Abstract
Common stress-related mental health disorders affect women more than men. Physical activity can provide protection against the development of future stress-related mental health disorders (i.e. stress resistance) in both sexes, but whether there are sex differences in exercise-induced stress resistance is unknown. We have previously observed that voluntary wheel running (VWR) protects both female and male rats against the anxiety- and exaggerated fear-like behavioral effects of inescapable stress, but the time-course and magnitude of VWR-induced stress resilience has not been compared between sexes. The goal of the current study was to determine whether there are sex differences in the time-course and magnitude of exercise-induced stress resistance. In adult female and male Sprague Dawley rats, 6 weeks of VWR produced robust protection against stress-induced social avoidance and exaggerated fear. The magnitude of stress protection was similar between the sexes and was independent of reactivity to shock, general locomotor activity, and circulating corticosterone. Interestingly, 3 weeks of VWR prevented both stress-induced social avoidance and exaggerated fear in females but only prevented stress-induced social avoidance in males. Ovariectomy altered wheel-running behavior in females such that it resembled that of males, however; 3 weeks of VWR still protected females against behavioral consequences of stress regardless of the absence of ovaries. These data indicate that female Sprague Dawley rats are more responsive to exercise-induced stress resistance than are males.
HIGHLIGHTS
The duration of wheel running required to enable stress resistance differs between the sexes in a behavior-dependent manner.
Wheel running enables rapid protection against stress-induced social avoidance in both male and female Sprague Dawley rats.
Wheel running enables protection against stress-induced exaggerated fear more readily in female Sprague Dawley rats compared to males.
Ovarian hormones are not necessary for stress-protection produced by 3 weeks of wheel running in female Sprague Dawley rats.
1. Introduction
Stress can precipitate mental health disorders such as post-traumatic stress disorder, anxiety, and depression. Not all individuals who experience stressful events will develop mental health disorders, in part due to experiential factors that influence susceptibility or resistance to stress (Ungar & Theron, Citation2020). One of these factors is physical activity. Longitudinal studies that include subjects of both sexes suggest that engaging in regular physical activity can reduce the incidence of stress-related psychiatric disorders in women and men (Chekroud et al., Citation2018; Harvey et al., Citation2018; Pearce et al., Citation2022). However, whether there are sex differences in the timing and/or magnitude of the protective effect of physical activity (i.e. stress resistance) is unknown. Subtle sex differences are difficult to identify using longitudinal designs, and interventional studies on stress resistance are limited by the fact that humans cannot ethically be exposed to the types of stressors that lead to stress-related mental health disorders. Given the higher rates of stress-related mental health disorders in women than men (Pavlidi et al., Citation2023; Steel et al., Citation2014), and sex disparities in response to experiential (Fallon et al., Citation2020) and pharmacological (Bigos et al., Citation2009) interventions, there is a critical need for understanding sex differences in exercise-induced stress resistance.
Animal models provide a means to study sex differences in stress resistance. Prior physical activity has been reported to reduce depression-like behavior more readily in female mice than in males (Elias et al., Citation2023; Munive et al., Citation2016; Naghibi et al., Citation2021). Additionally, there seems to be a sex difference in the duration of physical activity required to protect Sprague Dawley rats from the anxiety- and exaggerated fear-like effects of stress. Sedentary rats exposed to an inescapable stressor, such as inescapable tail shock (IS), display behaviors resembling symptoms of human stress-related psychiatric disorders (Maier & Watkins, Citation2005), including social avoidance (Christianson et al., Citation2008) and exaggerated fear responding (Baratta et al., Citation2007; Maier, Citation1990). These anxiety- and fear-like consequences of IS occur in both sexes of rats (Baratta et al., Citation2018). Male rats provided with in-cage running wheels (voluntary wheel running; VWR) for 6 weeks are protected from both anxiety- and fear-like behavioral outcomes of IS (Greenwood et al., Citation2003; Greenwood et al., Citation2012). We recently observed that VWR also protects female rats from the social avoidance and exaggerated fear produced by IS (Tanner et al., Citation2019). Three weeks of VWR is sufficient to enable protection against IS-induced social avoidance and exaggerated fear in females (Fallon et al., Citation2020), but 3 weeks of VWR fails to protect male rats from IS-induced exaggerated fear (Greenwood et al., Citation2005). Although these data are compelling, the duration of VWR required to prevent IS-induced social avoidance in males remains unknown, and no study has yet statistically compared exercise-induced stress resistance between the sexes, a requisite for identifying sex differences (Garcia-Sifuentes & Maney, Citation2021). Moreover, the role of ovarian hormones in mediating sex differences in exercise-induced stress resistance is unknown.
The goal of this study is to investigate exercise-induced stress resistance in both female and male rats, to determine if sex differences in the time course or magnitude of stress resistance exist. The role of ovarian hormones in exercise-induced stress resistance is determined by investigating the effects of ovariectomy. Associations between stress resistance and locomotor activity, corticosterone, and running distance are also explored. Results indicate that females are more responsive than are males to the protective effects of exercise against the development of exaggerated fear following stress. This information could inform the design of longitudinal clinical studies investigating potential sex differences in exercise-induced stress resistance.
2. Materials and methods
2.1. Animals and housing
Age-matched, adult female (n = 176) and male (n = 56) Sprague Dawley rats (Envigo, Indianapolis, IN, USA) were single-housed in Nalgene Plexiglas cages (45.5 × 24 × 21 cm, length × width × height) containing a locked running wheel (1.081 m circumference; Starr Life Sciences, Oakmont, PA) with food (Teklad 2020X rodent diet, Envigo) and water available ad libitum. Rats were single-housed to allow individual running data to be recorded. Prior work indicates that single vs. pair housing has no effect on the behavioral consequences of IS, and 6 weeks of VWR enables similar stress resistance in both single and pair-housed rats (Greenwood & Fleshner, Citation2011). Rats were maintained on a 12 h light-dark cycle (lights on 0600-1800) in a temperature- (22 °C) and humidity- (30%) controlled vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were allowed to acclimate to the housing conditions for 1 week prior to experimentation. Body weights were recorded weekly. Behavioral procedures occurred within the first 4 h of the light cycle at the University of Colorado Denver Auraria campus. The experiment measuring corticosterone was performed at the University of Colorado Boulder campus. All procedures were approved by the University of Colorado Denver or Boulder Institutional Animal Care and Use Committees.
2.2. Ovariectomy surgeries
Female rats were either bilaterally ovariectomized (OVX, n = 48) or underwent sham surgery (Sham, n = 48). Under ketamine (75.0 mg/kg i.p.) and medetomidine (0.5 mg/kg i.p.) anesthesia, a single dorsal incision was made in the rat’s skin followed by bilateral incisions through the muscle wall. Ovaries on each side of the rat were located, the uterine horns were ligated with absorbable Vicryl sutures (4-0, FS-2), and the ovaries were removed. The muscle incisions and skin incision were closed with absorbable sutures. Bupivacaine (2 mg/kg) was applied at the site of the muscle incisions prior to skin closure. Sham surgery procedures were the same as ovariectomy procedures, except the ovaries were left undisturbed. Injections of carprofen (5 mg/kg s.c.) and penicillin G (22,000 IU/rat s.c.) were administered at induction and every 24 h for 72 h after surgery. Rats recovered for 2 weeks prior to experimentation. Sham rats displaying abnormal estrous cycling and OVX rats displaying vaginal cytology consistent with proestrus or estrus were removed from the study.
2.3. Estrous phase identification
Estrous cycle phase was determined by vaginal lavage daily for 10 days following sham or OVX surgery and immediately following stress treatment (no stress or IS) and later behavioral testing. Vaginal epithelial cells were collected using a sterile, blunt-tipped eye dropper filled with ∼0.5 mL sterile-filtered 0.2% PBS-Brij solution (Brij 35 Solution 30%; Sigma-Aldrich, B4184). The vagina was gently flushed, and the collected fluid was placed onto a microscope slide for visualization under a 20X objective lens (Olympus BX53). The estrous cycle phase was identified by the morphology of the cells as previously described (Tanner et al. Citation2022). Males were handled for an equivalent period, if applicable.
2.4. Voluntary wheel running
Rats were randomly assigned to either 3 or 6 weeks of VWR or locked wheel (Sedentary) conditions. Following the acclimation period, wheels in the cages of VWR rats were unlocked for the duration of the experiments, while wheels in the cages of the Sedentary rats remained immobilized. No other cage enrichment was provided. Wheel running activity was recorded automatically every 1 min using VitalView Analysis software (Starr Life Sciences). Speed, number of running bouts, distance per bout, and bout length were calculated from data obtained during the active cycle only, using VitalView Analysis software (Starr Life Sciences) and Microsoft Excel macros.
2.5. Inescapable stress
After 3 or 6 weeks of Sedentary or VWR conditions, rats were randomly assigned to either remain undisturbed in their home cages (HC) or be exposed to inescapable tail shock (IS) as described in prior work (Tanner et al., Citation2019). Rats exposed to IS were placed in clear Plexiglas restraint tubes (8 × 18 cm, diameter x length) with their tails taped to a Plexiglas rod extending from the rear of the tube. Two copper strips were affixed to each tail and electrode paste was applied. The IS session consisted of 100 trials of 5 s tail shocks administered on a 60 s variable intertrial interval (ITI) by shock scramblers (Coulbourn Instruments, Allentown, PA, USA) controlled by Graphic State 3.0 software via a custom interface. The intensity of the shock was increased (50 trials at 1.3 mA followed by 50 trials at 1.6 mA) to account for stress-induced analgesia. Following IS, rats were immediately returned to their home cage.
2.6. Behavioral testing
2.6.1. Juvenile social exploration
Testing for juvenile social exploration (JSE) occurred 24 h after HC or IS as previously described (Baratta et al., Citation2018; Greenwood et al., Citation2012). Experimental rats were placed individually into separate Nalgene Plexiglas cages and transferred to a brightly lit testing room. After a 1 h habituation period, a juvenile same-sex conspecific (P28 ± 2 d) was added to the cage and exploratory behaviors (sniffing, pinning, and allogrooming) initiated by the adult experimental rat were recorded for 3 min by 2 observers blind to treatment conditions. Total interaction time was calculated by averaging the scores obtained by both observers with an inter-rater reliability of 96%. A 3 min baseline test for JSE was performed 72 h prior to HC or IS using the same procedures detailed above.
2.6.2. Shock-elicited fear
Shock-elicited fear was assessed immediately after the JSE test as previously described (Greenwood et al., Citation2012; Baratta et al., Citation2018). Rats were transported to a novel fear conditioning chamber (20″ × 10″ × 12″, length × width × height) with shock grid floors (Coulbourn Instruments) and were allowed to explore the chamber for 5 min before receiving 2 foot shocks (1 s, 0.8 mA) with a 1 min ITI. After the second foot shock, rats remained in the chambers for 20 min, while freezing behavior was scored every 10 s by an observer blind to treatment conditions. Freezing is a fear response in rats and thus is used as an index of fear. Behavior was recorded by overhead cameras and freezing was calculated from the videos by EthoVision XT software (Noldus, Leesburg, VA). The inter-rater reliability between human scoring and EthoVision XT was calculated at 95%. Time spent freezing (%) was calculated by averaging the freezing data obtained by the human scorer and EthoVision XT. Since females have been reported to express fear with high velocity “darting” movements (Gruene et al., Citation2015), the high-velocity movement was quantified by EthoVision XT during the 20 min post-shock period as an alternative fear behavior. High velocity movement during the two 1 sec foot shocks was quantified as a measure of shock reactivity. Rats were returned to their home cages following testing.
2.6.3. Locomotor activity
The day after behavioral testing, rats were placed for 1 h into locomotor activity chambers (17″ × 17″ × 12″ length × width × height; Med Associates, Fairfax, VT) which use beam breaks to calculate the total distance traveled (Tanner et al., Citation2022). Locomotor activity was also measured by EthoVision XT during the 5 min exposure to the conditioning chamber prior to the first foot shock when rats were tested for shock-elicited fear.
2.7. Corticosterone
Following 6 weeks of Sedentary or VWR conditions, female and male rats (n = 8/group) were exposed to IS. Whole blood was collected immediately prior to IS (baseline), immediately after IS, and 4 h after IS via tail nick. Samples were spun in a centrifuge for 5 min and the plasma supernatant was taken. Levels of corticosterone were determined using a commercially available rat-specific ELISA corticosterone kit (Enzo Life Sciences). The assay was performed according to the manufacturer’s instructions.
2.8. Data analyses
Body weight, running data, shock-elicited freezing across 2 min blocks, and plasma corticosterone levels were analyzed using repeated measures analysis of variance (ANOVA) with sex or surgery, exercise, and stress as factors, as appropriate. Time spent exploring the juvenile during JSE testing, average shock-elicited freezing, shock reactivity, and locomotor activity were analyzed with multifactorial ANOVA. Greenhouse-Geisser corrections were utilized if data did not meet assumptions of sphericity. Relationships between running distance (both average weekly distance run and average daily distance run during the last week of VWR) and behavioral endpoints were explored using regression analyses. Cohen’s ds effect sizes were calculated by dividing the mean difference by the pooled standard deviation. To produce positive effect sizes in Sedentary rats, mean differences for JSE were calculated by subtracting the means of the IS groups from respective HC groups, whereas mean differences for shock-elicited freezing were calculated by subtracting the means of the HC groups from respective IS groups. Bonferroni post-hoc analyses were performed when required. Group differences were considered significant when p < .05.
3. Results
3.1. Sex differences in the protective effects of VWR against behavioral consequences of inescapable stress
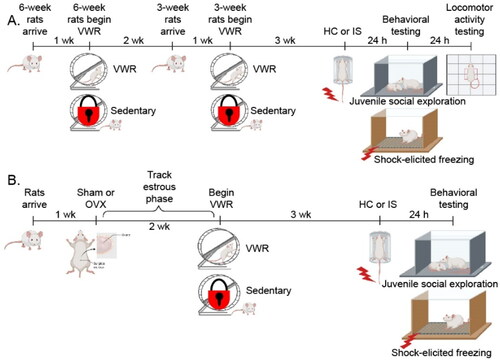
To investigate sex differences in exercise-induced stress resistance, female and male rats remained sedentary with locked wheels or were allowed voluntary access to running wheels for 3 or 6 weeks. The experimental design is shown in . Rats in the 3-week and 6-week conditions began VWR at the same age. Half of the sedentary rats arrived with the 6-week VWR groups, and the other half arrived with the 3-week VWR groups. No significant differences were observed between sedentary rats in the 3-week and 6-week groups, so these rats were combined into one sedentary group for each sex. Sedentary, 3-week VWR and 6-week VWR rats were randomly assigned to HC or IS conditions. Group sizes were 12/group for females and 8/group for males. More females were used than males to determine whether the estrous phase during IS or testing influences results in females. Six females were removed from the study due to injury (2) or data loss (4). Final group sizes were between 10–12/group for females and 8/group for males.
Figure 1. Experimental timelines. (A) Following 3 or 6 weeks of the locked wheel (Sedentary) or voluntary wheel running (VWR) conditions, female and male Sprague Dawley rats remained in their home cage (HC) or were exposed to inescapable tail shock stress (IS). Twenty-four hours later, rats were exposed to juvenile social exploration and shock-elicited freezing behavioral tests, followed by locomotor activity testing the next day. (B) Female rats received bilateral ovariectomy (OVX) or sham surgery (Sham) 2 weeks prior to the start of Sedentary or VWR. Rats were exposed to HC or IS following 3 weeks of VWR or Sedentary housing. Twenty-four hours after IS, rats were exposed to juvenile social exploration and shock-elicited freezing behavioral tests. The estrous cycle was monitored daily following surgery, as well as on the day of stress and behavioral testing. The figure was created using BioRender.
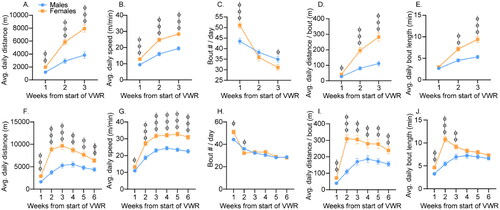
All rats gained weight over time (F(4,344) = 2358.1; p < .0001; Figure S1). Females gained less weight than males (interaction between sex and time: F(4,344) = 637.3; p < .0001), and VWR attenuated weight gain in both sexes (F(1,86) = 22.47; p < .0001). Females ran greater distances (3-week: (F(1,38) = 29.2; p < .0001; 6-week: F(1,34) = 26.76; p < .0001) and escalated more quickly (sex by time interaction, 3-week: F(2,76) = 17.2; p < .0001, ; 6-week: F(5,170) = 7.54; p < .0001, ) than males. Females also ran faster than males (3 week: F(1,38) = 35.27; p < .0001; 6-week: F(1,34) = 32.8; p < .0001) and running speed escalated in a manner similar to running distance (sex by time interaction, 3-week: F(2,76) = 14.5; p < .0001, ; 6-week: F (5,170) = 6.2; p < .0001; ). Number of running bouts was initially higher in females than in males (sex by time interaction, 3-week: F(2,76) = 11.1; p < .0001, ; 6-week: F(5,170) = 6.9; p < .0001, ) but decreased over time in both sexes (main effect of time, 3-week: F(2,76) = 61.7; p < .0001; 6-week: F(5,170) = 111.6; p < .0001). Distance per bout escalated rapidly before leveling off (sex by time interaction, 3-week: F(2,76) = 16.57; p < .0001; 6-week: F(5,170) = 7.9; p < .0001) and was higher in females than in males (3-week: F(1,38) = 27.5; p < .0001, ; 6-week: F(1,34) = 22.44; p < .0001, ). Bout length followed a similar pattern (sex by time interaction, 3-week: F(2,76) = 13.33; p < .0001, ; 6-week: F(5,170) = 14.05; p < .0001, ).
Figure 2. Sex differences in voluntary wheel running (VWR). Female and male, Sprague Dawley rats engaged in 3 (A–E) or 6 (F–J) weeks of VWR and average daily distance run during the active cycle (A and F), speed (B and G), number of bouts (C and H), distance run per bout (D and I), and bout length (E and J) were calculated. Data represent group means ± SEM. Females different from males: фp < .05, ффp < .001, фффp < .0001.
The effects of stress and VWR on JSE are shown in . IS reduced JSE in Sedentary rats of both sexes, and both 3 and 6 weeks of VWR prevented IS-induced reductions in JSE (interaction between exercise and stress: F(2,102) = 5.6; p = .004). Males spent more time interacting with juveniles than females (main effect of sex: F(1,102) = 63.6; p < .0001). As previously observed (Fallon et al. Citation2020, Tanner et al. Citation2019), the estrous phase during IS or behavioral testing had no impact on JSE in either sedentary or VWR rats. Cohen’s ds effect sizes shown in enable comparison of the effects of IS between groups.
Figure 3. Sex differences in exercise-induced stress resistance. Following 3 or 6 weeks of locked wheel (Sed) or voluntary wheel running (VWR) conditions, female and male Sprague Dawley rats were left undisturbed in their home cage (HC) or were exposed to inescapable tail shock stress (IS). Behavioral testing occurred 24 h later. (A) Time experimental rats spent interacting with a juvenile conspecific in the juvenile social exploration test. *p < .05; **p < .01. (B) Size of the IS effect on juvenile social exploration in each group. (C) Percent time males spent freezing before (pre) and for 20 min after 2 foot shocks in a fear conditioning chamber. Sed IS different from Sed HC: *p < .05, **p < .01; 3 wk VWR is different from 3 wk run HC: Δp < .05, ΔΔ p < .01. (D) Percent time females spent freezing before (pre) and for 20 min after 2 foot shocks in a fear conditioning chamber. Sed IS different from Sed HC: *p < .05; **p < .01; ***p < .001. (E) Average freezing during the post-shock freezing period. IS different from HC: **p < .01. (F) Size of the IS effect on shock-elicited freezing in each group. (G) Percent time spent engaging in locomotor activity during the 2 foot shocks in the fear conditioning chamber. (I) Distance traveled in locomotor activity chambers 48 h after HC or IS treatment. *p < .05; ***p < .001. Bars and symbols represent group means. Error bars express ± SEM. Small circles in bar graphs are individual data points.
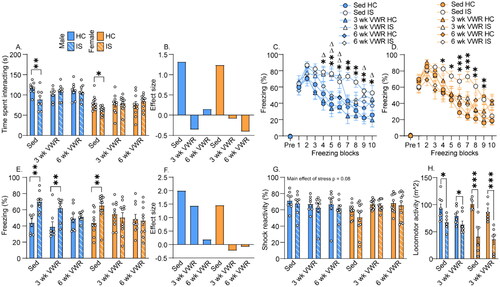
Both 3 and 6 weeks of VWR prevented IS-induced potentiated freezing in females (), but, similar to our prior report (Greenwood et al., Citation2005), only 6 weeks of VWR was protective in males (). Freezing prior to foot shock was negligible and did not differ between groups (). Repeated measures ANOVA revealed significant main effects of stress (F(1,102) = 15.7; p = .0001) and freezing blocks (F(9,918) = 84.8; p < .0001) and significant interactions between exercise and stress (F(2,102) = 4.28; p = .01) and sex and exercise (F(1,102) = 5.06; p = .02). Effect sizes of the IS effects in the various groups are shown in . Like JSE, an estrous phase during IS or behavioral testing had no impact on shock-elicited freezing in either sedentary or VWR rats. As in our prior work (Greenwood et al., Citation2003, Citation2012; Tanner et al., Citation2019), no significant correlations between running distance and IS outcomes were found.
High-velocity movement was quantified during the foot shocks as a measure of shock reactivity. Although there was a non-significant trend for IS to reduce locomotor reactivity to foot shock (F(2,102) = 5.05; p = .08), VWR had no effect on shock reactivity (main effect of exercise: F(2,102) = 0.7; p = .48; ). Negligible high-velocity movement was observed (mean = .065% ± .01%) during the 20-min post-shock observation period.
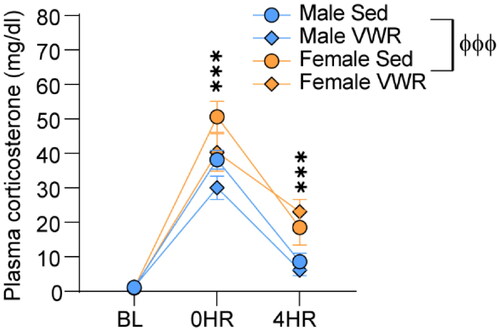
Locomotor activity was assessed the day after behavioral testing (2 d after IS). Only 3-week groups were included, since VWR enabled protection against at least one IS-induced behavior in both sexes after 3 weeks. IS reduced locomotor activity in all rats (main effect of stress: F(1,46) = 48.2; p < .0001; ). IS produced a larger reduction of locomotor activity in females compared to males (interaction between sex and stress: F(1,46) = 9.8; p = .003). No effect of VWR on locomotor activity was observed (). IS increased plasma corticosterone (main effect of time: F(2, 56) = 157.2; p < .0001; ). Levels of corticosterone were higher in females after stress (interaction between time and sex: F(2, 56) = 5.52; p = .006). VWR had no significant effect on corticosterone (main effect of exercise: F(1, 26) = 2.45; p = .12). Body weight and running data of rats used for assessment of corticosterone is shown in Figure S2.
3.2. Rapid stress resistance from VWR in females is independent of ovarian hormones during exercise
To establish the role of ovarian hormones in mediating sex differences in behavior, the hormones must be removed (Becker et al., Citation2005). Therefore, to determine the role of ovarian hormones during VWR in mediating the rapid stress resistance produced by 3 weeks of VWR in females, female rats received Sham or OVX surgery 2 weeks prior to the start of VWR (). After removal of Sham rats displaying abnormal estrous cycling or OVX rats displaying vaginal cytology consistent with proestrus or estrus, final group sizes were as follows: Sed Sham HC: 11, Sed Sham IS: 8, Sed OVX HC: 9, Sed OVX IS: 10, VWR Sham HC: 11, VWR Sham IS: 11, VWR OVX HC: 11, VWR OVX IS: 11. Sham rats included in the study had normal estrous cycling, while a lack of cycling was observed in OVX rats (). Rats gained weight over the course of the experiment (F(3,234) = 2572.4; p < .0001), but OVX rats gained more weight than sham (F(1,78) = 51.8; p < .0001). VWR attenuated weight gain in both sham and OVX groups (F(1,78) = 10.5; p = .001; Figure S3).
Figure 5. Effect of ovariectomy on voluntary wheel running behavior. Female Sprague Dawley rats received bilateral ovariectomy (OVX) or sham surgery (Sham) 2 weeks prior to the start of voluntary wheel running (VWR). (A) The estrous cycle was monitored for 10 d after OVX or Sham surgery. Sham rats cycled normally between metestrus/diestrus (Met/Di), proestrus (Pro) and estrus (Est), while OVX rats remained in Met/Di. Average daily running distance during the active cycle (B), speed (C), number of running bouts (D), distance run per bout (E), and bout length (F) were calculated. Data represent means ± SEM. Sham different from OVX: Φp < .05, ΦΦp < .01, ΦΦΦp < .0001.
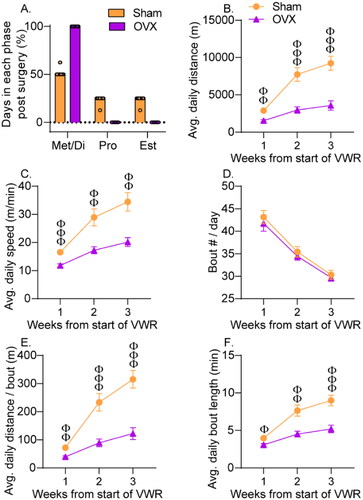
As observed previously (Gentry & Wade, Citation1976; Slonaker, Citation1924), OVX reduced distance run (F(1,42) = 26.5; p < .0001, ). In fact, the distance run by OVX females was comparable to that of males during the first 3 weeks of VWR (F(1,36) = 0.001; p = .92). Both sham and OVX escalated running distance over weeks (F(2,84) = 56.9; p < .0001), but sham rats escalated faster than OVX (F(2,84) = 15.48; p < .0001). Compared to sham, OVX also reduced running speed (main effect of surgery: F(1,42) = 15.8; p = .0003; surgery by time interaction: F(2,84) = 9.5; p = .0002; ), distance run per bout (main effect of surgery: F(1, 42) = 25.6; p < .0001; surgery by time interaction: F(2,84) = 15.43; p < .0001; ), and bout length (main effect of surgery: F(1,42) = 18.4; p < .0001; surgery by time interaction: F(2,84) = 10.9; p < .0001; ). The number of running bouts decreased over time (F(2,84) = 76.13; p < .0001), but OVX had no effect on number of bouts (F(1,42) = 0.63; p = .43; ).
Despite the lack of ovarian hormones and the reduction in running distance in OVX rats, 3 weeks of VWR still protected OVX females from IS-induced social avoidance and exaggerated fear. Exposure to IS reduced JSE in sedentary rats and 3 weeks of VWR again prevented this reduction, regardless of the presence of ovaries (exercise by stress interaction: F(1,74) = 9.1; p = .003; ). Cohen’s ds effect sizes shown in enable a comparison of the effects of IS on JSE between groups. Three weeks of VWR similarly protected females against IS-induced exaggerated freezing, again regardless of the presence of ovaries . Pre-shock freezing was negligible and did not differ between groups. Repeated measures ANOVA revealed significant interactions between exercise and stress (F(1,74) = 9.2; p = .003) and freezing blocks, exercise, and stress (F(9,666) = 2.8; p = .003). OVX increased shock-elicited freezing in VWR rats but not sedentary rats (interaction between surgery and exercise: F(1,74) = 4.8; p = .03). The size of the IS effects on shock-elicited freezing are shown in . No significant correlations were found between running distance and JSE or shock-elicited freezing in either sham or OVX rats.
Figure 6. Effect of ovariectomy on exercise-induced stress resistance. Two weeks after bilateral ovariectomy (OVX) or sham surgery (Sham), female Sprague Dawley rats were assigned to locked wheel (Sed) or voluntary wheel running (VWR) conditions. Three weeks later, rats were left undisturbed in their home cages (HC) or were exposed to inescapable tail shock stress (IS). (A) Time experimental rats spent interacting with a juvenile conspecific in the juvenile social exploration test. HC different from IS: *p < .05; **p < .01. (B) Size of the is effect on juvenile social exploration in each group. (C) Percent time Sed rats spent freezing over 2-min blocks. Sham Sed IS different from Sham Sed HC: *p < .05, **p < .01, ***p < .001. OVX Sed IS different from OVX Sed HC: Δ p < .05, ΔΔ p < .01. (D) Percent time VWR rats spent freezing over 2-min blocks. (E) Average percent time spent freezing during the 20-min post-shock freezing period. HC different from IS: *p < .05; ***p < .001. (F) Size of the IS effect on shock-elicited fear in each group. (G) Percent time rats spent engaged in locomotor activity in the fear conditioning chamber prior to the shock. (F) Percent time spent engaging in locomotor activity during the 2 foot shocks in the fear conditioning chamber.
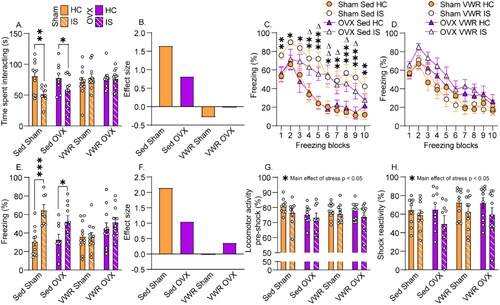
IS reduced locomotor activity during the 5 min prior to foot shock in the conditioning chambers (F(1,74) = 4.4; p = .04; ), but no effect of exercise was observed (F(1,74) = 0.18; p = .9). OVX tended to reduce locomotor activity, but this effect failed to reach significance (F(1,74) = 3.2; p = .08). IS also reduced locomotor reactivity during foot shock (F(1,74) = 6.14; p = .01), but no effects of OVX (F(1,74) = 0.5; p = .5) or VWR (F(1,74) = 2.76; p = .1) were observed (). Negligible high-velocity movement was observed (mean = 0.09% ± .01%) during the 20-min post-shock observation period.
4. Discussion
We report that VWR enables robust stress resistance in both sexes, but the duration of VWR required to enable stress resistance differs between the sexes in a behavioral outcome-dependent manner. Protection against stress-induced social avoidance is evident after 3 weeks of VWR in both sexes, but protection against exaggerated fear occurs more rapidly in females than it does in males. The magnitude of the stress-protective effects, when present, are similar between the sexes. In both sexes, stress resistance from VWR is independent of the effects of VWR on shock reactivity, locomotor activity, and corticosterone. Although females run greater distances, longer bouts, and faster speeds than males, the rapid stress-protective effects of VWR observed in females are independent of running distance and ovarian hormones at the time of exercise.
Here, we directly compare the stress-protective effects of VWR between sexes and find that sex differences in exercise-induced stress resistance are dependent on stress outcome. Protection against anxiety-related outcomes (e.g. social avoidance) from exercise develop similarly in females and males, while protection against maladaptive fear-related outcomes (e.g. exaggerated fear) develop more readily in females compared to males. Together with prior work suggesting that prior physical activity prevents depression-like behavior more readily in female mice than in males (Elias et al., Citation2023; Munive et al., Citation2016; Naghibi et al., Citation2021), these data suggest that female rats are more responsive to exercise-induced stress resistance than are males. Although this sex difference may be difficult to confirm in humans given the limitations of clinical studies on stress resistance, Kim et al. (Citation2019) found that transitioning from a sedentary lifestyle to a physically active one reduced the incidence of new depression within a year in women, but not in men (Kim et al., Citation2019). If additional clinical data can confirm the pre-clinical observations, then physical activity should be prioritized as a prophylactic strategy for women at risk for developing stress-related disorders.
It is interesting from a mechanistic perspective that females are more responsive than males to the protective effect of VWR against some of the behavioral consequences of IS, especially since other experiential strategies that are known to provide protection against IS in males fail to do so in females. Behavioral control over stress is another well-characterized experiential resistance factor. Male rats exposed to the same number, intensity, and duration of shocks as IS rats but able to exert control over the termination of the shocks by pressing a lever (escapable stress; ES), do not demonstrate social avoidance or exaggerated fear after the stressor (Christianson et al., Citation2008). In fact, ES protects male rats against the typical behavioral sequela of future IS (Maier, Citation2015). In contrast, female rats display social avoidance and exaggerated fear following both IS and ES (Baratta et al., Citation2018). Excessive activation of serotonin (5-HT) neurons in the dorsal raphe nucleus (DRN) is both necessary and sufficient for the behavioral outcomes of IS, including both social avoidance and exaggerated fear (Christianson. et al., Citation2008; Maier & Watkins, Citation2005). In males, ES recruits a circuit from the ventral medial prefrontal cortex (PFC) to the DRN which inhibits DRN 5-HT activity (Amat et al., Citation2005). Females learn to control the termination of the shocks during ES, but ES fails to recruit the PFC-DRN circuit in females, and thus ES hyperactivates DRN 5-HT neurons and produces the behavioral outcomes characteristic of IS (Baratta et al., Citation2018; McNulty et al., Citation2023). Interestingly, 6 weeks of VWR constrains the DRN 5-HT response to IS (Clark et al., Citation2015; Greenwood et al., Citation2003), but seems to do so through a mechanism independent of the PFC, since PFC lesions do not eliminate the protective effects of 6 weeks of VWR against the behavioral outcomes of IS (Greenwood et al., Citation2013). Differential requirement of the PFC could explain why VWR is effective at preventing IS outcomes in females, while females are resistant to the stress-buffering properties of ES.
Although no neural mechanisms were investigated in the current study, it is of interest to speculate how 3 weeks of VWR prevents some behavioral consequences of IS (e.g. social avoidance), but not others (e.g. exaggerated fear). Six weeks of VWR prevents hyperactivation and sensitization of DRN 5-HT neurons, so it is easy to conceptualize how 6 weeks of VWR prevents numerous behavioral outcomes of IS, all of which depend on DRN hyperactivation. However, 3 weeks of VWR fails to constrain the DRN 5-HT response to stress in males (Greenwood et al., Citation2005). This suggests VWR produces rapid adaptations in neural circuits involved in social avoidance.
The sensitized DRN caused by IS leads to excessive 5-HT efflux in the basolateral amygdala (BLA) during behavioral testing (Amat et al., Citation1998), where 5-HT2C receptor signaling is both necessary and sufficient for IS-induced social avoidance (Christianson et al., Citation2010) and exaggerated fear (Strong et al., Citation2011). The BLA is thought to mediate exaggerated fear and anxiety through separable systems. BLA projections to the central amygdala (CeA) drive fear responses, whereas BLA projections to the bed nucleus of the stria terminalis (BNST) contribute to anxiety (Davis et al., Citation2010), including social avoidance (Vantrease et al., Citation2022). While 3 weeks of VWR has no effect on IS-induced cFos in the BLA or CeA (Greenwood et al., Citation2005), it does alter neural activity in the BNST during IS (Greenwood et al., Citation2005). These data raise the possibility that rapid neural adaptations in the neural systems involved in anxiety (BNST) produced by VWR could act to prevent stress-induced anxiety by altering the BNST response to BLA input, despite the hyperactive DRN in 3-week VWR rats. Consistent with this possibility, Fox et al. (Citation2022) recently reported that 2 weeks of VWR reduces 5-HT2C mRNA in the BNST of male mice (Fox et al., Citation2022). Since 3 weeks of VWR prevents both social avoidance and exaggerated fear produced by IS, it is possible that 3 weeks of VWR simply constrains DRN 5-HT activity during stress in females. Future work should verify whether DRN constraint occurs more readily after the start of VWR in females compared to males.
Shock sensitivity, general locomotor activity, and circulating corticosterone were examined as potentially associated with exercise-induced stress resistance. VWR had no effect on locomotor reactivity to foot shocks in either sex, suggesting that alterations in shock sensitivity does not contribute to the stress-protective effects of VWR. Similar to reports that IS produces a long-term reduction in voluntary activity (Desan et al., Citation1988), we observed that IS reduces locomotor activity. IS-induced reduction in activity was equivalent between sedentary and VWR rats, and VWR had no effect on locomotor activity in non-stressed rats of either sex. These data suggest that VWR does not alter behavior by simply increasing activity or by buffering against IS-induced hypoactivity. Finally, VWR did not significantly alter IS-induced increases in corticosterone in either sex, corroborating prior observations that VWR does not impact the hypothalamic-pituitary-adrenal axis response to severe, acute stressors in male rats (Campeau et al., Citation2010; Fleshner, Citation2000). This observation indicates that the protective effect of VWR against IS-induced behavioral outcomes is independent of circulating corticosterone.
Female sex hormones have been reported to exert anxiolytic (Toufexis et al., Citation2004) and antidepressant (Bloch et al., Citation2000; Galea et al., Citation2001) effects, raising the possibility that ovarian hormones are involved in the observed sex differences in exercise-induced stress resistance. However, 3 weeks of VWR prevented IS-induced social avoidance and exaggerated fear in females lacking ovaries. Although it is possible that residual effects of ovarian hormones, or low circulating levels of the hormones themselves, lingered into the VWR period, these data strongly indicate that the rapid stress resistance produced by VWR in females is independent of the presence of ovarian hormones during exercise. Rather than the activational effects of circulating sex hormones, the sex differences in exercise-induced stress resistance could be explained by genetic differences or by organizational effects of hormones earlier in development.
We observed large sex differences in VWR behavior consistent with prior reports (Basso & Morrell, Citation2017; Eikelboom & Mills, Citation1988; Tanner et al., Citation2022). OVX eliminated sex differences in VWR behavior, yet 3 weeks of VWR still prevented IS-induced social avoidance and exaggerated fear. Together with prior and current observations that stress-buffering effects of VWR are not correlated with running distance, these data suggest that sex differences in exercise-induced stress resistance are not dependent on females running more than males.
In conclusion, the duration of VWR required to enable stress resistance differs between the sexes in a behavioral outcome-dependent manner. Exercise-induced protection against anxiety-like behavior develops rapidly in both sexes, evident after 3 weeks of VWR. In contrast, exercise-induced protection against exaggerated fear develops more readily in females than in males. Sex differences in exercise-induced stress resistance are not dependent on ovarian hormones during exercise, or sex differences in VWR behavior. These results suggest that women could be especially responsive to stress-protective effects of exercise.
Author contributions
Margaret Tanner: conceptualization, experimentation, analysis, writing the manuscript; Alyssa Hohorst: conceptualization, experimentation, analysis; Simone Mellert: experimentation, analysis; Esteban Loetz: experimentation; Michael Baratta: conceptualization, editing manuscript; Benjamin Greenwood: conceptualization, analysis, writing the manuscript. All authors approved the submitted manuscript.
Supplemental Material
Download MS Word (395.7 KB)Acknowledgements
We are grateful to Dr. Matthew Frank for assisting with corticosterone measurements.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be made available on request.
Additional information
Funding
Notes on contributors
Margaret K. Tanner
Margaret K. Tanner
received her PhD in Integrative Biology from the University of Colorado Denver, USA. She is currently a post-doctoral fellow in the Behavioral Exercise Neuroscience laboratory at the University of Colorado Denver.
Alyssa A. Hohorst
Alyssa A. Hohorst
is a doctoral candidate at the University of Colorado Denver, USA.
Simone M. Mellert
Simone M. Mellert
is a doctoral candidate at the University of Colorado Denver, USA.
Esteban C. Loetz
Esteban C. Loetz
is a professional research assistant in the Behavioral Exercise Neuroscience laboratory at the University of Colorado Denver, USA.
Michael V. Baratta
Michael V. Baratta
is an Assistant Professor in the Department of Psychology and Neuroscience at the University of Colorado Boulder, USA. His lab studies the neural basis of stress resilience.
Benjamin N. Greenwood
Benjamin N. Greenwood
is an Associate Professor in the Department of Psychology at the University of Colorado Denver, USA. He is director of the Behavioral Exercise Neuroscience laboratory.
References
- Amat, J., Baratta, M. V., Paul, E., Bland, S. T., Watkins, L. R., & Maier, S. F. (2005). Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience, 8(3), 1–12. https://doi.org/10.1038/nn1399
- Amat, J., Matus-Amat, P., Watkins, L. R., & Maier, S. F. (1998). Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Research, 812(1–2), 113–120. https://doi.org/10.1016/s0006-8993(98)00960-3
- Baratta, M. V., Christianson, J. P., Gomez, D. M., Zarza, C. M., Amat, J., Masini, C. V., Watkins, L. R., & Maier, S. F. (2007). Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience, 146(4), 1495–1503. https://doi.org/10.1016/j.neuroscience.2007.03.042
- Baratta, M. V., Leslie, N. R., Fallon, I. P., Dolzani, S. D., Chun, L. E., Tamalunas, A. M., Watkins, L. R., & Maier, S. F. (2018). Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. The European Journal of Neuroscience, 47(8), 959–967. https://doi.org/10.1111/ejn.13833
- Basso, J. C., & Morrell, J. I. (2017). Using wheel availability to shape running behavior of the rat towards improved behavioral and neurobiological outcomes. Journal of Neuroscience Methods, 290, 13–23. https://doi.org/10.1016/j.jneumeth.2017.07.009
- Becker, J. B., Arnold, A. P., Berkley, K. J., Blaustein, J. D., Eckel, L. A., Hampson, E., Herman, J. P., Marts, S., Sadee, W., Steiner, M., Taylor, J., & Young, E. (2005). Strategies and methods for research on sex differences in brain and behavior. Endocrinology, 146(4), 1650–1673. https://doi.org/10.1210/en.2004-1142
- Bigos, K. L., Pollock, B. G., Stankevich, B. A., & Bies, R. R. (2009). Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: an updated review. Gender Medicine, 6(4), 522–543. https://doi.org/10.1016/j.genm.2009.12.004
- Bloch, M., Schmidt, P. J., Danaceau, M., Murphy, J., Nieman, L., & Rubinow, D. R. (2000). Effects of gonadal steroids in women with a history of postpartum depression. The American Journal of Psychiatry, 157(6), 924–930. https://doi.org/10.1176/appi.ajp.157.6.924
- Campeau, S., Nyhuis, T. J., Sasse, S. K., Kryskow, E. M., Herlihy, L., Masini, C. V., Babb, J. A., Greenwood, B. N., Fleshner, M., & Day, H. E. W. (2010). Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. Journal of Neuroendocrinology, 22(8), 872–888. https://doi.org/10.1111/j.1365-2826.2010.02007.x
- Chekroud, S. R., Gueorguieva, R., Zheutlin, A. B., Paulus, M., Krumholz, H. M., Krystal, J. H., & Chekroud, A. M. (2018). Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: A cross-sectional study. The Lancet Psychiatry, 5(9), 739–746. https://doi.org/10.1016/S2215-0366(18)30227-X
- Christianson, J. P., Paul, E. D., Irani, M., Thompson, B. M., Kubala, K. H., Yirmiya, R., Watkins, L. R., & Maier, S. F. (2008). The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behavioural Brain Research, 193(1), 87–93. https://doi.org/10.1016/j.bbr.2008.04.024
- Christianson, J. P., Ragole, T., Amat, J., Greenwood, B. N., Strong, P. V., Paul, E. D., Fleshner, M., Watkins, L. R., & Maier, S. F. (2010). 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biological Psychiatry, 67(4), 339–345. https://doi.org/10.1016/j.biopsych.2009.09.011
- Clark, P. J., Amat, J., McConnell, S. O., Ghasem, P. R., Greenwood, B. N., Maier, S. F., & Fleshner, M. (2015). Running reduces uncontrollable stress-evoked serotonin and potentiates stress-evoked dopamine concentrations in the rat dorsal striatum. PLOS One, 10(11), e0141898. https://doi.org/10.1371/journal.pone.0141898
- Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–135. https://doi.org/10.1038/npp.2009.109
- Desan, P. H., Silbert, L. H., & Maier, S. F. (1988). Long-term effects of inescapable stress on daily running activity and antagonism by desipramine. Pharmacology, Biochemistry, and Behavior, 30(1), 21–29. https://doi.org/10.1016/0091-3057(88)90420-0
- Eikelboom, R., & Mills, R. (1988). A microanalysis of wheel running in male and female rats. Physiology & Behavior, 43(5), 625–630. https://doi.org/10.1016/0031-9384(88)90217-x
- Elias, E., Zhang, A. Y., White, A. G., Pyle, M. J., & Manners, M. T. (2023). Voluntary wheel running promotes resilience to the behavioral effects of unpredictable chronic mild stress in male and female mice. Stress, 26(1), 2203769. https://doi.org/10.1080/10253890.2023.2203769
- Fallon, I. P., Tanner, M. K., Greenwood, B. N., & Baratta, M. V. (2020). Sex differences in resilience: Experiential factors and their mechanisms. The European Journal of Neuroscience, 52(1), 2530–2547. https://doi.org/10.1111/ejn.14639
- Fleshner, M. (2000). Exercise and neuroendocrine regulation of antibody production: protective effect of physical activity on stress-induced suppression of the specific antibody response. International Journal of Sports Medicine, 21(Suppl 1), S14–S19. https://doi.org/10.1055/s-2000-1454
- Fox, J. H., Boucher, M. N., Abedrabbo, K. S., Hare, B. D., Grimmig, B. A., Falls, W. A., & Hammack, S. E. (2022). Exercise reduces the anxiogenic effects of meta-chlorophenylpiperazine: The role of 5-HT2C receptors in the bed nucleus of the stria terminalis. Frontiers in Synaptic Neuroscience, 14, 1067420. https://doi.org/10.3389/fnsyn.2022.1067420
- Galea, L. A., Wide, J. K., & Barr, A. M. (2001). Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behavioural Brain Research, 122(1), 1–9. https://doi.org/10.1016/s0166-4328(01)00170-x
- Garcia-Sifuentes, Y., & Maney, D. L. (2021). Reporting and misreporting of sex differences in the biological sciences. Elife, 10, e70817. https://doi.org/10.7554/eLife.70817
- Gentry, R. T., & Wade, G. N. (1976). Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. Journal of Comparative and Physiological Psychology, 90(8), 747–754. https://doi.org/10.1037/h0077246
- Greenwood, B. N., & Fleshner, M. (2011). Exercise, stress resistance, and central serotonergic systems. Exercise and Sport Sciences Reviews, 39(3), 140–149. https://doi.org/10.1097/JES.0b013e31821f7e45
- Greenwood, B. N., Foley, T. E., Burhans, D., Maier, S. F., & Fleshner, M. (2005). The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Research, 1033(2), 164–178. https://doi.org/10.1016/j.brainres.2004.11.037
- Greenwood, B. N., Foley, T. E., Day, H. E. W., Campisi, J., Hammack, S. H., Campeau, S., Maier, S. F., & Fleshner, M. (2003). Freewheel running prevents learned helplessness/behavioral depression: Role of dorsal raphe serotonergic neurons. The Journal of Neuroscience, 23(7), 2889–2898. https://doi.org/10.1523/JNEUROSCI.23-07-02889.2003
- Greenwood, B. N., Loughridge, A. B., Sadaoui, N., Christianson, J. P., & Fleshner, M. (2012). The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behavioural Brain Research, 233(2), 314–321. https://doi.org/10.1016/j.bbr.2012.05.017
- Greenwood, B. N., Spence, K. G., Crevling, D. M., Clark, P. J., Craig, W. C., & Fleshner, M. (2013). Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. The European Journal of Neuroscience, 37(3), 469–478. https://doi.org/10.1111/ejn.12044
- Gruene, T. M., Flick, K., Stefano, A., Shea, S. D., & Shansky, R. M. (2015). Sexually divergent expression of active and passive conditioned fear responses in rats. Elife, 4: e11352. https://doi.org/10.7554/eLife.11352
- Harvey, S. B., Overland, S., Hatch, S. L., Wessely, S., Mykletun, A., & Hotopf, M. (2018). Exercise and the prevention of depression: Results of the HUNT cohort study. The American Journal of Psychiatry, 175(1), 28–36. https://doi.org/10.1176/appi.ajp.2017.16111223
- Kim, S. Y., Park, J. H., Lee, M. Y., Oh, K. S., Shin, D. W., & Shin, Y. C. (2019). Physical activity and the prevention of depression: A cohort study. General Hospital Psychiatry, 60, 90–97. https://doi.org/10.1016/j.genhosppsych.2019.07.010
- Maier, S. F. (1990). Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. Journal of Experimental Psychology: Animal Learning and Cognition, 16(2), 137–149. https://doi.org/10.1037/0097-7403.16.2.137
- Maier, S. F. (2015). Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol Stress, 1, 12–22. https://doi.org/10.1016/j.ynstr.2014.09.003
- Maier, S. F., & Watkins, L. R. (2005). Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews, 29(4–5), 829–841. https://doi.org/10.1016/j.neubiorev.2005.03.021
- McNulty, C. J., Fallon, I. P., Amat, J., Sanchez, R. J., Leslie, N. R., Root, D. H., Maier, S. F., & Baratta, M. V. (2023). Elevated prefrontal dopamine interferes with the stress-buffering properties of behavioral control in female rats. Neuropsychopharmacology, 48(3), 498–507. https://doi.org/10.1038/s41386-022-01443-w
- Munive, V., Santi, A., & Torres-Aleman, I. (2016). A concerted action of estradiol and insulin like growth factor I underlies sex differences in mood regulation by exercise. Scientific Reports, 6, 25969. https://doi.org/10.1038/srep25969
- Naghibi, S., Shariatzadeh Joneydi, M., Barzegari, A., Davoodabadi, A., Ebrahimi, A., Eghdami, E., Fahimpour, N., Ghorbani, M., Mohammadikia, E., Rostami, M., & Salari, A.-A. (2021). Treadmill exercise sex-dependently alters susceptibility to depression-like behaviour, cytokines and BDNF in the hippocampus and prefrontal cortex of rats with sporadic Alzheimer-like disease. Physiology & Behavior, 241, 113595. https://doi.org/10.1016/j.physbeh.2021.113595
- Pavlidi, P., Kokras, N., & Dalla, C. (2023). Sex differences in depression and anxiety. Current Topics in Behavioral Neurosciences, 62, 103–132. https://doi.org/10.1007/7854_2022_375
- Pearce, M., Garcia, L., Abbas, A., Strain, T., Schuch, F. B., Golubic, R., Kelly, P., Khan, S., Utukuri, M., Laird, Y., Mok, A., Smith, A., Tainio, M., Brage, S., & Woodcock, J. (2022). Association between physical activity and risk of depression: A systematic review and meta-analysis. JAMA Psychiatry, 79(6), 550–559. https://doi.org/10.1001/jamapsychiatry.2022.0609
- Slonaker, J. R. (1924). The effect of pubescence, oestruation and menopause on the voluntary activity in the albino rat. American Journal of Physiology, 68, 294–315. https://doi.org/10.1152/ajplegacy.1924.68.2.294
- Steel, Z., Marnane, C., Iranpour, C., Chey, T., Jackson, J. W., Patel, V., & Silove, D. (2014). The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. International Journal of Epidemiology, 43(2), 476–493. https://doi.org/10.1093/ije/dyu038
- Strong, P. V., Christianson, J. P., Loughridge, A. B., Amat, J., Maier, S. F., Fleshner, M., & Greenwood, B. N. (2011). 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience, 197, 132–144. https://doi.org/10.1016/j.neuroscience.2011.09.041
- Tanner, M. K., Davis, J. K. P., Jaime, J., Moya, N. A., Hohorst, A. A., Bonar, K., Abrams, K. A., Jamil, N., Han, R., Hubert, T. J., Brown, N., Loetz, E. C., & Greenwood, B. N. (2022). Duration- and sex-dependent neural circuit control of voluntary physical activity. Psychopharmacology, 239(11), 3697–3709. https://doi.org/10.1007/s00213-022-06243-0
- Tanner, M. K., Fallon, I. P., Baratta, M. V., & Greenwood, B. N. (2019). Voluntary exercise enables stress resistance in females. Behavioural Brain Research, 369, 111923. https://doi.org/10.1016/j.bbr.2019.111923
- Toufexis, D. J., Davis, C., Hammond, A., & Davis, M. (2004). Progesterone attenuates corticotropin-releasing factor-enhanced but not fear-potentiated startle via the activity of its neuroactive metabolite, allopregnanolone. The Journal of Neuroscience, 24(45), 10280–10287. https://doi.org/10.1523/JNEUROSCI.1386-04.2004
- Ungar, M., & Theron, L. (2020). Resilience and mental health: how multisystemic processes contribute to positive outcomes. The Lancet Psychiatry, 7(5), 441–448. https://doi.org/10.1016/S2215-0366(19)30434-1
- Vantrease, J. E., Avonts, B., Padival, M., DeJoseph, M. R., Urban, J. H., & Rosenkranz, J. A. (2022). Sex differences in the activity of basolateral amygdalar neurons that project to the bed nucleus of the stria terminalis and their role in anticipatory anxiety. The Journal of Neuroscience, 42(22), 4488–4504. https://doi.org/10.1523/JNEUROSCI.1499-21.2022