Abstract
Besides significant benefits to physical health, exercise promotes mental health, reduces symptoms of mental illness, and enhances psychological development. Exercise can offset the impact of chronic stress, which is a major precursor to the development of mental disorders. The effects of exercise on chronic stress-induced behaviors are contradictory in preclinical studies, primarily due to the lack of data and sex-specific investigations. We sought to evaluate the effects of exercise on chronic stress-induced behavioral changes in both male and female mice. Mice were subjected to an Unpredictable Chronic Mild Stress (UCMS) paradigm with accessibility to running wheels for 2 h daily. Physiological and behavioral evaluations were conducted throughout the stress paradigm to determine if exercise blunts the effects of UCMS. Chronic stress induced voluntary wheel running (VWR) and weight loss in male and female mice. Compared to males, increased VWR was reported in females who also regained their weight lost by the end of the UCMS protocol. Exercise promoted resilience to stress-induced hyponeophagia in the novelty-suppressed feeding test and increased sucrose consumption. Exercise induced a sex-specific reduction in immobility and avoidance behavior in the tail suspension and open field tests and increased exploratory behavior in the light-dark test. These results indicate that exercise can promote resilience to the behavioral effects of chronic stress in males and females, and can affect behavior independent of chronic stress.
1. Introduction
Chronic stress is induced by various long-term internal and external stressors that can lead to a depressed mood, anhedonia, weight changes, sleep disturbances, among other symptoms (Otte et al., Citation2016). Unfortunately, chronic stress is a precursor of the most prevalent mental health disorders (NIMH, Citation2018), and the coronavirus (COVID-19) pandemic has resulted in quadrupling the number of adults reporting symptoms of chronic stress (Panchal & Kamal, Citation2021), and as a result, increased diagnosis of anxiety and depressive disorders have occurred.
Besides the known benefits to physical health, exercise is beneficial to mental health and enhances psychological development. There is a strong association between physical activity and decreased symptoms of chronic stress-induced psychiatric disorders (Zhao et al., Citation2020). For instance, low cardiorespiratory fitness is often associated with an increased risk of developing depression (Kandola et al., Citation2019), whereas decreased depression is reported in active adults (Salguero et al., Citation2011; Schuch et al., Citation2018; Zhao et al., Citation2020). There is an urgent need to better understand the effects of chronic stress and mechanisms of resilience. In rodent models, the VWR model of exercise can ameliorate the negative effects of chronic stress (Binder et al., Citation2004). The current literature that evaluates the benefit of exercise on stress-related behaviors is limited due to variables in stress and exercise paradigms. Most strikingly, the inclusion of females is lacking in current studies, even though it is well established that sex differences exist in mental disorders associated with chronic stress, including a difference in the incidence, onset, severity, episodes, and response to antidepressant treatments (Brody, Citation2018; Marcus et al., Citation2005). Therefore, in our study, male and female mice were subjected to UCMS while concurrently given daily access to running wheels for 2 h to determine if VWR can promote resilience to the behavioral effects of chronic stress. A battery of behavioral tests was conducted to measure the effect of stress and exercise on hyponeophagia, sucrose consumption, locomotor activity, and exploratory and avoidance behaviors. Our results indicate that VWR was increased in UCMS mice, with females voluntarily running more than males. Chronic stress induced weight loss in both sexes; however, females regained the lost weight. Exercise promoted resilience to the behavioral effects of chronic stress in both males and females and affected behavior in a sex-specific manner.
2. Materials and methods
2.1. Animals
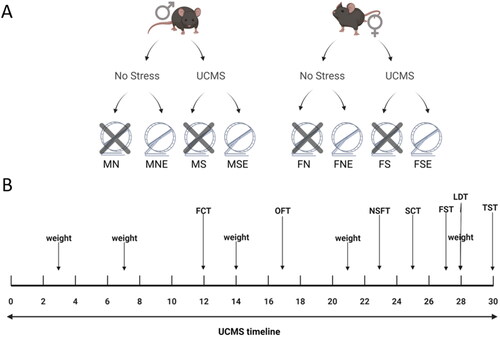
Male and female C57B6/J mice (n = 72) obtained from Jackson Laboratories were used in the experiments. All mice were housed in the same room in same-sex groups of 4 with ad libitum access to food and water and were maintained on a 12 h light/dark cycle (lights were on from 6 am to 6 pm). All experimental procedures were approved by St. Joseph’s University Institutional Animal Care and Use Committee. Mice weighed between 18 and 27 g and were 8–10 weeks old at the start of the UCMS protocol. The eight groups tested are referred to as male no stress no exercise (MN, n = 12); male no stress exercise (MNE, n = 8); male stress no exercise (MS, n = 8); male stress exercise (MSE, n = 8); female no stress no exercise (FN, n = 12); female no stress exercise (FNE, n = 8); female stress no exercise (FS, n = 8); female stress exercise (FSE, n = 8) ().
Figure 1. Experimental groups and UCMS timeline. (A) Mice were assigned to eight experimental groups based on variables of sex, stress, and exercise. (B) UCMS experimental timeline with physiological and behavioral evaluations. MN: male no stress; MNE: male no stress exercise; MS: male stress; MSE: male stress exercise: FN: Female no stress; FNE: Female no stress exercise; FS: Female stress; FSE: Female stress exercise. FCT: Food consumption test; OFT: open field test; NSFT: novelty suppressed feeding test; SCT: sucrose consumption test; FST: Forced swim test; LDT: light dark test; TST: tail suspension test.
2.2. UCMS
Mice were randomly assigned to two groups: no stress and UCMS. The UCMS group underwent the UCMS paradigm for 30 days. The UCMS protocol was adapted from previous studies (Manners et al., Citation2019). Briefly, for 30 consecutive days, mice were subjected to three stressors a day: in the morning, afternoon, and overnight in dedicated procedure rooms. Stressors persisted for 1–1.5 h in the morning and afternoon, and for 13 h overnight (Manners et al., Citation2019). Stressors included: a 45° cage tilt, food or water deprivation for 14 h, cage on a cold surface, new cage partners of the same sex, exposure to predator urine odor, wet bedding, cage shaking at 100 rotations per minute, restraint, social isolation, interruption of light/dark cycle, and stroboscope with lights off. Throughout the UCMS protocol, stressors remained random as no stressor was repeated on two consecutive days, and novelty was maintained by pairing stressors such as wet bedding and predator odor. Mice were returned to the animal colony room between stressors.
2.3. VWR
The VWR protocol was adapted and modified by Huang et al. (Huang et al., Citation2017). One day prior to UCMS, exercise and no-exercise groups were pre-exposed for 2 h to unlocked or locked running wheels respectively. On each day of the UCMS protocol, mice assigned to the exercise groups were permitted 2 h of access to a running wheel apparatus in a clear Perspex (20 × 20 × 35 cm) chamber. This setup allowed mice to be video recorded and tracked for time on the running wheel using the ANY-maze automated video tracking software (Stoelting Co. Wood Dale, IL). Running wheels were also connected to speedometers that enabled the recording of the average speed, total distance, and total time of VWR. VWR was conducted in-between the afternoon and evening stressors of the UCMS protocol, from the hours of 1:30 PM − 3:30 PM then 4:00 PM − 6:00 PM during the 12-h light phase. After the first run was completed (3:30 PM), mice were removed from the room which was then sanitized, and mice of the opposite sex were given wheel access from 4:00 PM–6:00 PM. VWR starting time was alternated between males and females on each day. The no-exercise groups were given access to locked wheels during the same time the exercise groups were given access to unlocked wheels throughout the duration of the experiment.
2.4. Physiological and behavioral studies
All behavioral evaluations were conducted during the light phase, between the hours of 8:00AM and 12:00 PM unless specified differently. Treatment conditions were randomly assigned, and animals were tested in an alternating order to account for the effect of time of day on behavior. Male and female mice were tested separately in order to avoid the influence of odors, pheromones, and vocalization between different sexes. Animals acclimated to testing rooms 1 h prior to behavioral assessment. All experimental procedures were completed as per the guidelines of the St. Joseph’s University Institutional Animal Care and Use Committee and were conducted in compliance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
2.4.1. Body weight measurement
The body weight of each mouse was evaluated the day prior to the UCMS procedure (baseline weight), then on days 3, 7, 14, 21, and 28 (). The percent change in weight from baseline weight is reported.
2.4.2. Food consumption test (FCT)
Food intake was measured on Day 12 () to determine if UCMS changed food consumption. Mice were individually housed overnight in a cage containing water, bedding, and pre-weighed food. Following 12 h, food and food remnants were collected and weighed to determine the amount consumed.
2.4.3. Open field test (OFT)
The OFT protocol was used to measure the locomotor activity, exploratory behaviors, and latency to enter an anxiogenic environment. The OFT was conducted on days 17, 18, and 19 of the UCMS paradigm (). After 1 h of acclimation to the testing room, mice were individually placed in a clear Perspex apparatus (40 × 40 × 35 cm) with an ambient room light intensity of 325 lux, and activity was recorded for 1 h. Latency to enter the center was recorded by ANY-maze.
2.4.4. Fecal droppings measurements
In order to assess the effect of stress and exercise on gastrointestinal motility, the bowel movement was assessed by counting mice fecal droppings in the clear Perspex apparatus (40x40x35 cm) following the 1-h OFT. The number of fecal droppings was then quantified by ImageJ.
2.4.5. Novelty suppressed feeding test (NSFT)
Hyponeophagia, the exposure to an anxiogenic environment suppressing feeding behavior, is a chronic stress-induced behavior in mice (Samuels & Hen, Citation2011). The NSFT was used to measure this behavior. On day 23 of the UCMS protocol (), all mice underwent the NSFT (Manners et al., Citation2019). Mice were food-deprived for 24 h prior to testing. On test day, mice were placed for 20 min in a box, in which 3 ml of highly palatable food (50% sweetened condensed milk solution) was placed in a petri dish in the center of the box, with 2500 lux light shining on the dish. Mice were video-recorded for the duration of the test, latency to first consumption was hand-scored by three blinded researchers, and the average time is reported.
2.4.6. Sucrose consumption test (SCT)
Chronic stress is known to reduce sensitivity to reward, which is observed in mouse models of chronic stress and in human depression (Forbes et al., Citation1996). Therefore, the effects of stress and exercise on sucrose consumption were assessed by SCT. On days 25 and 26 of the protocol (), UCMS and no stress mice respectively underwent the SCT adapted from Schmidt & Duman, and used in previous studies (Manners et al., Citation2019; Schmidt & Duman, Citation2010; Yohn & Blendy, Citation2017). Five days before testing, mice were familiarized with a 1% sucrose solution for 48 h. They were then gradually water-restricted for 4, 14, and 19 h before testing, to prevent neophobia during the SCT. On the day of testing, mice acclimated to individually housed cages containing home cage bedding for 1 h. Mice were then given access to a 1% sucrose solution for 1 h. On the following day, the amount of water drank in 1 h was recorded instead of sucrose. The weight of bottles containing water or sucrose was recorded prior to and after being placed in testing cages. The amount of sucrose consumed was normalized to the amount of water consumed in 1 h and to body weight.
2.4.7. Forced swim test (FST)
The FST was conducted in order to assess the effect of exercise on passive and active coping behavior during a swimming stressor. On day 27 of the UCMS protocol (), UCMS mice were individually placed in a plastic cylinder (23 cm tall × 14 cm diameter) containing 15 cm of water (22–24 °C) for 6 min. All movements, except for those necessary to balance the body, keep the head above the water, and drifting due to momentum, were considered mobile. The test was video recorded, and total immobility time was scored by three blinded researchers. The average time is reported.
2.4.8. Light dark test (LDT)
The LDT was conducted on day 28 for females and day 29 for males (). The apparatus consisted of two quadrants: one area with black Perspex walls and a black lid (20 × 40 × 35 cm) and one area with clear Perspex walls and no lid (20 × 40 × 35 cm). Light intensity of the light side was 325 lux. Mice acclimated to the room for 1 h before being individually placed in the dark portion of the box facing the entryway to the light portion. Mice were recorded for 15 min. Time spent in the light zone, number of entries to the light zone, and number of head entries to the threshold of the light zone were measured by ANY-maze.
2.4.9. Tail suspension test (TST)
On day 30 (), UCMS-exposed mice were subjected to the TST as a stressor. A small rubber tube was placed around the tails of the mice to prevent climbing. Mice were suspended from their tails from a metal rod for 6 min. The test was video recorded, and total immobility time was scored by three blinded researchers. The average time is reported.
2.5. Statistical evaluation
Statistical analysis was conducted using the GraphPad Prism software. A mixed-effects analysis that includes time (day) as the repeated measurement, was used to evaluate effect of stress and sex on VWR. A repeated-measure three-way ANOVA including time, stress and exercise variables was used to assess weight changes due to stress and exercise in males and females. Three-way ANOVA was used to evaluate the main effects and interaction of stress, exercise, and sex in the FCT, NSFT, SCT, OFT, fecal droppings and LDT. A two-way ANOVA was conducted in FST and TST to assess the effects of exercise and sex on mice mobility. Statistical outliers were determined using the two-sided Grubbs’ test (α = 0.05) and excluded from analysis. Excluded data points: VWR time: 1 MNE, 1 MSE and 1 FNE mice; VWR distance: 2 FN mice; NSFT: 1 MS and 1 FS mice; OFT latency to center: 1 FN and 1 FS mice; LDT light entries: 1 FN mouse; SCT: 2 FN and 1 FSE mice; FCT: 1 MS, 1 MSE and 1 FSE mice. Because multiple variables were assessed (stress, sex and exercise), we have assigned each to a different symbol for clearer analysis: the stress effect is represented by “*”, the sex effect by “α” and the exercise effect by “ξ”. The number of symbols refers to significance: one symbol corresponds to P ≤ 0.05, two symbols P ≤ 0.01, three symbols P ≤ 0.001, four symbols P ≤ 0.0001.
3. Results
3.1. Chronic stress increases VWR with females choosing to run more than males
Exercise mice had individual access to wheels for 2 h daily. To determine changes in running behavior, all running sessions were recorded by ANY-maze and by using speedometers attached to running wheels. Analysis of VWR by a mixed-effect model revealed a significant main effect of stress on VWR, increasing time spent on the wheel (F1,95=19.09, p < 0.0001), distance traveled (F1,89=44.01, p < 0.0001) and speed (F1,24=53.96, p < 0.0001) in both sexes (). We also identified a significant main effect of sex, as increased VWR parameters were observed in females (time: F1,95=133.5, p < 0.0001; distance: F1,89=141.2, p < 0.0001; speed: F1,24=14.73, p = 0.0008). VWR was significantly altered throughout the 4 weeks of UCMS, as demonstrated by mixed-effect model analysis revealing a significant main effect of time (week) on the time spent on the wheel (F3,95=14.8, p < 0.0001) and distance traveled (F2.819,83.63=19.83, p < 0.0001).
Figure 2. VWR is increased in females and is induced by chronic stress in both sexes. Exercise mice (n = 8 per group) had a 2 h daily access to wheels throughout the 4-week UCMS paradigm. Running sessions were recorded by ANY-maze and VWR parameters were assessed by speedometers attached to wheels. Averages of VWR (A) time, (B) distance and (C) speed of exercise mice were recorded on a daily basis, and weekly averages are plotted above. Error bars represent SEM. A Mixed-effects model analysis was performed on each VWR parameter and revealed significant main effects of stress “*” and sex “α”. The number of symbols reflects significance; three symbols: p ≤ 0.001, four symbols: p ≤ 0.0001.
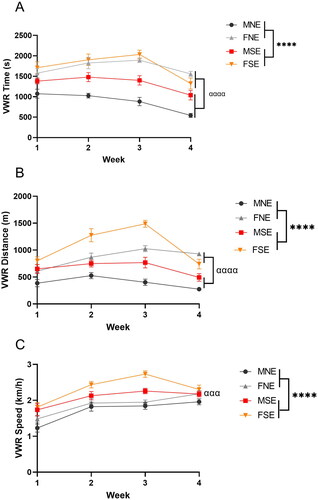
A significant stress × sex interaction was identified with time spent on the wheel (F1,95=14.7, p = 0.0002) (). Significant interactions of time × sex (F3,89=7.902, p < 0.0001), time × stress (F3,89=4.964, p = 0.0031), and time × sex × stress (F3,89=2.889, p = 0.0399) were reported with VWR distance (). No significant interactions of time, stress or sex were identified with speed.
3.2. Chronic stress induces weight loss and females regain lost weight over time
To assess stress-induced changes in body weight, mice were weighed on days 0, 3, 7, 14, 21, and 28 of UCMS (). Male and female data were analyzed separately using repeated-measures three-way ANOVA evaluating stress, exercise, and time.
In males, a repeated-measures three-way ANOVA revealed a significant main effect of stress (F1,44=65.27, p < 0.0001) and time (F5,159=19.00, p < 0.0001) on weight loss with no main effect of exercise (F1,44=1.412, p = 0.2411) (). Significant interactions of stress × time (F5,159=21.5, p < 0.0001), time × exercise (F5,159=3.963, p = 0.0021), stress × exercise (F1,44=5.368, p = 0.0252), and time × stress × exercise (F5,159=2.787, p = 0.0192) were also identified. Since time had a significant main effect on weight loss, we performed a Bonferroni post hoc test to assess on which day of UCMS weight loss was most significant. The statistical threshold for a significant difference in weight between any two days was p < 0.05. Post hoc test indicated that males lost the most weight on day 14 of UCMS (Bonferroni-corrected p < 0.0001), and maintained a significantly decreased weight between day 0 and 28 of UCMS (Bonferroni-corrected p < 0.0001) ().
Figure 3. Chronic stress induces weight loss in males and females. (A) male and (B) female mice (n = 8 or 12 per group) were weighed on days 0, 3, 7, 14, 21 and 28 of UCMS and percent change in weight from baseline weight is plotted above. Error bars represent SEM. Male and female data were analyzed separately. The effects of stress and exercise on weight were analyzed by repeated-measures three-way ANOVAs. Significant main effect of stress “*” and exercise “ξ” were identified. One symbol: p ≤ 0.05, four symbols: p ≤ 0.0001. (C) Food intake was measured on day 12. Following an overnight isolation, food was weighed per mouse to determine the amount of food consumed during a routine isolation stressor. Amount of food consumed was normalized to weight. Error bars represent SEM. A three-way ANOVA revealed a significant main effect of stress “*”. One symbol: Bonferroni-corrected p ≤ 0.05.
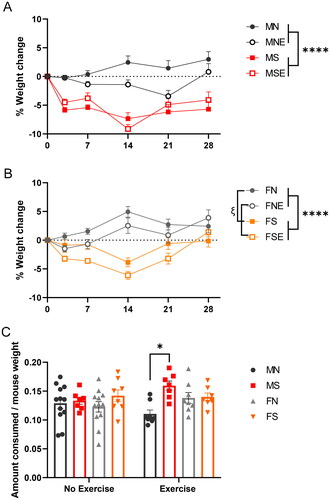
In females, a repeated-measures three-way ANOVA identified significant main effects of stress (F1,44=36.41, p < 0.0001), time (F2.885,92.32=11.19, p < 0.0001) and exercise (F1,44=6.042, p = 0.018) on weight loss (), and significant interactions of stress × time (F5,160=20.99, p < 0.0001), and time × exercise (F5,160=6.496, p < 0.0001). Similar to males, a Bonferroni post hoc test revealed that females lost the most weight on day 14 of UCMS (Bonferroni-corrected p = 0.0419). However, unlike males, females regained the majority of their lost weight by week 4, as no significant difference in weight was observed between days 0 and 28 of UCMS (Bonferroni-corrected P > 0.9999) ().
In order to determine if weight loss was due to consummatory changes, food consumption in a 12-h overnight session was measured. On day 12, mice were individually housed with ad libitum access to food and water, and the amount of food consumed was measured after 12 h. A three-way ANOVA analysis was performed and revealed a significant main effect of stress (F1,61=8.307, p = 0.0054) on increasing food consumption, and a significant interaction of sex × stress × exercise (F1,61=5.829, p = 0.0188) (). No significant main effects of exercise (F1,61=0.6572, p = 0.4207) or sex (F1,61=0.1779, p = 0.6746) were identified. This indicates that weight loss is likely not due to changes in food consumption.
3.3. Exercise promotes resilience to hyponeophagia and increases sucrose consumption
In order to determine if exercise could blunt the behavioral effects of chronic stress, we measured hyponeophagia, the inhibition of eating induced by exposure to an anxiogenic environment. To do so, we conducted a 15-min NSFT on day 23 of UCMS. Sweetened condensed milk was placed in the center of the testing arena, under a 2500 lux light and latency to first consumption was measured. A three-way ANOVA revealed a significant main effect of stress (F1,59=27.12, p < 0.0001) and exercise (F1,59=17.82, p < 0.0001) on latency to first consumption, and a significant interaction of stress × exercise (F1,59=8.590, p = 0.0048) (). No significant sex effect was observed (F1,59=2.679, p = 0.1070). A Bonferroni post hoc test was then conducted and revealed a stress-induced increase in latency to consume in no-exercise males (Bonferroni-corrected p = 0.0002) and females (Bonferroni-corrected p = 0.0108). Exercise protected from the effect of stress on latency to consume in stressed males (Bonferroni-corrected p = 0.010) and females (Bonferroni-corrected p = 0.0183).
Figure 4. Exercise promotes resilience to hyponeophagia and increases sucrose consumption. (A) NSFT was conducted on day 23 of the UCMS paradigm. Mice (n = 8 or 12 per group) were food-deprived for 24 h prior to test day, during which they were placed for 20 min in a box, with condensed milk solution placed under 2500 lux light in the center of the arena. Latency to first consumption was hand-scored by three blinded researchers and plotted above. Error bars represent SEM. The effects of stress, sex and exercise on latency were analyzed by a three-way ANOVA. Significant main effects of stress (F1,59=27.12, p < 0.0001) and exercise (F1,59=17.82, p < 0.0001) were identified. A Bonferroni post hoc test was then performed, and significant Bonferroni-corrected P values were plotted for stress “*” and exercise “ξ”. One symbol: Bonferroni-corrected p ≤ 0.05, three symbols: Bonferroni-corrected p ≤ 0.001. (B) SCT was performed on days 25 and 26 of UCMS. After several episodes of water restriction 4, 14, and 19 h before testing, mice (n = 8 or 12 per group) were given access to a 1% sucrose solution for 1 h. On the following day, testing was repeated with water instead of sucrose. Sucrose consumption was recorded and normalized to water consumption and body weight. Error bars represent SEM. The effects of stress, sex and exercise on sucrose consumption were analyzed by a three-way ANOVA. Significant main effects of stress “*” and exercise “ξ” were identified. Two symbols: p ≤ 0.01.
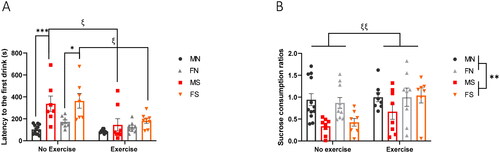
On day 25, the SCT was conducted to assess the effect of stress and exercise on sucrose consumption. After modest episodes of water restriction, sucrose consumption for 1 h was measured and normalized to water consumption and to body weight. A three-way ANOVA analysis revealed significant main effects of stress (F1,61=11.01, p = 0.0015) and exercise (F1,61=7.901, p = 0.0066) on sucrose consumption, with no significant effect of sex (F1,61=0.9126, p = 0.3432) (). Exercise ameliorated the stress-induced decrease in sucrose consumption in both males and females.
3.4. Exercise reduces avoidance behavior in the LDT with a sex-dependent effect in the OFT
The LDT and OFT were used to measure the effect of exercise on avoidance and exploratory behaviors in a chronic stress paradigm. In the LDT, mice were placed in the dark chamber and movement in the light area was recorded for 15 min. We measured the time spent in the light zone, the number of head entries into the threshold of the light zone, and the number of entries into the light zone. Three-way ANOVA analyses revealed a significant main effect of exercise increasing the time (F1,64=7.511, p = 0.0079), head entries (F1,64=20.72, p < 0.0001) and entries (F1,63=14.35, p = 0.0003) into the light zone of both sexes (). There was no main effect of stress on exploratory behaviors (light time: F1,64=0.8421, p = 0.3622; head entries: F1,64=0.2219, p = 0.6392; light entries: F1,63=1.675, p = 0.2004). A significant main effect of sex in time spent in light zone (F1,64=4.724, p = 0.0334) was revealed but not in number of entries (F1,63=0.1999, p = 0.6563) or head entries (F1,64=0.07168, p = 0.7898).
Figure 5. Exercise reduces avoidance behavior in LDT and OFT. The LDT was conducted on days 28 for females and day 29 for males (n = 8 or 12 per group). Mice were individually placed for 15 min in a box consisting of a dark and a light side and were recorded by ANY-maze. (A) Time spent in the light zone, (B) number of head entries to the threshold of the light zone, and (C) number of head entries to light zone were measured and averages are plotted above. Error bars represent SEM. A three-way ANOVA was performed on each of the LDT parameters and revealed significant main effects of sex “α” and exercise “ξ”. One symbol: P ≤0.05, two symbols: p ≤ 0.01, three symbols: p ≤ 0.001, four symbols: p ≤ 0.0001. (D) Mice (n = 8 or 12 per group) underwent OFT on days 17, 18, and 19 of the UCMS paradigm to measure the locomotor activity, exploratory behaviors, and latency to enter an anxiogenic environment. Mice were individually placed for 1 h in a clear Perspex apparatus and latency to enter the center was recorded by ANY-maze. We performed a three-way ANOVA and identified significant main effects of sex (F1,61=6.630, p = 0.0125) and exercise (F1,61=33.95, p < 0.0001) on latency to center entry. A Bonferroni post hoc test was then performed, and significant Bonferroni-corrected P values were plotted for sex “α” and exercise “ξ”. One symbol: p ≤ 0.05, two symbols: Bonferroni-corrected p ≤ 0.01, three symbols: Bonferroni-corrected p ≤ 0.001. (E) Locomotor activity during the OFT was measured by ANY-maze, analyzed by a three-way ANOVA, and plotted above. Error bars represent SEM. The only significant main effect observed was stress “*”. Three symbols: p ≤ 0.001. (F) The bowel movement was assessed by counting mice fecal droppings following the 1-h OFT. The number of fecal droppings was recorded then quantified by ImageJ. A three-way ANOVA revealed significant main effects of stress “*”, sex “α” and exercise “ξ”. One symbol: p ≤ 0.05, three symbols: p ≤ 0.001.
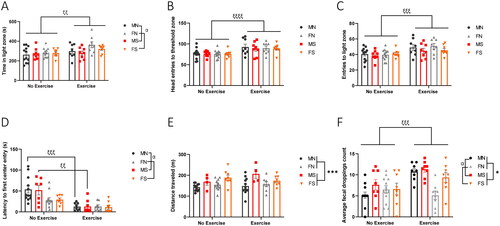
OFT was conducted on day 17 of UCMS. Locomotor activity and exploratory behavior were measured and analyzed by a three-way ANOVA. Significant main effects of exercise (F1, 61=33.95, p < 0.0001) and sex (F1,61= 6.630, p = 0.0125) on latency to center entry were identified, with a significant interaction of exercise × sex (F1, 61=6.051, p = 0.0167) (). Similar to LDT, no significant main effect of stress on exploratory behavior was observed in OFT (F1,61=0.004822, p = 0.9449). Bonferroni post hoc analysis revealed a sex-dependent effect of exercise on latency to center entry. Exercise reduced latency in no stress (p = 0.0003) and UCMS (p = 0.0036) males. This was not the case in no stress (Bonferroni-corrected p = 0.7752) or UCMS females (Bonferroni-corrected p = 0.6488) (). Locomotor activity in OFT was measured and analyzed by a three-way ANOVA. There was a significant main effect of stress on locomotor activity (F1,59=17.12, p = 0.0001) (), which was neither altered by exercise (F1,59=0.8815, p = 0.3516) nor sex (F1,59=0.03515, p = 0.8519).
Following OFT, fecal droppings were quantified using ImageJ. A three-way ANOVA revealed significant main effects of stress (F1,64=6.770, p = 0.0115), exercise (F1,64=14.03, p = 0.0004), and sex (F1,64=6.468, p = 0.0134) on increasing fecal droppings (). Significant interactions of sex × exercise (F1,64= 7.716, p = 0.0072) and sex × exercise × stress (F1,64=4.486, p = 0.0381) were also reported.
3.5. Exercise induces a sex-dependent decrease in immobility of UCMS mice during stressors
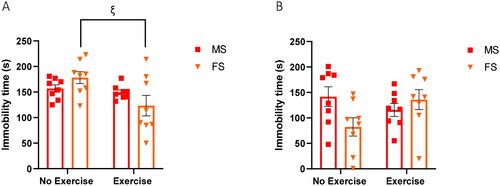
In order to evaluate the effect of exercise during a stressor, we conducted the FST and TST as part of the stress paradigm on days 27 and 30, thus only behaviors of UCMS mice were recorded. A two-way ANOVA analysis of the TST revealed a significant main effect of exercise (F1, 28=6.263, p = 0.0184) on immobility time. Bonferroni post hoc test revealed that this exercise effect was sex-dependent, as immobility was reduced in exercise females (Bonferroni corrected p = 0.0205) but not in males (Bonferroni corrected p = 0.9746) (). In FST, although no significant main effect of exercise (F1, 28=0.6058, p = 0.4429) or sex (F1,28=1.237, p = 0.2754) was observed, a two-way ANOVA analysis revealed a significant interaction of exercise × sex (F1,28=5.125, p = 0.0315) ().
Figure 6. Exercise reduces immobility of female stress mice during a stressor. (A) The TST was conducted on day 30 of UCMS. Stress mice (n = 8 per group) were suspended from their tails from a metal rod for 6 min. The test was video recorded, total immobility time was recorded by three blinded researchers and averages are plotted above. Error bars represent SEM. A two-way ANOVA analysis revealed a significant main effect of exercise (F1, 28= 6.263, p = 0.0184). A Bonferroni post hoc test was then performed, and significant Bonferroni-corrected P values were plotted for exercise “ξ”. One symbol: p ≤ 0.05. (B) The FST was conducted on day 27 of UCMS. Stress mice (n = 8 per group) were individually placed in a plastic cylinder containing 15 cm of water (22–24 °C) for 6 min. The test was video recorded, total immobility time was recorded by three blinded researchers and plotted above. Error bars represent SEM. A two-way ANOVA analysis revealed no significant main effect of sex or exercise, but a significant interaction of exercise × sex was identified (F1, 28= 5.125, p = 0.0315).
4. Discussion
To determine whether exercise could protect from the effects of chronic stress, male and female C57BL/6J mice were subjected to UCMS, with or without daily VWR. Our primary findings were that chronic stress increased VWR, and exercise blunted the stress-induced behavioral effects in males and females. Sex-specific behavioral differences were also identified in VWR, OFT and TST.
In this study, mice had daily access to running wheels for 2 h. This relatively short VWR period was sufficient to successfully ameliorate behavioral effects induced by the UCMS paradigm (Huang et al., Citation2017). VWR studies often provide unlimited access to running wheels, while mice are individually or group -housed which can promote competition for time on the wheel (Leduc et al., Citation2017), changes in social interactions, and an inability to track the performance of individual mice on the running wheel. Our experiment was designed to minimize isolation time and measure the running wheel parameters of individual mice throughout a UCMS protocol where stressors are conducted 3 times daily. This design preserved the ability to compare the running parameters of stress mice to no stress mice, with some limitations; notably, the VWR was conducted during the inactive light phase, when mice likely run less than during the active phase. This disruption, in addition to UCMS, might have interfered with the mice sleep cycle by providing an entertainment factor that shifts circadian rhythm. Nevertheless, this 2-h window was sufficient to rescue from the behavioral effects of UCMS in the NSFT and SCT, and impacted behavior in OFT, TST and LDT. Future studies could evaluate if increased access to VWR can rescue additional behaviors or had a more significant effect.
Although stress can increase overall rodent activity (Sibold et al., Citation2011), it typically reduces VWR (Desan et al., Citation1988; DeVallance et al., Citation2017). This variation in running wheel activity can result from a dysregulation in neural function due to chronic stress. UCMS-induced alteration in brain function has been reported in neural and behavioral tests (Alfarez et al., Citation2003; Desan et al., Citation1988; Di Chiara et al., Citation1999; Grippo, Francis, et al., Citation2005; Grippo, Sullivan, et al., Citation2005; Hill et al., Citation2012; Willner, Citation1997). A decrease in neuron signaling was also associated with chronic stress. This includes inhibition of the ventral tegmental area dopamine neuron activity, severe neuronal cell damage in the hippocampus and prefrontal cortex, downregulation of cannabinoid and glucocorticoid receptors, loss of plasticity, and induction of hippocampal apoptosis (Chang & Grace, Citation2014; Finsterwald & Alberini, Citation2014; Li et al., Citation2008; Song et al., Citation2006; Xu et al., Citation1997). These studies suggest that the stress-induced reduction in neural function might lead to a decrease in VWR. However, in our study which uniquely integrates voluntary exercise, physical and social stressors, and sex as variables, chronic stress increased VWR distance, speed, and time in both males and females. One possible reason for this contradiction from other reports could be the pre-exposure period; in our study, mice were pre-exposed to running wheels one day prior to the stress paradigm. However, others have conducted a 4-week long pre-exposure to VWR (DeVallance et al., Citation2017; Moraska & Fleshner, Citation2001). This extended pre-exposure period likely reduces the novelty of the wheel running experience, which could drive the behavioral changes in stressed mice. Additionally, the length of the stress paradigm may also impact behavior as these studies indicated that chronic stress, but not acute stress, reduced VWR.
Interestingly, studies including ours, report that female rodents exhibit increased VWR compared to males regardless of stress (Dworatzek et al., Citation2014; Jones et al., Citation1990; Konhilas et al., Citation2004; Citation2015; Nigro et al., Citation2021). Although the underlying reason for this sex difference remains poorly understood, sex hormones and neural circuits seem to significantly affect running behavior. When estrogen receptors alpha were deleted in female mice, their running performance was impaired (Dworatzek et al., Citation2014), and activity levels were reduced when females were treated with testosterone (Broida & Svare, Citation1984). Ovariectomizing female mice reduced running distance by more than 12-fold compared to control females, suggesting that gonadal hormones could be important drivers of sex differences in VWR (Basso & Morrell, Citation2017). The acquisition and maintenance of VWR involve unique sex-dependent neural substrates in the dorsomedial and dorsolateral striatum, which suggests that this brain region could be a target for sex-specific VWR studies (Tanner et al., Citation2022). Altogether, the increased voluntary running in females could provide an important coping mechanism that protects from stress-induced changes.
One expectation of the UCMS paradigm is weight loss or reduced weight gain compared to control mice (Pothion et al., Citation2004). We have also previously observed that weight loss in UCMS paradigms is not due to decreased food intake (Manners et al., Citation2019). Our current experiment supports these previous findings, as mice from the UCMS groups lost weight throughout the 30-day experiment, with females recovering a majority of their weight loss by day 28. Additionally, food intake was moderately increased in stress males, so weight loss was not due to decreased caloric intake. While weight loss due to stress does not positively correlate with food intake, other mechanisms involving activation of the sympathetic nervous system such as increased metabolism, changes in gut motility and absorption could promote weight loss. We incidentally observed increased fecal droppings in stressed mice, an effect that could be caused by stress-induced stimulation, leading to increased bowel movement and defecation, or to the dysregulation of serotonin (5-HT) concentration in the colon (Julio-Pieper et al., Citation2012). While exercise did influence behavioral effects of chronic stress, there was no effect on stress-induced weight loss and or increased fecal droppings, thus VWR did not protect mice from these physiological effects of chronic stress. Fecal corticosterone is an indicator of chronic stress (Ibarguen-Vargas et al., Citation2021). Future studies will incorporate measuring fecal corticosterone levels in exercise and stress groups to determine if there are corticosterone changes associated with the behavioral findings.
It is well established that exercise rescues mice from chronic stress-induced behaviors (Cao et al., Citation2022;; Huang et al., Citation2017; Luo et al., Citation2020; Yüksel et al., Citation2019) and ameliorates avoidance and exploratory behaviors (Cao et al., Citation2022; Duman et al., Citation2008; Wang et al., Citation2022). However, the benefit of exercise has not been well explored in both male and female populations, and there is limited data on emotionality or avoidance behaviors (Francois et al., Citation2022). Strikingly, we observed that 2 h of daily VWR protected from stress-induced hyponeophagia (NSFT) in both sexes, and increased sucrose consumption (SCT). The NSFT and SCT measure changes in consummatory behaviors. While it is possible for consummatory behaviors to be affected by the metabolic demands of exercise, there was no difference in the relative consumption of sucrose, or the latency to consume between the no stress no exercise and no stress exercise groups. We anticipated that UCMS would reduce exploratory behavior in the LDT and OFT, however, this was not observed in our study. We suspect that using a brighter light during this test would have produced the anticipated results. The UCMS model induces a variety of physical, behavioral, and neurobiological disturbances in mice that can be assessed through physiological and behavioral tests. By conducting a battery of behavioral tests, we assessed multiple behavioral changes associated with chronic stress. Although stress did not affect exploratory behavior, exercise alone reduced avoidance of light zones, with a sex-dependent effect in the OFT. This observation indicates that exercise has the potential to blunt the effects of chronic stress, and also affect behavior, independent of stressors. This effect on baseline behavior may support the role of exercise in the prevention of stress-induced behavioral changes in addition to the overall psychological and behavioral benefits of exercise (Lancel et al., Citation2003; Navarro et al., Citation2004).
We conducted a TST on UCMS mice to measure changes in escape or coping behaviors during a novel stressor. Interestingly, stressed females from the exercise group had reduced immobility time compared to stressed no-exercise females. No changes in locomotor activity were observed due to exercise in the OFT, indicating that reduced immobility time was specific to behavior associated with this novel stressor. In our study, these behavioral effects were only observed in females, which is consistent with others who reported decreased immobility of female mice in the TST from 5-HT1B knock-out mice (Jones & Lucki, Citation2005).
One mechanism by which exercise improves brain function is by modulating serotonin. Serotonin levels are affected by both voluntary and forced exercise paradigms, with reports indicating either increased or decreased activity and behavioral effects (Clark et al., Citation2015; Lin & Kuo, Citation2013). Forced exercise can be acutely stressful, but may have long-term protective effects which can differentially affect the 5-HT system in specific brain regions. Neurons that project from the dorsal raphe nucleus to the medial pre-frontal cortex (mPFC) are involved in regulating behaviors associated with depression (Warden et al., Citation2012), however, lesions of the mPFC had no effect on diminishing the protective potential of VWR (Greenwood et al., Citation2013). More investigation is necessary to determine the role of the serotonergic circuit in the mPFC in conferring the protective effects of exercise, and if this circuit is differentially affected in females versus males (Fallon et al., Citation2020; Tanner et al., Citation2019).
There are many approaches to investigating the relationship between exercise and chronic stress. In this present study, we sought to investigate the benefit of voluntary exercise during a chronic stress paradigm by conducting a battery of behavioral tests. In order to minimize the impact of repeated testing on mice behavior, tests were scheduled such that the least invasive ones (OFT and FCT) were conducted first, whereas the more stressful ones (TST and FST) were conducted at the end of the study, with at least 24 h between tests. Others have conducted experiments with variations on voluntary exercise versus involuntary, differences in length of chronic stressors, and pre-exposure to exercise versus no pre-exposure. These factors are important to consider because a variability in these factors could lead to contrasting behavioral outcomes, highlighting the complexity of the relationship between exercise and stress. One possible limitation in our study is that mice of the exercise groups were isolated daily in order to record running activity, which may have impacted behavior differently from mice that have access to running wheels within their home cage. Additionally, mice were pre-exposed to running wheels only one day prior to the beginning of the experiment, so it is possible that the protective effect of VWR might not be due to the exercise-induced physiological effects but to the novelty of the wheel running experience. Additional studies that compare exercise to exercise-induced physiological changes such as lactate levels, heart rate and other biomarkers could determine if protection is promoted by physiological changes, or by the novel exercise experience. We did not monitor the estrous cycle in female mice during this study; while stress and exercise affected female mice regardless of their estrous cycle stage. Accounting for the stage could have reduced variability, and the effect of each specific estrous cycle stage on time of VWR, changes in activity, and behavior would be interesting to consider in future studies including. Despite these limitations, voluntary exercise ameliorated outcomes in chronic stress conditions, and affected baseline behaviors of both males and females. Future studies could expand behavioral tests to investigate changes in cognitive and social behaviors, as both stress and exercise are known to affect memory, cognition, and social interactions.
Author contributions
EE: conceptualization; formal analysis; investigation; writing-original draft; and visualization. AYZ: Formal analysis; investigation; and writing-original draft. AGW: Formal analysis; investigation; and writing-original draft. MJP: Formal analysis and investigation. MTM: conceptualization; formal analysis; investigation; writing- review and editing; supervision; and project administration.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Elias Elias
Elias Elias received his undergraduate degree in Biology and his Master’s degree in Molecular Biology from the Lebanese American University. His research experience expands from cancer in vitro pharmacology to in vivo neurobiology. He is currently pursuing his PhD in Cell and Molecular Biology at Saint Joseph’s University in the laboratory of Dr. Melissa T. Manners conducting research on neuroinflammation and mechanisms of resilience to chronic stress.
Ariel Y. Zhang
Ariel Y. Zhang received her undergraduate degree in Integrative Neuroscience from Fordham University, and her master’s degree in Biomedical Science from Geisinger Commonwealth School of Medicine. She is currently pursuing a PhD in Cell and Molecular Biology from Saint Joseph’s University in the laboratory of Dr. Melissa T. Manners. Her graduate work focuses on chronic stress and neuroinflammation.
Abigail G. White
Abigail Grace White received her Bachelor of Science degree in Neuroscience from the University of the Sciences, now Saint Joseph’s University. During her undergraduate training she investigated the molecular and behavioral effects of chronic stress in the laboratory of Dr. Manners.
Matthew J. Pyle
Matthew J. Pyle received his Bachelor of Science degree in Biology from the University of the Sciences, now Saint Joseph’s University, where he conducted neurobiology research in the Manners Lab.
Melissa T. Manners
Melissa Taft Manners received her undergraduate degree in Biology from Rutgers University, and her PhD in Pharmacology and Physiology from Drexel University College of Medicine. She developed research expertise in stress neurobiology during her postdoctoral training in the laboratory of Dr. Julie Blendy at the University of Pennsylvania. Her laboratory has been conducting stress research since 2019 at University of the Sciences, now Saint Joseph’s University, and is continuing this research focus at Rowan University.
References
- Alfarez, D. N., Joëls, M., & Krugers, H. J. (2003). Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. The European Journal of Neuroscience, 17(9), 1–12. https://doi.org/10.1046/j.1460-9568.2003.02622.x
- Basso, J. C., & Morrell, J. I. (2017). Using wheel availability to shape running behavior of the rat towards improved behavioral and neurobiological outcomes. Journal of Neuroscience Methods, 290, 13–23. https://doi.org/10.1016/j.jneumeth.2017.07.009
- Binder, E., Droste, S. K., Ohl, F., & Reul, J. M. H. M. (2004). Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behavioural Brain Research, 155(2), 197–206. https://doi.org/10.1016/j.bbr.2004.04.017
- Brody, D. J. (2018). Prevalence of depression among adults aged 20 and over: United States, 2013–2016. NCHS Data Brief (303), 1–8.
- Broida, J., & Svare, B. (1984). Sex differences in the activity of mice: Modulation by postnatal gonadal hormones. Hormones and Behavior, 18(1), 65–78. https://doi.org/10.1016/0018-506x(84)90051-5
- Cao, Q., Wang, J., Hao, Y., Zhao, F., Fu, R., Yu, Y., Wang, J., Niu, R., Bian, S., & Sun, Z. (2022). Exercise ameliorates fluoride-induced anxiety- and depression-like behavior in mice: role of GABA. Biological Trace Element Research, 200(2), 678–688. https://doi.org/10.1007/s12011-021-02678-2
- Chang, C. H., & Grace, A. A. (2014). Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biological Psychiatry, 76(3), 223–230. https://doi.org/10.1016/j.biopsych.2013.09.020
- Clark, P. J., Amat, J., McConnell, S. O., Ghasem, P. R., Greenwood, B. N., Maier, S. F., & Fleshner, M. (2015). Running Reduces Uncontrollable Stress-Evoked Serotonin and Potentiates Stress-Evoked Dopamine Concentrations in the Rat Dorsal Striatum. PloS One, 10(11), e0141898. https://doi.org/10.1371/journal.pone.0141898
- Desan, P. H., Silbert, L. H., & Maier, S. F. (1988). Long-term effects of inescapable stress on daily running activity and antagonism by desipramine. Pharmacology, Biochemistry, and Behavior, 30(1), 21–29. https://doi.org/10.1016/0091-3057(88)90420-0
- DeVallance, E., Riggs, D., Jackson, B., Parkulo, T., Zaslau, S., Chantler, P. D., Olfert, I. M., & Bryner, R. W. (2017). Effect of chronic stress on running wheel activity in mice. PloS One, 12(9), e0184829. https://doi.org/10.1371/journal.pone.0184829
- Di Chiara, G., Loddo, P., & Tanda, G. (1999). Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biological Psychiatry, 46(12), 1624–1633. https://doi.org/10.1016/s0006-3223(99)00236-x
- Duman, C. H., Schlesinger, L., Russell, D. S., & Duman, R. S. (2008). Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research, 1199, 148–158. https://doi.org/10.1016/j.brainres.2007.12.047
- Dworatzek, E., Mahmoodzadeh, S., Schubert, C., Westphal, C., Leber, J., Kusch, A., Kararigas, G., Fliegner, D., Moulin, M., Ventura-Clapier, R., Gustafsson, J.-A., Davidson, M. M., Dragun, D., & Regitz-Zagrosek, V. (2014). Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovascular Research, 102(3), 418–428. https://doi.org/10.1093/cvr/cvu065
- Fallon, I. P., Tanner, M. K., Greenwood, B. N., & Baratta, M. V. (2020). Sex differences in resilience: Experiential factors and their mechanisms The European Journal of Neuroscience, 52(1), 2530–2547. https://doi.org/10.1111/ejn.14639
- Finsterwald, C., & Alberini, C. M. (2014). Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiology of Learning and Memory, 112, 17–29. https://doi.org/10.1016/j.nlm.2013.09.017
- Forbes, N. F., Stewart, C. A., Matthews, K., & Reid, I. C. (1996). Chronic Mild Stress and Sucrose Consumption: Validity as a Model of Depression. Physiology & Behavior, 60(6), 1481–1484. https://doi.org/10.1016/s0031-9384(96)00305-8
- Francois, M., Canal Delgado, I., Shargorodsky, N., Leu, C.-S., & Zeltser, L. (2022). Assessing the effects of stress on feeding behaviors in laboratory mice. eLife, 11, e70271. https://doi.org/10.7554/eLife.70271
- Greenwood, B. N., Spence, K. G., Crevling, D. M., Clark, P. J., Craig, W. C., & Fleshner, M. (2013). Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. The European Journal of Neuroscience, 37(3), 469–478. https://doi.org/10.1111/ejn.12044
- Grippo, A. J., Francis, J., Beltz, T. G., Felder, R. B., & Johnson, A. K. (2005). Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiology & Behavior, 84(5), 697–706. https://doi.org/10.1016/j.physbeh.2005.02.011
- Grippo, A. J., Sullivan, N. R., Damjanoska, K. J., Crane, J. W., Carrasco, G. A., Shi, J., Chen, Z., Garcia, F., Muma, N. A., & Van de Kar, L. D. (2005). Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology, ), 179(4), 769–780. https://doi.org/10.1007/s00213-004-2103-4
- Hill, M. N., Hellemans, K. G., Verma, P., Gorzalka, B. B., & Weinberg, J. (2012). Neurobiology of chronic mild stress: parallels to major depression. Neuroscience and Biobehavioral Reviews, 36(9), 2085–2117. https://doi.org/10.1016/j.neubiorev.2012.07.001
- Huang, P., Dong, Z., Huang, W., Zhou, C., Zhong, W., Hu, P., Wen, G., Sun, X., Hua, H., Cao, H., Gao, L., & Lv, Z. (2017). Voluntary wheel running ameliorates depression-like behaviors and brain blood oxygen level-dependent signals in chronic unpredictable mild stress mice. Behavioural Brain Research, 330, 17–24. https://doi.org/10.1016/j.bbr.2017.05.032
- Ibarguen-Vargas, Y., Leman, S., Palme, R., Belzung, C., & Surget, A. (2021). CRF-R1 antagonist treatment exacerbates circadian corticosterone secretion under chronic stress, but preserves HPA feedback sensitivity. Pharmaceutics, 13(12), 2114. https://doi.org/10.3390/pharmaceutics13122114
- Jones, L. C., Bellingham, W. P., & Ward, L. C. (1990). Sex differences in voluntary locomotor activity of food-restricted and ad libitum-fed rats. Implications for the maintenance of a body weight set-point. Comparative Biochemistry and Physiology. A, Comparative Physiology, 96(2), 287–290. https://doi.org/10.1016/0300-9629(90)90694-n
- Jones, M. D., & Lucki, I. (2005). Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology : official Publication of the American College of Neuropsychopharmacology, 30(6), 1039–1047. https://doi.org/10.1038/sj.npp.1300664
- Julio-Pieper, M., O’Mahony, C. M., Clarke, G., Bravo, J. A., Dinan, T. G., & Cryan, J. F. (2012). Chronic stress-induced alterations in mouse colonic 5-HT and defecation responses are strain dependent. Stress (Amsterdam, Netherlands), 15(2), 218–226. https://doi.org/10.3109/10253890.2011.607524
- Kandola, A., Ashdown-Franks, G., Stubbs, B., Osborn, D. P. J., & Hayes, J. F. (2019). The association between cardiorespiratory fitness and the incidence of common mental health disorders: A systematic review and meta-analysis. Journal of Affective Disorders, 257, 748–757. https://doi.org/10.1016/j.jad.2019.07.088
- Konhilas, J. P., Chen, H., Luczak, E., McKee, L. A., Regan, J., Watson, P. A., Stauffer, B. L., Khalpey, Z. I., McKinsey, T. A., Horn, T., LaFleur, B., & Leinwand, L. A. (2015). Diet and sex modify exercise and cardiac adaptation in the mouse. American Journal of Physiology. Heart and Circulatory Physiology, 308(2), H135–H145. https://doi.org/10.1152/ajpheart.00532.2014
- Konhilas, J. P., Maass, A. H., Luckey, S. W., Stauffer, B. L., Olson, E. N., & Leinwand, L. A. (2004). Sex modifies exercise and cardiac adaptation in mice. American Journal of Physiology. Heart and Circulatory Physiology, 287(6), H2768–2776. https://doi.org/10.1152/ajpheart.00292.2004
- Lancel, M., Droste, S. K., Sommer, S., & Reul, J. M. H. M. (2003). Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. The European Journal of Neuroscience, 17(10), 2171–2179. https://doi.org/10.1046/j.1460-9568.2003.02658.x
- Leduc, R. Y., Rauw, G., Baker, G. B., & McDermid, H. E. (2017). What goes around can come around: an unexpected deleterious effect of using mouse running wheels for environmental enrichment. J Am Assoc Lab Anim Sci, 56(2), 194–201.
- Li, S., Wang, C., Wang, W., Dong, H., Hou, P., & Tang, Y. (2008). Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sciences, 82(17-18), 934–942. https://doi.org/10.1016/j.lfs.2008.02.010
- Lin, T. W., & Kuo, Y. M. (2013). Exercise benefits brain function: the monoamine connection. Brain Sciences, 3(1), 39–53. https://doi.org/10.3390/brainsci3010039
- Luo, J., Tang, C., Chen, X., Ren, Z., Qu, H., Chen, R., & Tong, Z. (2020). Impacts of aerobic exercise on depression-like behaviors in chronic unpredictable mild stress mice and related factors in the AMPK/PGC-1α pathway. International Journal of Environmental Research and Public Health, 17(6), 2042. https://doi.org/10.3390/ijerph17062042
- Manners, M. T., Brynildsen, J. K., Schechter, M., Liu, X., Eacret, D., & Blendy, J. A. (2019). CREB deletion increases resilience to stress and downregulates inflammatory gene expression in the hippocampus. Brain, Behavior, and Immunity, 81, 388–398. https://doi.org/10.1016/j.bbi.2019.06.035
- Marcus, S. M., Young, E. A., Kerber, K. B., Kornstein, S., Farabaugh, A. H., Mitchell, J., Wisniewski, S. R., Balasubramani, G. K., Trivedi, M. H., & Rush, A. J. (2005). Gender differences in depression: Findings from the STAR*D study. Journal of Affective Disorders, 87(2-3), 141–150. https://doi.org/10.1016/j.jad.2004.09.008
- Moraska, A., & Fleshner, M. (2001). Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 281(2), R484–489. https://doi.org/10.1152/ajpregu.2001.281.2.R484
- Navarro, A., Gomez, C., López-Cepero, J. M., & Boveris, A. (2004). Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 286(3), R505–R511. https://doi.org/10.1152/ajpregu.00208.2003
- Nigro, P., Middelbeek, R. J. W., Alves, C. R. R., Rovira-Llopis, S., Ramachandran, K., Rowland, L. A., Møller, A. B., Takahashi, H., Alves-Wagner, A. B., Vamvini, M., Makarewicz, N. S., Albertson, B. G., Hirshman, M. F., & Goodyear, L. J. (2021). Exercise Training Promotes Sex-Specific Adaptations in Mouse Inguinal White Adipose Tissue. Diabetes, 70(6), 1250–1264. https://doi.org/10.2337/db20-0790
- NIMH. (2018). Depression. National Institute of Mental Health. https://www.nimh.nih.gov/health/topics/depression/
- Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., Mohr, D. C., & Schatzberg, A. F. (2016). Major depressive disorder. Nature Reviews Disease Primers, 2(1), 1–20. https://doi.org/10.1038/nrdp.2016.65
- Panchal, N., & Kamal, R. (2021). The Implications of COVID-19 for Mental Health and Substance Use. KFF. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
- Pothion, S., Bizot, J.-C., Trovero, F., & Belzung, C. (2004). Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behavioural Brain Research, 155(1), 135–146. https://doi.org/10.1016/j.bbr.2004.04.008
- Salguero, A., Martínez-García, R., Molinero, O., & Márquez, S. (2011). Physical activity, quality of life and symptoms of depression in community-dwelling and institutionalized older adults. Archives of Gerontology and Geriatrics, 53(2), 152–157. https://doi.org/10.1016/j.archger.2010.10.005
- Samuels, B. A., & Hen, R. (2011). Novelty-Suppressed Feeding in the Mouse. In T. D. Gould (Ed.), Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests., Volume II (pp. 107–121). Humana Press. https://doi.org/10.1007/978-1-61779-313-4_7
- Schmidt, H. D., & Duman, R. S. (2010). Peripheral BDNF Produces Antidepressant-Like Effects in Cellular and Behavioral Models. Neuropsychopharmacology : official Publication of the American College of Neuropsychopharmacology, 35(12), 2378–2391. https://doi.org/10.1038/npp.2010.114
- Schuch, F. B., Vancampfort, D., Firth, J., Rosenbaum, S., Ward, P. B., Silva, E. S., Hallgren, M., Ponce De Leon, A., Dunn, A. L., Deslandes, A. C., Fleck, M. P., Carvalho, A. F., & Stubbs, B. (2018). Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. The American Journal of Psychiatry, 175(7), 631–648. https://doi.org/10.1176/appi.ajp.2018.17111194
- Sibold, J. S., Hammack, S. E., & Falls, W. A. (2011). C57 mice increase wheel-running behavior following stress: preliminary findings. Perceptual and Motor Skills, 113(2), 605–618. https://doi.org/10.2466/06.16.20.PMS.113.5.605-618
- Song, L., Che, W., Min-Wei, W., Murakami, Y., & Matsumoto, K. (2006). Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacology, Biochemistry, and Behavior, 83(2), 186–193. https://doi.org/10.1016/j.pbb.2006.01.004
- Tanner, M. K., Davis, J. K. P., Jaime, J., Moya, N. A., Hohorst, A. A., Bonar, K., Abrams, K. A., Jamil, N., Han, R., Hubert, T. J., Brown, N., Loetz, E. C., & Greenwood, B. N. (2022). Duration- and sex-dependent neural circuit control of voluntary physical activity. Psychopharmacology, 239(11), 3697–3709. https://doi.org/10.1007/s00213-022-06243-0
- Tanner, M. K., Fallon, I. P., Baratta, M. V., & Greenwood, B. N. (2019). Voluntary exercise enables stress resistance in females. Behavioural Brain Research, 369, 111923. https://doi.org/10.1016/j.bbr.2019.111923
- Wang, X., Wang, Y., Chen, J., Li, J., Liu, Y., & Chen, W. (2022). Aerobic exercise improves motor function and striatal MSNs-Erk/MAPK signaling in mice with 6-OHDA-induced Parkinson’s disease. Experimental Brain Research, 240(6), 1713–1725. https://doi.org/10.1007/s00221-022-06360-4
- Warden, M. R., Selimbeyoglu, A., Mirzabekov, J. J., Lo, M., Thompson, K. R., Kim, S.-Y., Adhikari, A., Tye, K. M., Frank, L. M., & Deisseroth, K. (2012). A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nature, 492(7429), 428–432. https://doi.org/10.1038/nature11617
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology, ), 134(4), 319–329. https://doi.org/10.1007/s002130050456
- Xu, L., Anwyl, R., & Rowan, M. J. (1997). Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature, 387(6632), 497–500. https://doi.org/10.1038/387497a0
- Yohn, N. L., & Blendy, J. A. (2017). Adolescent chronic unpredictable stress exposure is a sensitive window for long-term changes in adult behavior in mice. Neuropsychopharmacology : official Publication of the American College of Neuropsychopharmacology, 42(8), 1670–1678. https://doi.org/10.1038/npp.2017.11
- Yüksel, O., Ateş, M., Kızıldağ, S., Yüce, Z., Koç, B., Kandiş, S., Güvendi, G., Karakılıç, A., Gümüş, H., & Uysal, N. (2019). Regular aerobic voluntary exercise increased oxytocin in female mice: the cause of decreased anxiety and increased empathy-like behaviors. Balkan Medical Journal, 36(5), 257–262. https://doi.org/10.4274/balkanmedj.galenos.2019.2018.12.87
- Zhao, J.-L., Jiang, W.-T., Wang, X., Cai, Z.-D., Liu, Z.-H., & Liu, G.-R. (2020). Exercise, brain plasticity, and depression. CNS Neuroscience & Therapeutics, 26(9), 885–895. https://doi.org/10.1111/cns.13385

