, M. M., Klumpers, F., & Roelofs, K. (2019). Frontal control over automatic emotional action tendencies predicts acute stress responsivity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(11), 975–983. https://doi.org/10.1016/j.bpsc.2019.06.011
- Kircanski, K., Sisk, L. M., Ho, T. C., Humphreys, K. L., King, L. S., Colich, N. L., Ordaz, S. J., & Gotlib, I. H. (2019). Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Development and Psychopathology, 31(3), 1011–1022. https://doi.org/10.1017/S0954579419000555
- Kraaijenvanger, E. J., Pollok, T. M., Monninger, M., Kaiser, A., Brandeis, D., Banaschewski, T., & Holz, N. E. (2020). Impact of early life adversities on human brain functioning: A coordinate-based meta-analysis. Neuroscience and Biobehavioral Reviews, 113, 62–76. https://doi.org/10.1016/j.neubiorev.2020.03.008
- Kudielka, B. M., & Kirschbaum, C. (2005). Sex differences in HPA axis responses to stress: a review. Biological Psychology, 69(1), 113–132. https://doi.org/10.1016/j.biopsycho.2004.11.009
- Kudielka, B. M., Schommer, N. C., Hellhammer, D. H., & Kirschbaum, C. (2004). Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology, 29(8), 983–992. https://doi.org/10.1016/j.psyneuen.2003.08.009
- Kuniecki, M., Urbanik, A., Sobiecka, B., Kozub, J., & Binder, M. (2002). Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiologiae Experimentalis, 63(1), 39–48.
- Lai, C. L. J., Lee, D. Y. H., & Leung, M. O. Y. (2021). Childhood adversities and salivary cortisol responses to the trier social stress test: A systematic review of studies using the Children Trauma Questionnaire (CTQ). International Journal of Environmental Research and Public Health, 18(1), 29.
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1999). International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida, Center for Research in Psychophysiology.
- Leal, A. S. M., & Silvers, J. A. (2021). Neurobiological markers of resilience to early-life adversity during adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(2), 238–247. https://doi.org/10.1016/j.bpsc.2020.08.004
- LeDoux, J. E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23(1), 155–184. https://doi.org/10.1146/annurev.neuro.23.1.155
- Liu, Y., Huang, H., McGinnis-Deweese, M., Keil, A., & Ding, M. (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience. 32(42), 14563–14572. https://doi.org/10.1523/JNEUROSCI.3109-12.2012
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Review Neuroscience, 10, 434–445. https://doi.org/10.1038/nrn2639
- Machlin, L., Miller, A. B., Snyder, J., McLaughlin, K. A., & Sheridan, M. A. (2019). Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Frontiers in Behavioral Neuroscience, 13, 80. https://doi.org/10.3389/fnbeh.2019.00080
- McCrory, E. J., De Brito, S. A., Sebastian, C. L., Mechelli, A., Bird, G., Kelly, P. A., & Viding, E. (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21, 947–948.
- McLaughlin, K. A., Sheridan, M. A., & Lambert, H. K. (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews, 47, 578–591. https://doi.org/10.1016/j.neubiorev.2014.10.012
- Moreno-López, L., Ioannidis, K., Askelund, A. D., Smith, A. J., Schueler, K., & Van Harmelen, A. L. (2020). The resilient emotional brain: A scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 392–402. https://doi.org/10.1016/j.bpsc.2019.12.008
- Pine, D. S., Mogg, K., Bradley, B. P., Montgomery, L., Monk, C. S., McClure, E., … Kaufman, J. (2005). Attention bias to threat in maltreated children: Implications for vulnerability to stress-related psychopathology. American Journal of Psychiatry, 162(2), 291–296. https://doi.org/10.1176/appi.ajp.162.2.291
- Pollak, S. D., Klorman, R., Thatcher, J. E., & Cicchetti, D. (2001). P3b reflects maltreated children’s reactions to facial displays of emotion. Psychophysiology, 38(2), 267–274. https://doi.org/10.1111/1469-8986.3820267
- Pollak, S. D., Messner, M., Kistler, D. J., & Cohn, J. F. (2009). Development of perceptual expertise in emotion recognition. Cognition, 110(2), 242–247. https://doi.org/10.1016/j.cognition.2008.10.010
- Pruessner, J. C., Kirschbaum, C., Meinlschmid, G., & Hellhammer, D. H. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. https://doi.org/10.1016/S0306-4530(02)00108-7
- Puetz, V. B., Viding, E., Palmer, A., et al. (2016). Altered neural response to rejection-related words in children exposed to maltreatment. Journal of Child Psychology and Psychiatry, 57(10), 1165–1173. https://doi.org/10.1111/jcpp.12595
- Reidy, J., & Richards, A. (1997). Anxiety and memory: A recall bias for threatening words in high anxiety. Behaviour Research and Therapy, 35(6), 531–542. https://doi.org/10.1016/S0005-7967(97)00001-6
- Sabatinelli, D., Lang, P. J., Keil, A., & Bradley, M. M. (2007). Emotional perception: Correlation of functional MRI and event-related potentials. Cerebral Cortex, 17(5), 1085–1091. https://doi.org/10.1093/cercor/bhl017
- Semlitsch, H. V., Anderer, P., Schuster, P., & Presslich, O. (1986). A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology, 23(6), 695–703. https://doi.org/10.1111/j.1469-8986.1986.tb00696.x
- Sergerie, K., Chochol, C., & Armony, J. L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 32(4), 811–830. https://doi.org/10.1016/j.neubiorev.2007.12.002
- Shackman, J. E., Shackman, A. J., & Pollak, S. D. (2007). Physical abuse amplifies attention to threat and increases anxiety in children. Emotion, 7(4), 838. https://doi.org/10.1037/1528-3542.7.4.838
- Spielberger, C. D. (1983). Manual for the State-Trait Anxiety Inventory (STAI Form Y). Consulting Psychologists.
- Stoltenborgh, M., Bakermans‐Kranenburg, M. J., Alink, L. R., & van IJzendoorn, M. H. (2015). The prevalence of child maltreatment across the globe: Review of a series of meta‐analyses. Child Abuse Review, 24(1), 37–50. https://doi.org/10.1002/car.2353
- Swartz, J. R., Knodt, A. R., Radtke, S. R., & Hariri, A. R. (2015). A neural biomarker of psychological vulnerability to future life stress. Neuron, 85(3), 505–511. https://doi.org/10.1016/j.neuron.2014.12.055
- Teicher, M. H., & Parigger, A. (2015). The ‘Maltreatment and Abuse Chronology of Exposure’(MACE) scale for the retrospective assessment of abuse and neglect during development. PLOS One, 10(2), e0117423. https://doi.org/10.1371/journal.pone.0117423
- Teicher, M. H., & Samson, J. A. (2016). Annual research review: enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, 57(3), 241–266. https://doi.org/10.1111/jcpp.12507
- Teicher, M. H., Samson, J. A., Anderson, C. M., & Ohashi, K. (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. https://doi.org/10.1038/nrn.2016.111
- Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. https://doi.org/10.1038/nrn2647
- Vallet, W., Hone-Blanchet, A., & Brunelin, J. (2020). Abnormalities of the late positive potential during emotional processing in individuals with psychopathic traits: A meta-analysis. Psychological Medicine, 50(12), 2085–2095. https://doi.org/10.1017/S0033291719002216
- van Dongen, J. D. M., Brazil, I. A., van der Veen, F. M., & Franken, I. H. A. (2018). Electrophysiological correlates of empathic processing in individuals with psychopathic meanness traits. Neuropsychology, 32(8), 996–1006. https://doi.org/10.1037/neu0000477
- Vandewalle, G., Middleton, B., Rajaratnam, S. M. W., Stone, B. M., Thorleifsdottir, B., Arendt, J., & Dijk, D. J. (2007). Robust circadian rhythm in heart rate and its variability: Influence of exogenous melatonin and photoperiod. Journal of Sleep Research, 16(2), 148–155. https://doi.org/10.1111/j.1365-2869.2007.00581.x
- Viola, T. W., Salum, G. A., Kluwe-Schiavon, B., Sanvicente-Vieira, B., Levandowski, M. L., & Grassi-Oliveira, R. (2016). The influence of geographical and economic factors in estimates of childhood abuse and neglect using the childhood trauma questionnaire: A worldwide meta-regression analysis. Child Abuse & Neglect, 51, 1–11. https://doi.org/10.1016/j.chiabu.2015.11.019
- Wu, J., Liu, Y., Fang, H., Qin, S., Kohn, N., & Duan, H. (2021). The relationship between childhood stress and distinct stages of dynamic behavior monitoring in adults: Neural and behavioral correlates. Social Cognitive and Affective Neuroscience, 16(9), 937–949. https://doi.org/10.1093/scan/nsab041
- Xin, Y., Wu, J., Yao, Z., Guan, Q., Aleman, A., & Luo, Y. (2017). The relationship between personality and the response to acute psychological stress. Scientific Reports, 7(1), 1–8.
- Xin, Y., Yao, Z., Wang, W., Luo, Y., Aleman, A., & Wu, J. (2020). Recent life stress predicts blunted acute stress response and the role of executive control. Stress, 23(3), 359–367. https://doi.org/10.1080/10253890.2019.1687684
- Zhang, H., Yao, Z., Lin, L., Sun, X., Shi, X., & Zhang, L. (2019). Early life stress predicts cortisol response to psychosocial stress in healthy young adults. PsyCh Journal, 8(3), 353–362. https://doi.org/10.1002/pchj.278
- Zhang, J.-F., Shi, Z.-B., Zhao, P.-L., & Wang, L. (2012). Posttraumatic growth and related factors in junior middle school students after the Wenchuan earthquake. Chinese Mental Health Journal, 26(5), 357–362.