Abstract
Early life adversity (ELA) heightens the risk for anxiety disorders (which are characterized by heightened fear and avoidance behaviors), with females being twice as likely as males to develop pathology. Pavlovian fear conditioning tasks have been used to study possible mechanisms supporting endophenotypes of pathology. Identification of sex and ELA selective effects on the nature of behavioral responding in these paradigms may provide a unique window into coping strategies in response to learned fear to guide more mechanistic studies. The goals of this study were two-fold; First, to test if male and female mice employed different coping strategies in response to threat learning using different conditioning parameters (low, medium, and high intensity foot shocks). Second, to test if ELA in the form of limited bedding and nesting (LBN) altered the behavioral response of mice to conditioning. Mice received 6 tone/foot-shock pairings at one of three different foot-shock intensities (0.35 mA; 0.57 mA; 0.7 mA). Freezing, darting, and foot-shock reactivity were measured across trials. During conditioning, control-reared female mice exhibited significantly higher rates of darting behavior compared to control males at nearly all shock intensities tested. LBN rearing decreased the proportion of darting females to levels observed in males. Thus, ELA in the form of LBN significantly diminished the recruitment of active versus passive coping strategies in female mice but did not generally change male responding. Additional work will be required to understand the neural basis of these behavioral effects. Findings extending from this work have the potential to shed light on how ELA impacts trajectories of regional brain development with implications for sex-selective risk for behavioral endophenotypes associated with pathology and possibly symptom presentation.
1. Introduction
Early life adversity (ELA) is associated with increased risk for the development of affective pathology, including elevated risk for generalized anxiety disorder (GAD) and post-traumatic stress disorder (PTSD). Significant sex disparities exist in the incidence of these disorders, with females having a two-fold higher risk compared to males (Gater et al., Citation1998; Kessler et al., Citation1994; Weissman et al., Citation1994). Further, significant sex differences have been reported in symptom presentation and risk for comorbid and subclinical pathology (Altemus et al., Citation2014; Breslau et al., Citation1997; Hankin, Citation2009; Maeng & Milad, Citation2015; Nolen-Hoeksema et al., Citation1999). The mechanism supporting sex disparities in risk and symptom profiles for adversity-associated pathology is poorly understood. The effects of ELA on symptom development have been proposed to be the result of altered development of key regions supporting the processing of emotional cues and those that regulate the physiological and behavioral response to threat. Prior work has found significant sex differences following ELA rearing on neural and behavioral development (Demaestri et al., Citation2020; Grassi-Oliveira et al., Citation2016; Honeycutt et al., Citation2020; Manzano Nieves et al., Citation2020; Peña et al., Citation2019), possibly contributing to sex-selective behavioral profiles indicative of pathology. Approaches using model systems to determine the impact of ELA on sex disparities in behavioral development and future response to stress may provide insights into the neurobiological basis of sex selective risk for ELA associated pathology. Here, we assessed the impact of sex and ELA on the expression of active versus passive coping responses to threat-associated cues in a Pavlovian fear conditioning paradigm.
Threat-associated learning, where either a cue or a context is associated with an aversive experience, is a common paradigm used to measure threat-learning, threat assessment, threat generalization, and emotional regulation in both humans and animals (Paré et al., Citation2004; Quirk et al., Citation1996). In responding to threat-associated cues, two coping strategies have been proposed, an active coping strategy (fight or flight) and a passive coping strategy (immobilization or freezing) (reviewed in Steimer, (Citation2002)). The “selection” of coping strategy has been argued to depend on the proximity and magnitude of the threat. Sex differences have been identified in the engagement of active versus passive coping strategies in a cue-associated learning paradigm in rats (Gruene et al., Citation2015; Mitchell et al., Citation2022; Trott et al., Citation2022). This sex disparity manifests in elevated active coping strategies (darting behavior) in females (rapid escape-like movements in response to the presentation of a cue previously paired with a foot shock) compared to males (Colom-Lapetina et al., Citation2019; Gruene et al., Citation2015). Sex differences in the behavioral response to this basic form of learning could indicate engagement of different circuits regulating the response to threat and the selection of coping strategies in males relative to females. Sex disparities in the engagement of differing coping strategies could provide insights into sex differences in symptom presentation in stress-associated pathology and identify possible neural substrates of risk for pathology (Altemus et al., Citation2014; Gobinath et al., Citation2014). For example, prior work has identified differential engagement of longitudinal columns of the periaqueductal gray and their modulation by regions of the medial and orbitofrontal cortex in the recruitment of passive versus active coping strategies (Bandler et al., Citation2000; Keay & Bandler, Citation2001). In response to ELA, we have identified significant and sex selective effects on the development and functioning of subclasses of cells in several of these same regions (Goodwill et al., Citation2018; Manzano Nieves et al., Citation2020). Sex-selective effects on the maturation and functioning of these circuits following ELA may impact the behavioral response to threat and contribute to sex-selective risk for the development of behavioral profiles that contribute to symptoms underlying anxiety or depression. However, to date, the effects of ELA on possible sex differences in engagement of active versus passive coping strategies remain poorly understood.
The studies presented here were designed to test several specific questions. First, do sex differences in coping strategies in responses to threat-associated learning exist in mouse models? Second, is the behavioral response of male and female mice to threat-associated stimuli sensitive to the magnitude of the threat? Finally, does rearing under ELA conditions (in the form of limited bedding and nesting) alter the behavioral response of male or female mice to cue-associated threat? To test these questions, a Pavlovian conditioning paradigm was used where a tone was associated with a foot shock and the behavioral response to conditioning, recall, and the shock were assessed. Separate groups of animals were conditioned to tone/shock pairings of differing intensities to determine if sex differences in the engagement of active versus passive coping strategies were dependent upon the magnitude of the shock. To assess the effects of ELA rearing on threat-associated learning, separate groups of mice were reared in ELA conditions in the form of limited bedding and nesting (LBN), a paradigm that has been associated with a female selective increase in depressive-like behavior (Goodwill et al., Citation2019) and attentional deficits (Goodwill et al., Citation2018).
Consistent with prior work in rats, female control-reared mice, engaged in higher rates of darting to threat-associated cues than males. This effect was present for all levels of shock that were capable of eliciting an aversive response. Together, these data indicate that sex differences in darting are also present in mice and are not dependent upon the magnitude of the threat. Further, no sex differences were detected in shock reactivity, suggesting that differing behavioral profiles were not due to differences in pain threshold or the aversive nature of the shock. Finally, ELA in the form of LBN ablated the sex difference in the proportion of darters, with fewer LBN females engaging in darting behavior compared to control females. The current results indicate that ELA in the form of LBN had a significant effect on the development and recruitment of active versus passive coping strategies in female mice and altered the female-typical recruitment of a more active coping strategy to a more passive coping strategy. Additional work will be required to understand the impact of ELA on the development of brain centers supporting these behaviors. Such work has the potential to identify altered trajectories of regional brain development in response to ELA with implications for sex-selective risk for behavioral markers of pathology and possibly symptom presentation.
2. Methods
2.1. Subjects
A total of 178 C57BL/6N virgin mice were used for this study. Animals were randomly assigned to control or LBN rearing. All animals were bred in-house and weaned and sex-segregated (2–4 mice per cage) at age (postnatal day (PND) 21). Animals were housed on a 12:12 h light:dark cycle in a temperature and humidity-controlled room in Thoren cages and had ad libitum access to food and water throughout the study. All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Limited bedding and nesting
For early life adversity (ELA) conditions, at PND 4, pups and dam were transferred from the standard home cage to a cage with a wire mesh floor and provided with limited bedding (3 × 4 cm cotton nestlet) and ad libitum access to food and water. Animals remained under these conditions from PND 4 to PND 11. Following the ELA conditions, at PND 11, mice were returned to standard housing conditions with full bedding and nesting. Control-reared mice remained in their standard home cage with cob bedding and a 4 × 4 cm cotton nestlet. Previous work with the limited bedding model of ELA has shown that Limited bedding and nesting (LBN) rearing leads to fragmented maternal care, with dams exiting and returning to the nest more frequently than standard housed dams (Gallo et al., Citation2019; Rice et al., Citation2008).
2.3. Fear conditioning
To assess the effect of sex and rearing conditions on threat responding, young adult mice (PND 50-65; ) were conditioned to a tone shock association (Citation2018). Mice were randomly assigned to be conditioned at 1 of 3 foot-shock intensities (0.35 mA, 0.57 mA, 0.70 mA). To begin the fear conditioning protocol, mice were placed in a conditioning chamber (Context A; coconut odor) and allowed to acclimate and explore the chamber for 2-minutes. Following the acclimation period, mice were presented with 6 tones (30 s, 4 KHz, 75 dB), each co-terminating with a 1-second footshock (intensity of shock depended on the experiment). Tones were separated by a 60-second inter-trial interval (ITI). To assess conditioned memory recall, mice were placed in a second chamber (context B; banana odor), 24-hours post conditioning. During the memory recall test, mice were acclimated to the recall chamber for 2 minutes, followed by 2 tones separated by a 60s ITI. Their behavior was digitally recorded for analysis. The conditioning recall chamber (context B) differed from the conditioning chamber (context A) in color, floor texture, and smell.
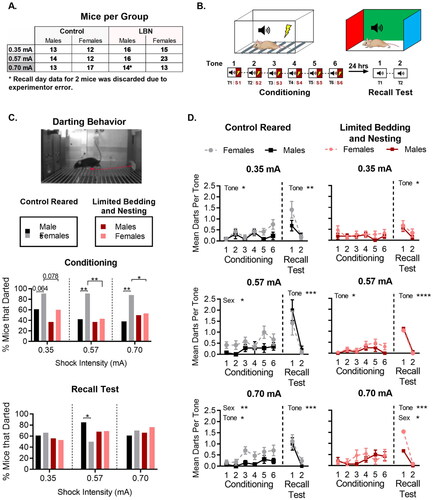
Figure 1. Sex difference in darting behavior is decreased by LBN rearing. (A) Number of mice in each group during conditioning and recall. Mice were randomly assigned to control or limited bedding rearing at birth. The number of mice per group was randomly established. (B) Depiction of conditioning protocol. (C) Image of a mouse darting during a tone presentation (top), the per cent of mice that darted at least once during conditioning for all foot-shock intensities tested (middle), and the percent of mice that darted at least once during memory recall for mice conditioned at different foot-shock intensities (bottom). (D) fear conditioning and recall test for control-reared (left) and LBN-reared (right), male and female mice. N per group ranged from 12–23 mice (exact n shown in A). For panel C (bottom), data is presented as the total percent of mice of each sex and rearing condition. For panel D, data is presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
2.4. Behavioral analysis
Percent freezing was analyzed with the activity tracker module of Ethovision 11. The parameters used in the module were: averaging interval of 4 samples, number of states equaled 2, the threshold was set at inactive below 0.03%, state duration threshold excluded instances of immobility that were shorter than 0.10s. Fidelity of results was validated by hand scoring from recorded videos by an observer who was blind to the treatment condition and shock intensity.
Darting behavior was analyzed through the use of the velocity analysis module of Ethovision 11. The parameters used in the module were: start velocity equal to 45 cm/s and stop velocity equal to 0.50 cm/s. In pilot studies using this system, rearing behaviors could be misclassified as darting (false positive). Thus, to remove all misclassification of rearing as darting, an observer blind to condition, shock intensity, and sex, independently reviewed all videos and verified each instance of darting to exclude false positive calls.
Footshock reactivity was measured with the velocity analysis module of Ethovision 11. The mean velocity of mice during the 1-second delivery of foot-shock was used to determine the presence and magnitude of the response of each mouse to a given shock.
2.5. Data analysis
Data was analyzed using Prism software (Prism, GraphPad Software, La Jolla, CA, USA) and SPSS (IBM). Graphs and images were made using GraphPad Software. Either three-way analysis of variance (ANOVA) or two-way repeated measures analysis of variance (ANOVA) followed by Sidak’s multiple comparison test (simple effects) were used to assess sex differences within control and LB-reared groups. A chi-square analysis was used to assess differences in the distribution of darter and non-darters across sex and rearing conditions. For all analyses, a minimum alpha of 0.05 was set.
3. Results
3.1. LBN rearing ablates the elevated darting observed in female mice
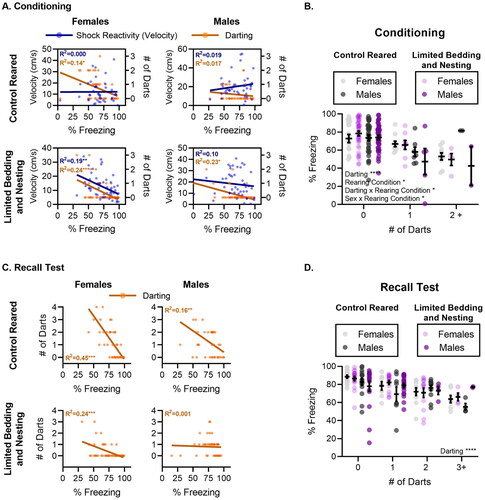
Separate cohorts of control and LBN-reared male or female mice underwent Pavlovian fear conditioning at one of three different shock intensities (0.35 mA, 0.57 mA or 0.70 mA). For conditioning, mice were placed in context A and presented with six tones that each co-terminated with a one-second foot shock (). A recall test was then performed 24-hours post conditioning. For recall, mice were placed in context B and presented with two tones. The percentage of mice that engaged in darting behavior was calculated separately for conditioning and recall. Data is presented as percent darters for ease of viewing, however, all chi-square analyses were conducted using the fraction of darters in each group.
To test if levels of darting behavior were different for females compared to male mice, the portion of control female darters to control male darters during conditioning was compared. Consistent with work in rats, control-reared female mice were significantly more likely to engage in darting behavior than control-reared males during conditioning. A significant main effect of sex was found at the 0.57 mA (X21 = 6.80, p = 0.0091) and 0.70 mA (X21 = 8.21, p = 0.0042) shock intensities but not 0.35 mA (X21 = 3.10, p = 0.078; ). As expected, a greater proportion of females exhibited darting behaviors. However, sex differences in darting during conditioning were dependent on shock intensity, as no sex difference was observed at the lowest shock intensity. When sex effects on darting behavior during the recall phase were compared, a greater proportion of female darters were found only at the 0.57 mA shock intensity (X21 = 3.86, p = 0.049).
To test if early life adversity in the form of LBN rearing altered darting behavior, the percent of control-reared mice that engaged in darting behavior were compared with mice reared under LBN conditions. In females, LBN rearing led to a significant decrease in the percent of darters when conditioning occurred at medium (0.57 mA X21 = 7.63, p = 0.0057) and high (0.70 mA X21 = 4.45, p = 0.034) foot-shock intensities but not when they were conditioned at low (0.35 mA X21 = 3.48, p = 0.062) foot-shock intensities. Although LBN decreased the percent of female darters during conditioning, similar effects were not observed when mice were tested on memory recall. No effect of LBN rearing was observed on the percentage of female darters during recall (0.35 mA X21 = 0.49, p = 0.48; 0.57 mA X21 = 1.29, p = 0.25; 0.70 mA X21 = 0.00084, p = 0.97). When comparing males during conditioning, LBN rearing did not impact the percentage of males that darted during conditioning. Furthermore, no effect of LBN rearing was found for the proportion of male darters during recall.
In summary, control-reared females were more likely to engage in darting behavior than males at higher shock intensities. Further, LBN rearing significantly diminished darting behavior in female mice, ablating sex differences in this behavior.
3.2. Sex difference in darting behavior emerge during conditioning for control reared mice
Engagement in darting behavior was assessed during the presentation of the tones in the conditioning phase of the task. To determine if levels of darting were present prior to initial tone shock pairing, or if levels of darting were altered by accumulating experience with prior shocks, the number of darts per tone was quantified for control male and female mice and LBN reared male and female mice during the conditioning phase (). To verify that levels of darting were comparable prior to conditioning, planned t-tests were used to compare males and females within each condition at each foot-shock intensity. Prior to conditioning, control males and females had similar levels of darting at 0.35 mA (t(23) = 0.056, p = 0.95) and 0.70 mA (t(28) = 0.78, p = 0.44) but not at 0.57 mA (t(24) = 2.19, p = 0.038). Thus, unexpectedly, control-reared females used for conditioning at 0.57 mA were more prone to darting even before any conditioning took place, which should be taken into consideration when interpreting sex differences at the 0.57 mA foot-shock intensity. For ELA-reared male and female mice, no statistical differences in darting behaviors were observed during the first tone, prior to conditioning taking place at any of the shock intensities (0.35 mA: t(29) = 1.2, p = 0.24; 0.57 mA: t(37) = 0.83, p = 0.41; 0.70 mA: t(25) = 0.32, p = 0.75).
For control-reared mice ( Left Column), a 2-way ANOVA was used to test for the effects of sex and tone experience on the frequency of darting. A main effect of sex was observed at medium (0.57 mA: F(1, 24) = 5.34, p = 0.029) and high (0.70 mA: F(1,28) = 7.95, p = 0.0087) shock intensities, but did not reach significance at the lowest shock intensity (0.35 mA: F(1, 23) = 3.75, p = 0.065), with female engaging in higher rates of darting than males. A significant effect of tone was also observed at the lowest (0.35 mA- F(3.094, 71.16) = 2.81, p = 0.0438) and highest (0.70 mA- F(3.917, 109.7) = 3.06, p = 0.0203) shock intensities, but not at the medium shock intensity (0.57 mA- F(3.556, 85.34) = 1.95, p = 0.115), with increased incidence of darting as tones progressed. The failure to detect a significant effect at 0.57 mA may have been the result of the unusually high number of mice that darted during the first tone, prior to any tone/shock association in that group. Despite the main effects of sex and tone, no tone-by-sex interaction was found for any of the foot-shock intensities tested (0.35 mA: F(5, 115) = 1.10, p = 0.364; 0.57 mA: F(5, 120) = 0.80, p = 0.548; 0.70 mA: F(5, 140) = 0.76, p = 0.579).
For LBN-reared mice ( Right Column), no main effect of sex was found during the conditioning phase at any of the shock intensities tested (0.35 mA: F(1, 29) = 1.17, p = 0.28; 0.57 mA: F(1, 37) = 1.38, p = 0.246; 0.70 mA: F(1, 25) = 0.15, p = 0.698). A main effect of tone was observed at 0.57 mA (F(2.904, 107.5) = 4.00, p = 0.0103), with increasing engagement in darting behavior observed over successive trials. However, no effect of tone was found for the other foot-shock intensities tested (0.35 mA: F(3.309, 95.97) = 0.83, p = 0.487; 0.70 mA: F(3.980, 99.49) = 1.97, p = 0.103). Finally, no tone x sex interaction was observed at any of the foot-shock intensities tested (0.35 mA: F(5, 145) = 0.74, p = 0.591; 0.57 mA: F(5, 185) = 0.66, p = 0.653; 0.70 mA: F(5, 125) = 1.52, p = 0.187).
To test for the effects of rearing conditions in female mice, we used a 2-way ANOVA. The analysis revealed a main effect of condition between control and LBN females at medium foot-shock intensities (0.57 mA: F(1, 33) = 4.56, p = 0.040), but not low (0.35 mA: F(1, 25) = 0.48, p = 0.491) or high (0.70 mA: F(1, 28) = 0.71, p = 0.404) foot-shock intensities. No significant effect of tone was observed for low foot-shock intensities (0.35 mA: F(3.68, 92.14) = 1.33, p = 0.265). However, a significant main effect of tone was found at medium and high foot-shock intensities (0.57 mA: F(3.78, 125) = 3.65, p = 0.0086; 0.70 mA: F(3.93, 110.2) = 3.12, p = 0.018). Together, the main effects of tone suggest that darting in females increases with CS-US pairing only at medium and high foot-shock intensities. No significant interaction between tone and condition was observed at any of the foot-shock intensities tested (0.35 mA: F(5, 125) = 2.14, p = 0.064; 0.57 mA: F(5, 165) = 1.17, p = 0.324; 0.70 mA: F(5, 140) = 0.69, p = 0.629).
When testing for effects of rearing in control and LBN-reared males, we did not find a significant main effect of condition at any of the 3 foot-shock intensities tested (0.35 mA: F(1, 27) = 0.079, p = 0.780; 0.57 mA: F(1, 28) = 0.73, p = 0.399; 0.70 mA: F(1, 25) = 1.04, p = 0.317). No main effect of tone was observed at low or medium foot-shock intensities (0.35 mA: F(3.71, 100.4) = 1.71, p = 0.156; 0.57 mA: F(2.97, 83.41) = 1.43, p = 0.238). However, a main effect of tone was found for male mice conditioned under high foot-shock intensity (0.70 mA: F(2.97, 74.28) = 3.17, p = 0.029), suggesting that male mice increase darting with accumulating US-CS presentations but only at high foot-shock intensities. Lastly, no interaction between tone and condition was found for male control and LBN mice at any of the foot-shock intensities tested (0.35 mA: F(5, 135) = 0.53, p = 0.749; 0.57 mA: F(5, 140) = 1.04, p = 0.396; 0.70 mA: F(5, 125) = 0.51, p = 0.762).
3.3. Sex difference in darting behavior during recall are observable in LBN mice conditioned at high foot-shock intensity
Consistent with published work (Gruene et al., Citation2015; Mitchell et al., Citation2022; Trott et al., Citation2022), control-reared mice exhibited elevated levels of darting behavior on the first tone of the recall test ( Left). However, the darting behavior decreased dramatically between the first tone and the second tone of the recall test, with the results of a 2-way ANOVA showing a significant effect of tone presentation on darting behavior at all 3 shock intensities tested (0.35 mA: F(1, 23) = 12.56, p = 0.0017; 0.57 mA: F(1, 24) = 16.57, p = 0.0004; 0.70 mA: F(1, 28) = 16.70, p = 0.0003). In the control-reared mice, darting was observed as a conditioned response that appeared to extinguish quickly. No main effects of sex (0.35 mA: F(1, 23) = 2.09, p = 0.161; 0.57 mA: F(1, 24) = 1.02, p = 0.321; 0.70 mA: F(1, 28) = 0.67, p = 0.417) or sex x tone interaction (0.35 mA: F(1, 23) = 2.66, p = 0.116; 0.57 mA: F(1, 24) = 0.34, p = 0.560; 0.70 mA: F(1, 28) = 0.065, p = 0.800) were observed during the recall test for control reared mice.
Surprisingly, and contrary to findings from the conditioning phase, high levels of darting behavior were observed in the first tone of the recall test in our LBN-reared mice ( Right). The darting response during recall in LBN-reared mice quickly dissipated by the second tone of the recall test, with a significant main effect of tone being observed at all shock intensities tested (0.35 mA: F(1, 29) = 5.27, p = 0.0290; 0.57 mA: F(1, 37) = 48.55, p < 0.0001; 0.70 mA: F(1, 23) = 21.74, p = 0.0001). When sex differences were tested for in the LBN-reared mice, females had significantly higher levels of darting compared to males when conditioning had occurred at high foot-shock intensities (main effect of sex- 0.70 mA: F(1, 23) = 5.44, p = 0.028). As expected, Sidak’s multiple comparison tests revealed that the sex differences were due to the high darting frequency of females to the first tone of recall (t 46 = 2.85, p = 0.012), and not to the second (t 46 = 0.25, p = 0.961). This main effect of sex during recall was not observed in LBN mice conditioned at the low (0.35 mA: F(1, 29) = 0.79, p = 0.378) or medium (0.57 mA: F(1, 37) = 0.18, p = 0.670) intensities. This was also surprising, given the failure to observe significant effects of sex during the conditioning phase in LBN mice. Thus, LBN rearing appeared to blunt sex-specific effects on darting when the threat (foot-shock) was present, but not in its absence. No sex by tone interaction (0.35 mA: F(1, 29) = 0.096, p = 0.758; 0.57 mA: F(1, 37) = 0.003, p = 0.950; 0.70 mA: F(1, 23) = 3.03, p = 0.094) was observed during the recall test for LBN reared mice.
We then used 2-way ANOVA to test if LBN and control-reared females differed in darting behavior during the recall test. There was no significant main effect of condition (0.35 mA: F(1, 25) = 1.38, p = 0.250; 0.57 mA: F(1, 33) = 0.90, p = 0.348; 0.70 mA: F(1, 28) = 0.30, p = 0.586) for any of the 3 foot-shock intensities tested. A main effect of tone was observed (0.35 mA: F(1, 25) = 11.12, p = 0.0027; 0.57 mA: F(1, 33) = 25.51, p < 0.0001; 0.70 mA: F(1, 28) = 19.21, p = 0.0001), with increasing levels of darting across tones for the 3 foot-shock intensities tested. There was no tone x condition interaction for any of the shock intensities tested (0.35 mA: F(1, 25) = 3.72, p = 0.065; 0.57 mA: F(1, 33) = 0.37, p = 0.542; 0.70 mA: F(1, 28) = 1.17, p = 0.288).
We also tested the effects of LBN or control rearing on darting behavior in males during the memory recall portion of the task. No significant main effect of condition was found at any of the shock intensities tested (0.35 mA: F(1, 27) = 0.65, p = 0.424; 0.57 mA: F(1, 28) = 3.88, p = 0.058; 0.70 mA: F(1, 23) = 1.08, p = 0.307). A main effect of tone was observed for each of the 3 foot-shock intensities tested (0.35 mA: F(1, 27) = 7.39, p = 0.011; 0.57 mA: F(1, 28) = 25.97, p < 0.0001; 0.70 mA: F(1, 23) = 27.18, p < 0.0001). Further, a significant interaction between tone and condition was found at the low foot-shock intensity (0.35 mA: F(1, 27) = 0.005, p = 0.942), but not at the medium (0.57 mA: F(1, 28) = 1.67, p = 0.206), or high (0.70 mA: F(1, 23) = 1.08, p = 0.307) footshock intensities.
3.4. Sex and LBN do not impact level of freezing during cued-threat acquisition
Significant effects of sex were found on the likelihood of darting as well as the level of darting during conditioning. To test whether this was associated with differences in levels of freezing observed during cued-threat acquisition and recall, the percent time mice engaged in freezing behavior was measured during each tone presentation. Freezing behavior was measured in the same cohorts of mice used to assess darting behavior (male and female control and LBN reward mice conditioned with low, medium, or high shock intensities- ).
Figure 2. Traditional freezing measurements do not reveal sex differences in control or LBN-reared mice. Freezing curves for auditory cue conditioning and recall test comparing control male and female mice conditioned at low (0.35 mA), medium (0.57 mA), and high (0.70 mA) foot-shock intensities for (A) control reared and (B) LBN reared mice. Individual tone data is shown for freezing during foot-shock conditioning and recall tests. N per group ranged from 12–23 mice (exact n shown in ). Data is presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
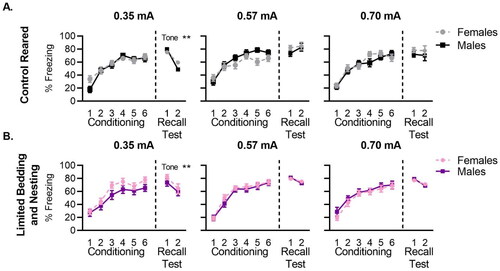
For control-reared mice (), no main effect of sex was found at any of the foot-shock intensities tested (0.35 mA: F(1, 23) = 0.33, p = 0.566; 0.57 mA: F(1, 24) = 1.94, p = 0.175; 0.70 mA: F(1, 28) = 0.47, p = 0.496) with males and females displaying similar levels of freezing across conditioning. A main effect of tone presentation was observed at all foot-shock intensities (0.35 mA: F(3.998, 91.95)=37.52, p < 0.0001; 0.57 mA: F(3.829, 91.90)=27.79, p < 0.0001; 0.7 mA: F(3.471, 97.18)=33.80, p < 0.0001), with levels of freezing increasing with successive tone/shock pairings. No sex x tone interaction was observed during conditioning at any of the foot-shock intensities tested (0.35 mA F(5, 115)= 1.90, p = 0.098; 0.57 mA F(5, 120)= 2.17, p = 0.061; 0.70 mA F(5, 140) = 1.27, p = 0.278). Thus, while sex differences were observed in the likelihood to dart and frequency of darting, this did not result in any sex differences in overall levels of freezing behavior in control-reared mice.
For LBN-reared mice (), no main effect of sex was found for freezing behavior at any of the foot-shock intensities tested (0.35 mA: F(1, 29) = 3.78, p = 0.0616; 0.57 mA: F(1, 37) = 0.46, p = 0.498; 0.70 mA: F(1, 25) = 0.98, p = 0.329). As with control mice, a significant effect of tone was found at all shock intensities tested (0.35 mA: F(3.774, 109.4) = 26.95, p < 0.0001; 0.57 mA: F(4.160, 153.9) = 69.21, p < 0.0001; 0.70 mA: F(3.921, 98.01) = 25.94, p < 0.0001) with both male and female LBN reared mice engaging in increasing levels of freezing with successive tone/shock pairings. No tone x sex interaction was observed in LBN-reared mice at any of the shock intensities tested (0.35 mA: F(5, 145) = 0.54, p = 0.744; 0.57 mA: F(5, 185) = 0.45, p = 0.806; 0.70 mA: F(5, 125) = 0.25, p = 0.936).
3.5. Sex nor rearing condition altered levels of freezing during cued recall
Following conditioning, mice were returned to their home cage for 24-hours. They were then placed in a chamber that they had previously been exposed to, but never been shocked in. In this chamber, mice were presented twice with the conditioning tone but received no foot-shocks. The levels of freezing in response to the tones were measured to assess behavioral response of the mice to the tone as a means of measuring memory of the tone-shock association.
Separate two-way ANOVAs were carried out on recall test data for control-reared mice () and LBN-reared mice (). No main effects of sex were found for either the control (0.35 mA: F(1, 23) = 0.22, p = 0.637; 0.57 mA: F(1, 24) = 0.98, p = 0.330; 0.70 mA: F(1, 28) = 1.10, p = 0.303) or LBN reared mice (0.35 mA: F(1, 29) = 1.16, p = 0.290; 0.57 mA: F(1, 37) = 0.011, p = 0.914; 0.70 mA: F(1, 23) = 0.000065, p = 0.993) at any of the foot-shock intensities tested. This suggests that freezing (an index of conditioned memory recall) was not affected by the sex of the mouse. This is consistent with previously published data from our lab showing no sex difference in auditory fear conditioning in mice (Manzano Nieves et al., Citation2020). However, a main effect of tone was observed for control (0.35 mA: F(1, 23) = 13.92, p = 0.0011) and LBN (0.35 mA: F(1, 29) = 9.86, p = 0.0039) mice that were conditioned to the lowest foot-shock intensity. No main effect of tone was observed at any other foot-shock intensity tested for either control (0.57 mA: F(1, 24) = 1.27, p = 0.270; 0.70 mA: F(1, 28) = 0.06, p = 0.808) or LBN (0.57 mA: F(1, 37) = 2.38, p = 0.130; 0.70 mA: F(1, 23) = 3.34, p = 0.080) mice. The main effect of tone observed at low but not other foot-shock intensities provides evidence that mice could distinguish between the intensity of the US, despite very similar fear conditioning curves. No main sex by-tone interaction was observed in control (0.35 mA: F(1, 23) = 1.48, p = 0.235; 0.57 mA: F(1, 24) = 0.40, p = 0.531; 0.70 mA: F(1, 28) = 0.007, p = 0.932) or LBN reared mice (0.35 mA: F(1, 29) = 0.12, p = 0.729; 0.57 mA: F(1, 37) = 0.23, p = 0.627; 0.70 mA: F(1, 23) = 0.202, p = 0.657).
3.6. Increasing foot-shock intensity increases behavioral response to shock across groups
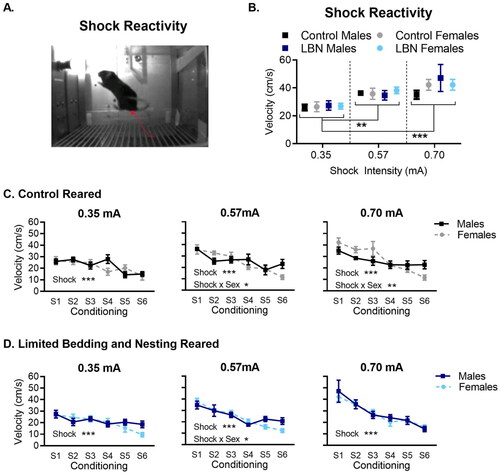
Curves depicting levels of freezing behavior during conditioning () show similar changes in freezing behavior across tone shock pairings for the low, medium, and high shock intensities. To determine if mice could differentiate between the shock intensities being administered, the mean velocity in response to the 1-second foot-shock delivery was analyzed () as a measure of the shock reactivity. First, the response (velocity) to the initial shock presentation was analyzed (). A three-way ANOVA was used to test for differences in foot-shock reactivity across foot-shock intensities. A significant main effect of foot-shock intensity was observed (F(2, 166) = 13.95, p < 0.0001) with the velocity of mice increasing in response to shocks of increased intensity. A Tukey’s post hoc comparison showed a significantly different reaction of mice to 0.57 mA (p = 0.0019) and 0.70 mA (p < 0.0001) compared with the response observed to a 0.35 mA shock. No significant difference between 0.57 mA and 0.70 mA (p = 0.14) was found. No effect of group (F(3, 166) = 0.55, p = 0.645) or group x foot shock interaction (F(6, 166) = 0.54, p = 0.774) were found. Together these findings suggest that mice were able to distinguish between the low and medium and low and high shock intensities, but may not differentially respond to the medium versus high foot-shock intensities.
Figure 3. Shock reactivity increases as foot-shock intensity increases. (A) Image of a mouse reacting to a foot-shock. (B) Mice, across all groups, react with a higher velocity to 0.57 and 0.70 mA when compared to 0.35 mA. (C) In control-reared mice at the higher shock intensities, males show a shallow decline compared to females in the magnitude of responding across successive tone-shock pairings (D) for LBN-reared mice, males and females show similar changes in behavioral response to successive shocks at 0.35 mA and 0.70 mA, with males showing a slightly shallower decrease in shock responding at the 0.57 mA intensity. N per group ranged from 12–23 mice (exact n shown in ). Data is presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
3.7. Mice habituate to repeated shocks, but this is not affected by sex, rearing condition, or shock intensity
In prior work, our lab found that females have an altered sensitivity to foot shock compared to males (Manzano-Nieves et al., Citation2018), as measured by the threshold to induce a measurable behavioral response to shock. However, in those studies, the minimum shock intensity required to elicit a behavioral jump response was approximately 0.10 − 0.15 mA. That shock intensity was far below even the weakest foot-shock intensity used to condition mice in the current study. To test if the behavioral response to shocks changed with repeated shock exposure at different intensities and if sex or rearing condition impacted the response to repeated shocks, the velocity of mice in response to each of the six successive foot shocks administered during conditioning was measured.
For control-reared mice (), a main effect of shock was found for all three shock intensities used (0.35 mA: F(4.449, 102.3) = 6.46, p < 0.0001; 0.57 mA: F(4.461, 107.1) = 12.03, p < 0.0001; 0.70 mA: F(3.025, 84.70) = 12.17, p < 0.0001), with the velocity of mice decreasing in response to successive shocks. No main effect of sex was found for any of the foot-shock intensities used (0.35 mA: F(1, 23) = 0.28, p = 0.597; 0.57 mA: F(1, 24) = 0.24, p = 0.624; 0.70 mA: F(1,28) = 0.48, p = 0.481). However, significant sex-by-shock interaction did emerge at the high and middle foot-shock intensities (0.70 mA: F(5,140) = 3.17, p = 0.0096 and 0.57 mA: F(5,120) = 2.76, p = 0.021) but not at the lowest shock intensity (0.35 mA: F(5,115) = 2.12, p = 0.067). In general, the reactivity of females was slightly higher than males during the initial shocks and lower than males by the final two shocks, contributing to the significant interaction.
For LBN reared mice (), a main effect of shock was observed at all foot-shock intensities (0.35 mA: F(4.599, 133.4) = 5.74, p = 0.0001; 0.57 mA: F(4.194, 155.2) = 23.35, p < 0.0001; 0.70 mA: F(2.098,52.44) = 15.43 p < 0.0001) with LBN mice showing decreasing behavioral reactivity to successive shocks. No main effect of sex was found for foot-shock reactivity at any of the foot-shock intensities tested (0.35 mA: F(1, 29) = 0.63, p = 0.432; 0.57 mA: F(1, 37) = 0.36, p = 0.548; 0.70 mA: F(1,25) = 0.087 p = 0.769). A sex x shock interaction was found at the middle shock intensity 0.57 mA (F(5, 185) = 2.52, p = 0.0306) but not at 0.35 mA (F(5, 145) = 1.52, p = 0.186) or 0.70 mA (F(5,125) = 0.24, p = 0.942). As with control-reared mice, female LBN mice showed a more robust response to the initial shocks and showed greater habituation, ending slightly lower than males, contributing to the interaction.
3.8. Increased darting is correlated with decreased freezing
Darting requires mice to move, while freezing requires mice to inhibit mobility. Therefore, it has been argued that darting may influence levels of freezing (e.g. a mouse with high levels of darting would have low levels of freezing). Recent work by Shansky and colleagues (Mitchell et al., Citation2022), suggests this to be the case. Here, Pearson correlation and Spearman Correlation analysis were used in an attempt to understand the relationship between darting and other assessed variables (such as freezing). To test this, correlation analysis for each sex of each rearing conditioning (4 separate analysis) were completed. In each analysis, data for all shock intensities was grouped together. For example, the Darting variable contains the darting values for mice conditioned at 0.35 mA, 0.57 mA, and 0.70 mA. Only the data for the 6th tone of conditioning and the first tone of the recall test were used in the analysis. This was done in an attempt to capture learning effects. A separate variable with the shock intensity that mice were conditioned at (shock intensity) was used to analyze if variables correlated with the shock intensity at mice were conditioned. For correlation analysis, we conducted both a Pearson correlation (PC) which assumes normality of distribution and a Spearman correlation (SC) which does not. For , only the Spearman correlation analysis is shown, the analysis that accounts for the large number of non-darters that were included in the analysis. Additionally, we used a simple linear regression analysis to analyze the relationship between freezing and darting, and freezing and foot-shock velocity. Lastly, we used a 3-Way ANOVA to further explore the relationship between freezing and different levels of darting behaviors.
Figure 4. Darting behavior tends to be negatively correlated with freezing behavior. (A) Spearman correlation matrices for control and LBN-reared male and female mice during conditioning. A negative correlation between darting and freezing behavior was observed for control-reared females, LBN females, and LBN males. LBN females also showed a significant negative correlation between foot-shock reactivity and freezing. N per group control females = 41, control males = 40, LBN females = 51, LBN males = 46. (B) Spearman correlation matrices for control and LBN-reared male and female mice during recall test. A negative correlation between darting and freezing behavior was observed for control-reared females, control-reared males, and LBN females. LBN females also showed a significant positive correlation between foot-shock intensity and darting behavior. Both a Spearman correlation analysis (shown here and in the results section) and a Pearson correlation analysis (results section only) were used to assess the correlations between darting, freezing, foot-shock reactivity (shock reactivity) and foot-shock intensity (shock intensity). For analysis, data from all 3 foot-shock intensities are pooled for all variables. N per group controls females = 41, control males = 40, LBN females = 51, LBN males = 44. Spearman correlations analysis. *p < 0.05, **p < 0.01, ***p < 0.001.
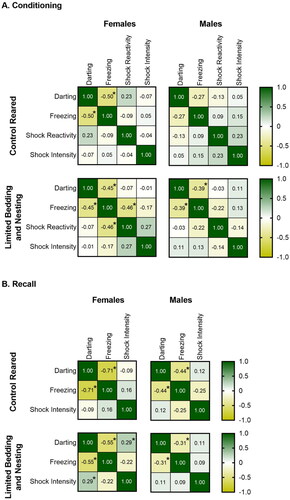
Both the Spearman (SC) and the Pearson (PC) correlation analysis of the behavioral variables for mice during the conditioning phase of the task () revealed a significant negative correlation between darting and freezing behavior in control females (PC: r(39) = −0.38, p = 0.013; SC: r(39) = −0.50, p = 0.001), LBN females (PC: r(49) = −0.50, p = 0.00018; SC: r(49) = −0.45, p = 0.001), and LBN males (PC: r(44) = −0.38, p = 0.013; SC: r(44) = −0.39, p = 0.0072), with mice that froze more engaging in less darting. A significant correlation was found between shock reactivity and freezing only in LBN females (PC: r(49) = −0.44, p = 0.001; SC: r(49) = −0.46, p = 0.001), suggesting that LBN females that have increased reactivity (jumping) in response to the foot-shock, but tend to exhibit lower freezing. Of note, the relationship between darting and freezing did not depend on the level of shock intensity used.
As a follow-up to the correlation analysis, we used a simple linear regression analysis to assess whether freezing behavior was related to the measures of darting and foot-shock reactivity (velocity) during fear conditioning (). Similar to the findings from the correlation analysis, the linear regression between freezing and foot-shock reactivity had a non-zero slope in LBN females (F(1, 49) = 12.04, p = 0.001) but not LBN males (F(1, 44) = 0.60, p = 0.441), control males (F(1, 38) = 0.77, p = 0.384), or control females (F(1, 39) = 0.0003, p = 0.985). The linear regression between freezing and darting had a non-zero slope in LBN females (F(1, 49) = 16.31, p = 0.0002), LBN males (F(1, 44) = 13.58, p = 0.0006), and control females (F(1, 39) = 6.83, p = 0.012), but not control males (F(1, 38) = 0.69, p = 0.411). To further explore if freezing levels were modulated by sex, rearing, and frequency of darting (zero, once, or more than two times) on the last trial of conditioning, we conducted a 3-way ANOVA (). This analysis revealed a significant main effect of darting (F(2, 166) = 13.58, p < 0.0001), of rearing condition (F(2, 166) = 3.92, p = 0.049), and significant interactions between darting and rearing condition (F(2, 166) = 3.21, p = 0.042) and sex by rearing condition (F(2, 166) = 4.28, p = 0.040). Together this analysis suggests that freezing generally tends to decrease as darting behaviors increase and that both sex and rearing conditions influence these effects.
Figure 5. Freezing tends to decrease as darting increases. (A) Scatter plot and linear regression showing the relationship between the velocity of mice in response to foot-shock and their freezing behavior (y-axis), and the relationship between mouse darting behavior and their freezing behavior (right y-axis), during the last tone of conditioning. (B) Graph showing the individual values and the mean values for mice that darted 0, 1, or more than 2 times during the last conditioning tone. The data shows that freezing decreases as darting increases. Significant main effects are displayed in the figure. Individual values are represented by circles, mean and SEM are displayed above individual values. For A and B, N per group are control females = 41, control males = 40, LBN females = 51, LBN males = 46. (C) Scatter plot and linear regression showing the relationship between mouse darting behavior and freezing behavior (y-axis), during the first tone of the recall test. (D) Graph showing the individual and the mean percent freezing for mice that darted 0, 1, 2, or more than 3 times during the first recall tone. The data shows that freezing decreases as darting increases. Significant main effects are displayed in the figure. Individual values are represented by circles and mean and SEM are displayed above individual values. For B and C, N per group controls females = 41, control males = 40, LBN females = 51, LBN males = 44. For analysis, data from all 3 foot-shock intensities are pooled for all variables. For A and C simple linear regression analysis were conducted. For B and D a 3-way ANOVA was used to analyze changes of freezing at different darting levels. *p < 0.05, **p < 0.01, ***p < 0.001.
Using both Pearson and Spearman correlation analysis for behavior during the recall test (), a significant negative correlation was found between freezing and darting for control reared females (PC: r(39) = −0.67, p < 0.0001; SC: r(39) = −0.70, p < 0.0001), control reared males (PC: r(38) = −0.40, p = 0.009; SC: r(38) = −0.44, p = 0.004), and LBN females (PC: r(49) = −0.51, p = 0.0001; SC: r(49) = −0.55, p < 0.0001). LBN males had a significant negative correlation between darting and freezing during conditioning, and the analysis of recall test correlations led to mixed results. PC analysis suggested no correlation between darting and freezing in LBN males, while SC resulted in a significant correlation between darting and freezing behavior (PC: r(42) = −0.04, p = 0.798; SC: r(42) = −0.31, p = 0.039). Lastly, a significant positive correlation between shock intensity and darting was found during recall for LBN females only (PC: r(49) = 0.29, p = 0.038; SC: r(49) = 0.29, p = 0.039). This indicates that LBN females darted more during recall as the shock intensity during conditioning increased. Next, we assessed the relationship between freezing and darting using linear regression to analyze if variables produce a line with a non-zero slope (). We found a significant relationship between freezing and darting in LBN females (F(1, 49) = 16.31, p = 0.0002), control males (F(1, 38) = 7.57, p = 0.009), control females (F(1, 39) = 32.39, p < 0.0001), but not LBN males (F(1, 42) = 0.066, p = 0.798). This finding suggests that freezing decreases as darting increases in all groups except LBN males. To further explore the relationship between sex, rearing condition, and darting frequency on the freezing levels, we conducted a 3-way ANOVA on the freezing levels of the first tone of the recall test (). Our analysis only revealed a main effect of darting (F(3, 160) = 9.542, p < 0.0001), suggesting that the freezing level decreased as darting frequency increased.
4. Discussion
The present study was designed to examine the impact of sex, conditioning parameters, and early life adversity (ELA) on the expression of active and passive coping strategies in response to threats in mice. Consistent with previous reports in rats (Colom-Lapetina et al., Citation2019; Gruene et al., Citation2015; Mitchell et al., Citation2022), control-reared female mice were more likely to engage in and exhibited higher levels of, active coping strategies (darting) than control reared males. The female darting bias was present at all shock intensities used for conditioning and darting rates increased with accumulating tone-shock pairings. Interestingly, ELA in the form of LBN rearing ablated this sex difference in the proportion of mice that were darters, with the probability that an LBN-reared female mouse being classified as a darter being indistinguishable from either control or LBN-reared males. Based on other metrics of shock reactivity and freezing behavior, the effects of sex and ELA on darting did not appear to be due to changes in the salience of the shock used for conditioning or changes in the general mobility of the animal.
The behavioral response to threat can include active (e.g. escape/avoid) and passive (e.g. freeze/hide) (De Boer et al., Citation1990) forms of responding. The selected/reflexive response has been proposed to depend on the context (De Boer et al., Citation1990), intensity (Corrêa et al., Citation2019; Fanselow and Bolles, Citation1979; Ponder et al., Citation2007; Sigmundi et al., Citation1980), and proximity of the threat (Choi and Kim, Citation2010), with distinct but overlapping circuits mediating the diversity of behavioral responses. In the current study, separate groups of mice were conditioned at one of three different shock intensities (0.35 mA, 0.57 mA, or 0.70 mA) and a wide array of measures were collected, including freezing, darting, and shock reactivity. Based on our prior work, the three levels of shock that were chosen for testing were well above the threshold to elicit a strong behavioral response in both male and female mice (Manzano-Nieves et al., Citation2018) and within the range of standard shock intensities used by the field for threat-associated learning in mice.
Consistent with prior work in rats (Colom-Lapetina et al., Citation2019; Greiner et al., Citation2019; Gruene et al., Citation2015; Mitchell et al., Citation2022), in control-reared mice, female mice were significantly more likely to engage in darting and darted more than males at all shock intensities tested. However, within sex, no significant effect of shock intensity was observed for either freezing or darting behaviors during conditioning. Both freezing and darting increased as tone shock pairings progressed, indicating that these responses required experience with a negative stimulus (shock) and could be augmented by additional experience. For shock reactivity (velocity of mice during the 1-second shock presentation), a main effect of shock intensity was found, with mice engaging higher velocity responses to shocks of higher intensity. However, while the magnitude of the immediate response to the shock varied by shock intensity, this did not lead to differences in either darting or freezing behavior during the tone presentations at any of the shock intensities tested. Based on these observations, like rats, mice show significant sex differences in the expression of active forms of threat responding and are more likely than males to engage in an active coping strategy (darting) in anticipation of the delivery of an aversive stimulus. Thus, this sex bias in darting appears to replicate across two of the most commonly used vertebrate species to study the neurobiology of disease (mice and rats) and may be indicative of sex differences in the neural circuitry underlying behavioral repertoires expressed in anticipation of threat. For example, the selection of active and passive fear behaviors is thought to be mediated by the engagement of distinct subpopulations of inhibitory neurons in the central amygdala (Fadok et al., Citation2017; Gozzi et al., Citation2010; Tye et al., Citation2011). However, these effects have previously only been characterized in males. A greater understanding of sex disparities in the neural underpinnings regulating these behaviors will be important.
When interpreting the darting behavior findings, it is important to consider both the frequency and manifestation of this behavior. The average number of darts was 0.5–0.8 darts for the last tones of conditioning. This is similar to levels of darting observed in rats in the initial study by the Shansky lab (Gruene et al., Citation2015), and previously published studies in mice (Trott et al. Citation2022). The generally low levels of darting can impact the group variance and make the interpretation of statistical comparisons across groups challenging. In the current study, the n for each group ranged from 12-23 mice. Across groups, between 25-90% of mice darted, and darting did not typically occur in every trial. It is possible that collecting data from a larger sample of animals may have revealed additional sex and rearing effects. Furthermore, there are differences in the behaviors exhibited by mice and rats that could make comparisons of darting behavior across species complicated. While rats lunge forward when they receive a footshock, mice typically jump, which we had anticipated would make mice more prone to exhibiting darting behavior. However, the levels of darting in mice were actually quite similar to levels reported in rats despite this difference. In addition, when comparing results across species, phenotypic differences in the way that animals engage in specific behavior must also be accounted for. For example, freezing behavior between rats and mice has subtle differences, with rats engaging in a highly stereotypic and sustained freezing posture, whereas freezing in mice often contains subtle twitches and micromovements and shorter freezing bout durations, but comparable total levels of freezing. These differences do not always lead to differences in absolute levels of freezing or differential response to varying foot-shock intensities between species (Laine et al. Citation2022; Laxmi et al. Citation2003). Thus, underlying species-specific behaviors or methodological differences in quantifying freezing or darting may underlie some of the divergent behavioral outcomes between mice and rats.
Traditionally, in Pavlovian fear conditioning paradigms (LeDoux, Citation1994; Rescorla, Citation1988), the level of freezing in response to a conditioned stimulus (CS = tone) that has been paired with a potent unconditioned stimulus (US = electric foot-shock) has been used as an indicator of strength of learning. However, additional active components of the fear response have been identified (e.g. tail rattling, vocalizations, and brief rapid movements in anticipation of threat-darting). While assessment of levels of freezing has been critical for dissecting the neurobiological underpinning of threat-associated learning, understanding the additional behaviors engaged in by animals during the threat-learning paradigm has the potential to provide a richer understanding of the neural circuitry underlying threat evaluation and behavioral response to threat anticipation. For example, sex differences have been identified in the engagement of active components of fear responding (e.g. darting), with females being more likely to dart than males, but no overall differences in levels of freezing. In those same studies, female rats that exhibited higher levels of darting than males, showed enhanced extinction retention on subsequent days of testing. Such factors may be especially relevant when considering sex as a biological variable for stress-associated illness, given that females have a near two-fold increased risk of developing trauma- and stress-associated pathology, including post-traumatic stress disorder (PTSD), depression, and anxiety disorders (Breslau et al., Citation1997; Kessler et al., Citation2005; Naninck et al., Citation2015). Sex differences in the deployment or gating of a coping strategy in the face of real or perceived threat have been associated with vulnerability to stress-induced pathology (de Boer et al., Citation2017). In instances of traumatic experiences, the presentation of specific behaviors during the traumatic event – such as peritraumatic dissociation or tonic immobility – correlate highly with the subsequent development of PTSD, underscoring the need for studies investigating the factors that influence the selection of response strategies (coping strategies) in the face of real or perceived threat. Here, as with rats, female mice engaged in higher rates of darting behavior than males in anticipation of threat. Furthermore, increased darting correlated with decreased freezing behaviors, suggesting that darting behavior influenced levels of freezing behavior. However, darting in response to a conditioned stimulus is far less common and requires more careful observation to quantify than freezing. Here, we interpret the increase in darting in females as a differential response to the conditioned stimulus. However, this interpretation is somewhat complicated given the quick decay in darting behavior during memory recall, where we observed darting during the first tone but almost no darting during the second tone of memory recall. This effect may be the result of the rapid extinction of this behavior, or maybe a consequence of the generally low occurrence of this behavior leading to difficulty in observing or detecting group differences as the levels of expression of darting decline across trials. Additional studies may be required that have increased sample size, increased duration of presentation of the CS, or additional CS presentations to further pursue this question. Follow-up experiments may also be required to assess if darting behavior also shows spontaneous recovery following a long interval between testing sessions, similar to what is found for freezing behavior. This would help clarify whether the observed effects are in fact a conditioned response and if these effects are stable over time. The assessment of sex differences in behavioral response to anticipated threat and the neural underpinning underlying these different endophenotypes may provide a fertile testing ground to understand sex disparities in risk and/or symptom development. Future studies will be important to understanding the contribution of these differences to future risk for behavioral profiles of stress-associated illness.
A history of stress exposure (e.g. ELA) has been shown to significantly increase the risk for the development of neuropsychiatric disease, increasing the risk for the development of depression, anxiety, and PTSD. Here, the impact of ELA in the form of resource scarcity (limited bedding and nesting- LBN) on behavior of mice in the Pavlovian fear conditioning paradigm were tested. Notably, LBN rearing significantly decreased the proportion of females that engaged in darting behavior in anticipation of threat. The percent of LBN-reared female darters was significantly lower than control-reared females and indistinguishable from either control or LBN-reared males (). However, an analysis of female darting behavior across US-CS presentations did not reveal a significant difference in learned darting behavior between LBN and control-reared females. This suggests that LBN significantly lowers the number of female darters but does not change group differences in darting behavior. An important caveat to this interpretation is the low incidence of darting behavior in general, an average of less than 1 dart per tone. It is possible that with increased sample size there would be sufficient power to detect group differences in darting across these phases of testing.
As cataloged by Lesuis et al., (Citation2019), mice may exhibit differential levels of freezing in response to varying foot-shock frequencies and intensities, suggesting that shock intensity may alter the behavioral response profile of mice. To address this issue, LBN effects on levels of freezing at the different shock intensities were assessed, and no effect of LBN or shock intensity was found for levels of freezing. Further, the effects of shock intensity and rearing condition on darting behavior were assessed. The diminished proportion of darters observed in LBN-reared females compared to control females was present at all foot-shock intensities tested. Thus, LBN effects on female darters were not related to changes in freezing or shock intensity.
In prior studies, our lab has found that LBN rearing could impact sensitivity to foot-shock, as evidenced by an increased threshold to vocalization, but not jump in response to foot-shock delivery (Manzano-Nieves et al., Citation2018). The foot-shock intensities chosen for the current study were well above the minimum shock intensity required to elicit a significant response from both male and female mice. Here, LBN rearing did not impact the physical response of females, relative to males, to the delivery of foot-shocks, and the diminished levels of darting observed in the LBN females were present even at the highest shock intensity tested. Thus, lower levels of darting in LBN females were not likely due to the diminished salience of the shock used for training. Based on these observations, it appears that rearing under ELA conditions significantly impacts the behavior response to threat anticipation in mice and that these effects may be due to alterations in the development of brain centers supporting the behavioral anticipation of threat. It is still unclear if such effects may explain sex differences in relative risk for stress-associated pathology. However, the LBN rearing model has been shown to lead to a female-specific risk of developing behavioral profiles consistent with depressive-like disorder and altered development of brain centers that are critical for threat evaluation and threat responding (Bath et al., Citation2016; Bolton et al., Citation2018; Goodwill et al., Citation2019; Manzano Nieves et al., Citation2020; Manzano-Nieves et al., Citation2018; Marschner et al., Citation2008; Rice et al., Citation2008; Tzanoulinou et al., Citation2014). Future studies will be required to understand the mechanisms by which ELA alters active coping strategies in females and the potential contribution of such effects to symptom development in stress-associated pathology.
Author contributions
GMN designed experiments. GMN conducted experiments. GMN and MB sorted and analyzed the data. GMN and KGB wrote the manuscript, with edits provided by MB.
Acknowledgements
Authors would like to thank members of the Bath Lab for their comments on the figures and manuscript. Very special thanks to Kathleen Huntzicker for her comments, edits, and work on the manuscript. Thanks to Roberto Aponte Rivera for comments on the manuscript. We would also like to thank Jose Colom Lapetina for helping verify some of the darting videos and providing a second opinion on what constituted darting. This work was funded by the National Institutes of Health (NIMH- R01-MH115049- KGB; R01-MH115914-KGB and K00-MH123183- GMN)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Gabriela Manzano Nieves
Gabriela Manzano Nieves is a Postdoctoral Fellow at Weill Cornell Medicine, New York, USA. Her work spans developmental and behavioral neuroscience, focusing on how changes in neuronal computations across a lifespan can bias behavior.
Marilyn Bravo
Marilyn Bravo is a medical student at the David Geffen School of Medicine in the UCLA/Charles R. Drew University of Medicine Medical Education Program. She graduated with Honors from Brown University in 2018 with a Sc.B in Neuroscience. Her research interests lie in neural development and mood disorders.
Kevin G. Bath
Dr. Kevin G. Bath is an Associate Professor of Medical Psychology in Psychiatry at Columbia University Medical Center, Research Scientist VI at New York State Psychiatric Institute (NYSP I), and Director of the Rodent Behavioral Core facility at NYSP I. His research interests include the impact of early life adversity on neurobehavioral development, sex differences in response to adversity, and evolutionary biology.
References
- Altemus, M., Sarvaiya, N., & Neill Epperson, C. (2014). Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology, 35(3), 1–15. https://doi.org/10.1016/j.yfrne.2014.05.004
- Bandler, R., Keay, K. A., Floyd, N., & Price, J. (2000). Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Research Bulletin, 53(1), 95–104. https://doi.org/10.1016/s0361-9230(00)00313-0
- Bath, K., Manzano-Nieves, G., & Goodwill, H. (2016). Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Hormones and Behavior, 82, 64–71. https://doi.org/10.1016/j.yhbeh.2016.04.010
- Bolton, J. L., Molet, J., Regev, L., Chen, Y., Rismanchi, N., Haddad, E., Yang, D. Z., Obenaus, A., & Baram, T. Z. (2018). Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biological Psychiatry, 83(2), 137–147. https://doi.org/10.1016/j.biopsych.2017.08.023
- Breslau, N., Davis, G. C., Andreski, P., Peterson, E. L., & Schultz, L. R. (1997). Sex differences in posttraumatic stress disorder. Archives of General Psychiatry, 54(11), 1044–1048. https://doi.org/10.1001/archpsyc.1997.01830230082012
- Choi, J.-S., & Kim, J. J. (2010). Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21773–21777. https://doi.org/10.1073/pnas.1010079108
- Colom-Lapetina, J., Li, A. J., Pelegrina-Perez, T. C., & Shansky, R. M. (2019). Behavioral diversity across classic rodent models is sex-dependent. Frontiers in Behavioral Neuroscience, 13, 45. https://doi.org/10.3389/fnbeh.2019.00045
- Corrêa, M. d S., dos Santos Corrêa, M., dos Santos Vaz, B., Grisanti, G. D. V., de Paiva, J. P. Q., Tiba, P. A., & Fornari, R. V. (2019). Relationship between footshock intensity, post-training corticosterone release and contextual fear memory specificity over time. Psychoneuroendocrinology, 110, 104447. https://doi.org/10.1016/j.psyneuen.2019.104447
- de Boer, S. F., Buwalda, B., & Koolhaas, J. M. (2017). Untangling the neurobiology of coping styles in rodents: Towards neural mechanisms underlying individual differences in disease susceptibility. Neuroscience and Biobehavioral Reviews, 74(Pt B), 401–422. https://doi.org/10.1016/j.neubiorev.2016.07.008
- De Boer, S. F., Slangen, J. L., & Van der Gugten, J. (1990). Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiology & Behavior, 47(6), 1089–1098. https://doi.org/10.1016/0031-9384(90)90357-a
- Demaestri, C., Pan, T., Critz, M., Ofray, D., Gallo, M., & Bath, K. G. (2020). Type of early life adversity confers differential, sex-dependent effects on early maturational milestones in mice. Hormones and Behavior, 124, 104763. https://doi.org/10.1016/j.yhbeh.2020.104763
- Fadok, J. P., Krabbe, S., Markovic, M., Courtin, J., Xu, C., Massi, L., Botta, P., Bylund, K., Müller, C., Kovacevic, A., Tovote, P., & Lüthi, A. (2017). A competitive inhibitory circuit for selection of active and passive fear responses. Nature, 542(7639), 96–100. https://doi.org/10.1038/nature21047
- Fanselow, M. S., & Bolles, R. C. (1979). Naloxone and shock-elicited freezing in the rat. Journal of Comparative and Physiological Psychology, 93(4), 736–744. https://doi.org/10.1037/h0077609
- Gallo, M., Shleifer, D. G., Godoy, L. D., Ofray, D., Olaniyan, A., Campbell, T., & Bath, K. G. (2019). Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Frontiers in Behavioral Neuroscience, 13, 167. https://doi.org/10.3389/fnbeh.2019.00167
- Gater, R., Tansella, M., Korten, A., Tiemens, B. G., Mavreas, V. G., & Olatawura, M. O. (1998). Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Archives of General Psychiatry, 55(5), 405–413. https://doi.org/10.1001/archpsyc.55.5.405
- Gobinath, A. R., Mahmoud, R., & Galea, L. A. M. (2014). Influence of sex and stress exposure across the lifespan on endophenotypes of depression: focus on behavior, glucocorticoids, and hippocampus. Frontiers in Neuroscience, 8, 420. https://doi.org/10.3389/fnins.2014.00420
- Goodwill, H. L., Manzano-Nieves, G., Gallo, M., Lee, H.-I., Oyerinde, E., Serre, T., & Bath, K. G. (2019). Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology, 44(4), 711–720. https://doi.org/10.1038/s41386-018-0195-5
- Goodwill, H. L., Manzano-Nieves, G., LaChance, P., Teramoto, S., Lin, S., Lopez, C., Stevenson, R. J., Theyel, B. B., Moore, C. I., Connors, B. W., & Bath, K. G. (2018). Early life stress drives sex-selective impairment in reversal learning by affecting parvalbumin interneurons in orbitofrontal cortex of mice. Cell Reports, 25(9), 2299–2307.e4. https://doi.org/10.1016/j.celrep.2018.11.010
- Gozzi, A., Jain, A., Giovannelli, A., Bertollini, C., Crestan, V., Schwarz, A. J., Tsetsenis, T., Ragozzino, D., Gross, C. T., & Bifone, A. (2010). A neural switch for active and passive fear. Neuron, 67(4), 656–666. https://doi.org/10.1016/j.neuron.2010.07.008
- Grassi-Oliveira, R., Honeycutt, J. A., Holland, F. H., Ganguly, P., & Brenhouse, H. C. (2016). Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology, 71, 19–30. https://doi.org/10.1016/j.psyneuen.2016.04.016
- Greiner, E. M., Müller, I., Norris, M. R., Ng, K. H., & Sangha, S. (2019). Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behavioural Brain Research, 368, 111903. https://doi.org/10.1016/j.bbr.2019.111903
- Gruene, T. M., Flick, K., Stefano, A., Shea, S. D., & Shansky, R. M. (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. eLife, 4. https://doi.org/10.7554/eLife.11352
- Hankin, B. L. (2009). Development of sex differences in depressive and co-occurring anxious symptoms during adolescence: descriptive trajectories and potential explanations in a multiwave prospective study. Journal of Clinical Child and Adolescent , 38(4), 460–472. https://doi.org/10.1080/15374410902976288
- Honeycutt, J. A., Demaestri, C., Peterzell, S., Silveri, M. M., Cai, X., Kulkarni, P., Cunningham, M. G., Ferris, C. F., & Brenhouse, H. C. (2020). Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. eLife, 9. https://doi.org/10.7554/eLife.52651
- Keay, K. A., & Bandler, R. (2001). Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neuroscience and Biobehavioral Reviews, 25(7-8), 669–678. https://doi.org/10.1016/s0149-7634(01)00049-5
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. https://doi.org/10.1001/archpsyc.62.6.593
- Kessler, R. C., McGonagle, K. A., Nelson, C. B., Hughes, M., Swartz, M., & Blazer, D. G. (1994). Sex and depression in the National Comorbidity Survey. II: Cohort effects. Journal of Affective Disorders, 30(1), 15–26. https://doi.org/10.1016/0165-0327(94)90147-3
- Laine, M. A., Mitchell, J. R., Rhyner, J., Clark, R., Kannan, A., Keith, J., Pikus, M., Bergeron, E., Ravaglia, I., Ulgenturk, E., Shinde, A., & Shansky, R. M. (2022). Sounding the alarm: Sex differences in rat ultrasonic vocalizations during Pavlovian fear conditioning and extinction. ENeuro, 9(6), ENEURO.0382-22.2022. https://doi.org/10.1523/ENEURO.0382-22.2022.
- Laxmi, T. R., Stork, O., & Pape, H. C. (2003). Generalisation of conditioned fear and its behavioural expression in mice. Behavioural Brain Research, 145(1-2), 89–98. https://doi.org/10.1016/S0166-4328(03)00101-3
- LeDoux, J. E. (1994). Emotion, memory and the brain. Scientific American, 270(6), 50–57. https://doi.org/10.1038/scientificamerican0694-50
- Lesuis, S. L., Lucassen, P. J., & Krugers, H. J. (2019). Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacology, 149, 195–203. https://doi.org/10.1016/j.neuropharm.2019.01.010
- Maeng, L. Y., & Milad, M. R. (2015). Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Hormones and Behavior, 76, 106–117. https://doi.org/10.1016/j.yhbeh.2015.04.002
- Manzano Nieves, G., Bravo, M., Baskoylu, S., & Bath, K. G. (2020). Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. eLife, 9. https://doi.org/10.7554/eLife.55263
- Manzano-Nieves, G., Gaillard, M., Gallo, M., & Bath, K. G. (2018). Early life stress impairs contextual threat expression in female, but not male, mice. Behavioral Neuroscience, 132(4), 247–257. https://doi.org/10.1037/bne0000248
- Marschner, A., Kalisch, R., Vervliet, B., Vansteenwegen, D., & Büchel, C. (2008). Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. The Journal of Neuroscience, 28(36), 9030–9036. https://doi.org/10.1523/JNEUROSCI.1651-08.2008
- Mitchell, J. R., Trettel, S. G., Li, A. J., Wasielewski, S., Huckleberry, K. A., Fanikos, M., Golden, E., Laine, M. A., & Shansky, R. M. (2022). Darting across space and time: parametric modulators of sex-biased conditioned fear responses. Learning & Memory, 29(7), 171–180. https://doi.org/10.1101/lm.053587.122
- Naninck, E. F. G., Hoeijmakers, L., Kakava-Georgiadou, N., Meesters, A., Lazic, S. E., Lucassen, P. J., & Korosi, A. (2015). Chronic early-life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus, 25(3), 309–328. https://doi.org/10.1002/hipo.22374
- Nolen-Hoeksema, S., Larson, J., & Grayson, C. (1999). Explaining the gender difference in depressive symptoms. Journal of Personality and Social Psychology, 77(5), 1061–1072. https://doi.org/10.1037//0022-3514.77.5.1061
- Paré, D., Quirk, G. J., & Ledoux, J. E. (2004). New vistas on amygdala networks in conditioned fear. Journal of Neurophysiology, 92(1), 1–9. https://doi.org/10.1152/jn.00153.2004
- Peña, C. J., Smith, M., Ramakrishnan, A., Cates, H. M., Bagot, R. C., Kronman, H. G., Patel, B., Chang, A. B., Purushothaman, I., Dudley, J., Morishita, H., Shen, L., & Nestler, E. J. (2019). Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nature Communications, 10(1), 5098. https://doi.org/10.1038/s41467-019-13085-6
- Ponder, C. A., Kliethermes, C. L., Drew, M. R., Muller, J., Das, K., Risbrough, V. B., Crabbe, J. C., Gilliam, T. C., & Palmer, A. A. (2007). Selection for contextual fear conditioning affects anxiety-like behaviors and gene expression. Genes, Brain, and Behavior, 6(8), 736–749. https://doi.org/10.1111/j.1601-183X.2007.00306.x
- Quirk, G. J., Armony, J. L., Repa, J. C., Li, X. F., & LeDoux, J. E. (1996). Emotional memory: a search for sites of plasticity. Cold Spring Harbor Symposia on Quantitative Biology, 61, 247–257.
- Rescorla, R. A. (1988). Behavioral studies of Pavlovian conditioning. Annual Review of Neuroscience, 11(1), 329–352. https://doi.org/10.1146/annurev.ne.11.030188.001553
- Rice, C. J., Sandman, C. A., Lenjavi, M. R., & Baram, T. Z. (2008). A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology, 149(10), 4892–4900. https://doi.org/10.1210/en.2008-0633
- Sigmundi, R. A., Bouton, M. E., & Bolles, R. C. (1980). Conditioned freezing in the rat as a function of shock intensity and CS modality. Bulletin of the Psychonomic Society, 15(4), 254–256. https://doi.org/10.3758/BF03334524
- Steimer, T. (2002). The biology of fear- and anxiety-related behaviors. Dialogues in Clinical Neuroscience, 4(3), 231–249. https://doi.org/10.31887/DCNS.2002.4.3/tsteimer
- Trott, J. M., Hoffman, A. N., Zhuravka, I., & Fanselow, M. S. (2022). Conditional and unconditional components of aversively motivated freezing, flight and darting in mice. eLife, 11. https://doi.org/10.7554/eLife.75663
- Tye, K. M., Prakash, R., Kim, S.-Y., Fenno, L. E., Grosenick, L., Zarabi, H., Thompson, K. R., Gradinaru, V., Ramakrishnan, C., & Deisseroth, K. (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature, 471(7338), 358–362. https://doi.org/10.1038/nature09820
- Tzanoulinou, S., Riccio, O., de Boer, M. W., & Sandi, C. (2014). Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Translational Psychiatry, 4(7), e410–e410. https://doi.org/10.1038/tp.2014.54
- Weissman, M. M., Bland, R. C., Canino, G. J., Greenwald, S., Hwu, H. G., Lee, C. K., Newman, S. C., Oakley-Browne, M. A., Rubio-Stipec, M., & Wickramaratne, P. J. (1994). The cross-national epidemiology of obsessive compulsive disorder. The Cross National Collaborative Group. J. Clin. Psychiatry, 55(Suppl), 5–10.

