Abstract
As endocrine hormones, glucocorticoids (GCs) play a pivotal role in numerous physiological processes, including mammary growth and lactation, circulatory metabolism, and responses to external stimuli. In the dairy industry, milk production from cows or goats is important for newborns and economic benefits. However, the milk yields from ruminant animals are always affected by the extent of mammary development, mammary disease, stress, or changes in metabolism. Thus, it is necessary to clarify how GCs changes in ruminants affect ruminant mammary gland function and mammary disease. This review summarizes the findings identifying that GCs modulate mammary gland development before lactation, but the stress-induced excessive release of GCs leads to milk production loss. In addition, the manner of GCs release may change under different concentrations of metabolites or during mastitis or inflammatory challenge. Nevertheless, exogenous GCs administration to animals may alleviate the clinical symptoms of mastitis. This review demonstrates that GCs offer a fascinating contribution to both physiologic and pathogenic conditions of the mammary gland in ruminant animals. Characterizing and understanding these changes or functions of endogenous and exogenous GCs in animals will be crucial for developing more endocrine regulators and therapies for improving milk production in ruminants.
1. Introduction
Glucocorticoids (GCs) are hormones secreted by the adrenal cortex and released following activation of the hypothalamic-pituitary-adrenocortical (HPA) axis. GCs in mammalian species are generally classified as cortisol (e.g. dairy cows and goats) and corticosterone (e.g. rodents). GCs are important regulators of metabolism and stress and play a vital role in a variety of events, including tissue development (Mostyn et al., Citation2003), control of energy balance, regulation of the immune system (Reiske et al., Citation2020), stress adaptation and behavioral regulation (Dobson & Smith, Citation2000). Modern artificial synthesized glucocorticoids, such as prednisolone, fluticasone, budesonide and dexamethasone, are based on the structure of cortisol (hydrocortisone) and are added alone or with other drugs to treat inflammatory or infectious diseases such as mastitis (Ziv et al., Citation1998), alcoholic hepatitis (Kendrick et al., Citation2010), and severe eyelid edema (Tuncel et al., Citation2013). GCs regulate physiological processes by binding to glucocorticoid receptors (GRs), which then trigger subsequent DNA binding-dependent or protein–protein interactions to control gene expression (Wolfgang Doppler, Citation1989; Reichardt et al., Citation2001). In fact, GCs exist in plasma in two forms: most GCs are bound to two plasma proteins of globulin (CBG, more than 80% of GCs) and serum album (10%-15% of GCs), whereas only approximately 5% of GCs are free (FC) and are biologically active after binding to GRs (Lewis et al., Citation2005). However, the proportion of FC relative to total cortisol (TC) is not constant, as the capacity and affinity of cortisol may change, which may alter the amount of circulating FC.
In the modern dairy industry, mammary gland development and milk production play key roles in fetal growth and economic income. However, potential limiting factors such as mammary growth, mammary disease, external stimuli-induced stress, and nutrition constraints could largely lead to endocrine and metabolic disorders as well as reduce milk yields. GCs modulate organ development, metabolism, and the immune system. Here, we summarize current insights into the role of GCs in mammary development and the effect of stress-induced GCs release on milk production. We also focused on the change in the GCs concentration under different metabolic conditions and inflammatory mammary disease. Finally, we review the cooperation of GCs with other hormones, factors or drugs under different mammary conditions. Thus, much future work should be performed to explore the mechanisms of GCs on mammary growth, function and disease to improve milk production in the dairy industry.
2. Mammary gland-specific glucocorticoid function
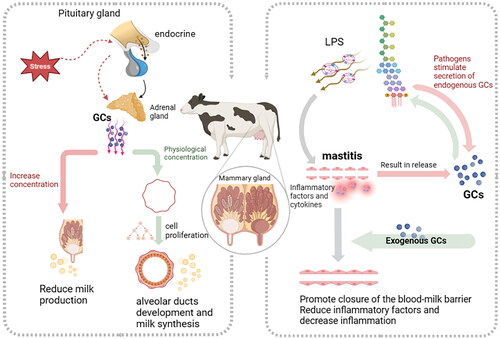
The neurohumoral feedback regulatory activities and functions of GCs in vivo are first described. GCs secretion is triggered by the hypothalamic–pituitary–adrenal (HPA) axis in response to both physical and psychological stress. First, stress signals rapidly stimulate adrenocorticotropin-releasing hormone (CRH) and pressor neurons in the paraventricular nucleus of the hypothalamus (PVN), which then induce anterior pituitary adrenocorticotropic hormone (ACTH) secretion, leading to the production of adrenocortical GCs. However, sustained high levels of endogenous GCs are detrimental, and rapid negative GC feedback is essential to terminate HPA axis activation. The release of endogenous GCs is part of a general stress response to adverse conditions, while milk production usually decreases when animals are stressed, and this acute effect may involve the rerelease of adrenaline. To prevent detrimental effects of endogenous GC secretion on the inhibition of lactation and maternal responsiveness, the HPA axis is often attenuated during parturition through autoregulation (Good et al., Citation2005).
In vivo, GCs orchestrate the activity of the HPA axis via GR and the mineralocorticoid receptor (MR). These two receptors are members of the steroid/thyroid/retinoic acid receptor (STR) family and share 94% amino acid identity in the DNA binding domain and 57% identity in the ligand-binding domain in humans (Arriza et al., Citation1987). MR has higher affinity for GCs (e.g. cortisol in humans and corticosterone in rats) than GR and is largely occupied under basal GC conditions, whereas GR occupancy is increased when GC levels rise during the circadian peak or following stress (Reul & de Kloet, Citation1985; Reul et al., Citation2000). These results suggest that MR may be involved in basal activity and the onset of stress-induced HPA axis activity, while GR primarily mediates negative feedback signals of elevated GCs and drives the termination of HPA axis activity (de Kloet et al., Citation2018). Postnatal mammary development, such as ductal morphogenesis, alveolar budding, and lobular formation, lactation, and involution, is tightly regulated by systemic levels of hormones such as estrogen (E), progesterone (P), prolactin (PRL) and GCs, as well as their receptors (Topper & Freeman, Citation1980). Mice lacking either PRL or PRLR fail to undergo lobuloalveolar development and do not lactate, while in vitro, endogenous GCs were demonstrated to act synergistically with PRL in mammary epithelial cells (MECs) to regulate mammary differentiation and milk protein expression (Rosen et al., Citation1999). GCs mainly exert their function through binding with GRs, which play vital roles in the mammary gland and use different modes of action to control development and milk protein (Reichardt et al., Citation2001); MR is present in mammary ducts and secretory ducts but not in myoepithelial and mesenchymal cells in the human mammary gland (Sasano et al., Citation1992). MR can compensate for the absence of GR to induce β-casein gene expression in the presence of PRL and insulin at some stage of mammary development in GR-/- mice (Kingsley-Kallesen et al., Citation2002).
2.1. The function of glucocorticoids in regulating mammary gland development
Mammary development can be divided into several important stages, such as prepuberty, puberty, pregnancy, and lactation. Each stage is under strict hormonal control, but corticosteroids, which act as metabolic hormones on the mammary gland, are not the most critical hormones for mammary development, and they usually promote mammary development during late gestation (Borellini & Oka, Citation1989; Neville et al., Citation2002; Svennersten-Sjaunja & Olsson, Citation2005). Generally, in vivo, GCs regulate physiological processes by binding to the GR to modulate subsequent gene expression and protein–protein interactions. A study in DNA binding-defective GR mice found that a lack of the DNA binding function of GR impaired the ductal development of the mammary gland in virgin females, while a lack of GR in lactating females resulted in normally differentiated mammary glands and was fully capable of milk protein production (Reichardt et al., Citation2001). Exogenous GC administration in female rats reduced the ductal extension and branching of the mammary gland as well as decreased the total volume of the mammary epithelium in a dose-dependent manner (Zhu et al., Citation1998). This suggests that the GC/GR pathway in vivo is more important for the development of the nonlactating mammary gland than that of the lactating mammary gland. However, exogenous treatment with GCs inhibits estrogen receptor-induced proliferation in the mammary epithelium in MCF-7 cells (Yang et al., Citation2017).
In ruminant animals, elevated endogenous cortisol and exogenous cortisol addition does not alter gene expression of insulin-like growth factor type 1 (IGF1), insulin-like growth factor-binding protein 3 and 5 (IGFBP3 and IGFBP5) in experimental goat mammary tissue, nor does it alter mammary tissue proliferation and apoptosis, while in vivo, injection of high exogenous cortisol levels could increase the expression of BAX gene and decrease the number of the epithelial cells while low cortisol levels have no effect on cell proliferation or cell apoptosis in mammary tissue (Bomfim et al., Citation2018). However, in vitro, GCs can indirectly affect the mammary gland via the GR pathway by enhancing the effects of the PRL/STAT5 pathway, which has been shown to be important for mammary gland development and milk production (Kobayashi et al., Citation2016). The administration of hexoestrol and progesterone in combination with prolactin (bovine), growth hormone (bovine) and adrenocorticotrophin to ovariectomized-hypophysectomized goats can promote significant mammary lobulo-alveolar growth (Cowie et al., Citation1966). These results demonstrated that GCs play a role in mammary development or in combination with other hormones, but this effect may vary depending on the different sensitivity of animals to endocrine GC release or exogenous GC addition ().
Table 1. Summary of the effects of GCs on milk yield in different species.
2.2. Glucocorticoids regulate milk synthesis and components
PRL, growth hormone (GH), and thyroid hormone (TH) play major roles in the synthesis of mammary milk during lactation. As exogenous GCs, cortisol (hydrocortisone and dexamethasone are GC analogs) is often used as one of the added components in the lactation-inducing system (including PRL, insulin, and hydrocortisone) when cultured goat or cow mammary epithelial cells (MECs) in vitro (Liu et al., Citation2014; Kung et al., Citation2015).
Here, we provide an overview of several in vitro experiments applying exogenous GCs. Casein and α-lactalbumin are the major milk proteins synthesized by lactating mammary glands in ruminants, while PRL is required to induce these two major milk protein genes. The accumulation of casein in organ culture of the mammary gland or in mammary epithelial cells under a lactation-inducing system with epidermal growth factor (EGF) plus PRL or insulin plus PRL is low, whereas the addition of exogenous cortisol to this culture system resulted in a dose-dependent increase in casein content, and the greatest accumulation occurred at a cortisol concentration of approximately 3 × 10−6 M (Ono & Oka, Citation1980b; Kobayashi et al., Citation2017b). However, the addition of cortisol alone or in combination with insulin or PRL did not result in an increase in casein synthesis, while the presence of PRL, insulin and cortisol had a dose-dependent effect on the accumulation of α-lactalbumin in cultured mammary explants in vitro (a moderate amount of cortisol doubled the amount of α-lactalbumin; a higher cortisol concentration of more than 10−7 M had an inhibitory effect on α-lactalbumin expression) (Ono & Oka, Citation1980b, Citation1980a). It was also discovered that other exogenous GCs, including dexamethasone, deoxycorticosterone, cortisone and prednisolone, promoted albumin accumulation at lower concentrations (10−9 M to 10−8 M) and inhibited albumin accumulation at higher concentrations (10−7 M to 10−6 M) (Ono & Oka, Citation1980b). In various tissues, endogenous GCs have been shown to have catabolic effects, leading to reduced synthesis of proteins and nucleic acids as well as amino acid and glucose uptake (Homer et al., Citation1990; Virgin et al., Citation1991; Sangild et al., Citation1993). A previous study reported that insulin and cortisol endogenously modulate the channeling of nutrients between anabolic and anti-anabolic aspects of maternal body protein metabolism, whereas TH and cortisol endogenously modulate nutrient partitioning toward milk production and visceral protein synthesis (Motil et al., Citation1994).
Soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins could regulate the intracellular trafficking and exocytosis of casein micelles, which are packaged into secretory vesicles and then transported into apical plasma membranes in alveolar MECs (McNew, Citation2008). SNARE proteins contain vesicular-SNARE proteins (VAMPs), which are localized on the vesicle membrane, and target-SNARE proteins (Syntaxins), which are localized on the apical or basolateral membranes (Rothman, Citation1994). The major milk proteins of caseins are secreted by exocytosis, while milk fat globules are released by budding and enwrapped by the apical membrane. A study reported that administration of an exogenous GC analog of dexamethasone in MECs not only increased milk protein and milk content but also upregulated the expression of SNARE proteins such as SNAP-23, VAMP-8, and Syntaxin-12 (Kobayashi et al., Citation2017b). These results demonstrated that GCs not only play a key role in the synthesis of milk proteins but also regulate the intracellular transportation and exocytosis of milk components.
2.3. Glucocorticoid response to external stimuli, which may reduce milk yields
In ruminants, GH predominates in lactation, PRL acts both directly on mammary epithelial cells and mammary epithelial cytokines to activate a variety of transcription factors (Svennersten-Sjaunja & Olsson, Citation2005), and exogenous treatment of GC/GR on MECs acts as an enhancer of PRLR/STAT5 in vitro (Kobayashi et al., Citation2016). Multiple in vivo stress modeling experiments can be illustrative. In cows under acute nutritional stress, exogenous dexamethasone is able to reduce milk production and energy deficiency without affecting immune function in vivo (Ollier et al., Citation2016), while transport stress elevates endogenous cortisol levels, which results in increased blood immune cell numbers, such as leucocytes, neutrophils and monocytes, and reduced milk yields and milk quality owing to an increase in milk somatic cell counts (SCCs) in vivo (Hong et al., Citation2019). For cows in early lactation, endogenous cortisol can stimulate gluconeogenesis, which particularly supports the high demand for glucose during early lactation (Gross et al., Citation2015). In goats, variations in milk cortisol concentration may be associated with milk production level but was not correlated with milk components (fat, total protein, and lactose) (Díaz et al., Citation2013). Previous studies in lactating ewes or goats in vivo have also reported that acute stress- or heat stress-induced increases in endogenous cortisol release, while reduced milk production and milk lactose, and increased somatic cell counts (SCC) in milk (Mehdid et al., Citation2019; Hooper et al., Citation2021). In addition, IGF1 stimulates milk production in goats, and dexamethasone has also been shown to reduce plasma IGF1 concentrations in cows. These results may suggest that GCs inhibit milk yields. However, after acute stress, the time for plasma cortisol within-day variation is not consistent with that for milk cortisol, and there was no within-day difference in SCC, milk composition, and overall milk yield other than blood glucose, which increased between the acute stress group and the control group (Romero et al., Citation2015). These results demonstrated that endocrine GC release affects milk yields, but these controversial results of GCs on goat and cow milk production may be due to different testing times, animal ages, and parities.
3. Function of glucocorticoids in relation to mastitis
Mastitis is a common mammary infectious disease usually occurring in dairy animals such as cows (Runciman et al., Citation2010), goats (Gabli et al., Citation2019), and camels (Hadef et al., Citation2022). Animals suffering from this disease often present with a range of clinical and subclinical symptoms: clinical mastitis can be characterized by hardening and swelling of the breast, pain on palpation, and visible changes in the color and texture of the milk, while subclinical mastitis presents mammary inflammation with nonvisible signs and can be detected by specific tests such as the California mastitis test (CMT), somatic cell count (SCC), and microbiological examination (Abdel Gadir Atif et al., Citation2006; Gao et al., Citation2019). Animal mastitis has been considered one of the major limitations to animal farming and can cause great economic loss and impact public health. The major pathogens causing mastitis are environmental bacteria such as Staphylococcus aureus, Streptococcus agalactiae, and Escherichia coli (Dalanezi et al., Citation2020). The study reported that endocrine GC changes occurred mainly in the early phases of inflammation, but the accompanying increase in rectal temperature occurred slightly later and lasted until the end of the observation period (Gross et al., Citation2021). The anti-inflammatory properties of dexamethasone have also been verified in numerous investigations (Lohuis et al., Citation1988; Zhu et al., Citation1998; Purba et al., Citation2020). When mastitis occurred, intramuscular dexamethasone reduced local clinical symptoms of inflammation, and dexamethasone-treated cows demonstrated reduced neutrophilia and reduced milk loss (Lohuis et al., Citation1988).
3.1. The role of glucocorticoids in treating mastitis
Inflammation in cow mastitis is mediated by inflammatory cytokines such as IL-1β, TNF-α, IL-6 and other chemokines, which are affected by the inactivation of transcription factors, such as NF-κB or STAT6 (Ershun et al., Citation2014; Akhtar et al., Citation2020). Natural and synthetic GCs have long been used as anti-inflammatory and immunosuppressive agents to treat acute and chronic inflammation as well as certain leukemias and in immunosuppressive regimens in humans, small animals, or ruminants (Stein et al., Citation2010; Olivry et al., Citation2015; Plessers et al., Citation2015; Formica et al., Citation2019; Horby et al., Citation2021; Koster et al., Citation2022). The mechanism by which GCs suppress inflammatory responses is through inhibiting the synthesis of proinflammatory cytokines and inflammatory cytokines and altering immune cell function (Barnes, Citation1998; Shimba & Ikuta, Citation2020). It has been reported that GCs inhibit the production of cytokines in a broad range and have a selective influence on leukocyte subsets in bovines: peripheral lymphocytes of dexamethasone-treated calves showed substantial inhibition of the mRNA and protein expression of IFN-γ, IL-1b, IL-2, IL-3, IL-4, IL-8 and tumor necrosis factor-a (TNF-α) (Spies et al., Citation2010; Maeda et al., Citation2011); treatment with dexamethasone induced depletion of γδ T-cell subsets and increased the number of CD14+ cells, whereas it had no effect on the number of CD8+ and CD14+ αβ T cells in bovines (Moiré et al., Citation2002; Menge & Dean-Nystrom, Citation2008). These studies indicate that GCs may regulate proinflammatory cytokines and leukocytes to affect the immune system.
Several GCs, such as prednisolone, dexamethasone, and isoflurane, have been applied or investigated as an adjacent treatment to antibiotics in treating cow mastitis (Lohuis et al., Citation1988; Ziv et al., Citation1998; Wagner & Apley, Citation2004). The researchers found that the combination use of ampicillin, colistin, dexamethasone, or cefapirin with prednisolone has a synergistic effect on alleviating clinical symptoms, reducing leukocyte or bacteria density in tissue and milk, and accelerating the recovery of milk quality of endotoxin-induced or E. coli-induced mastitis (Ziv et al., Citation1998; Sipka et al., Citation2013); In addition, intramuscular administration of dexamethasone in Escherichia coli mastitis reduced milk production loss and clinical signs of inflammation and diminished inhibition of rumen amplitude (Lohuis et al., Citation1988); however, a more recent study showed no effects when endotoxin-induced mastitis was treated with isoflupredone alone (Wagner & Apley, Citation2004). The blood milk barrier (BMB) in the mammary gland allows the exchange of components between blood and milk to provide the optimal milk composition for the offspring (Wellnitz & Bruckmaier, Citation2021). However, the integrity of the BMB is reduced during mastitis, which causes milk loss and an increased degree of inflammation since immune cells, milk constituents, and bacteria can transfer between blood and milk. GCs such as prednisolone have been reported to accelerate the recovery of BMB integrity, reduce SCC levels and the number of migrating immune cells, and block the production of proinflammatory cytokines in cows challenged with LPS or lipoteichoic acid, suggesting their function in restoring milk quality (Stahn et al., Citation2007; Wall et al., Citation2016). These studies indicate that some GCs, such as prednisolone and dexamethasone, used alone or with other drugs could alleviate the cell response or clinical symptoms of mastitis caused by endotoxin or bacteria.
3.2. Endocrine glucocorticoid response to metabolic and inflammatory challenges in dairy cows
Cortisol releases in an episodic and pulsatile manner underlying a circadian rhythm in normal cows. Intramammary LPS administration (mastitis model) usually stimulates the immune system, accompanied by distinct metabolic and endocrine changes. The HPA axis is known to endogenously respond to various types of metabolic and inflammatory stressors, and the release of cortisol or corticosterone from this system is a primary adaptive regulator in response to adverse conditions such as metabolic stimulation or inflammation (Beerda et al., Citation2004). In early lactating dairy cows, the occurrence of negative energy balance (NEB) is characterized by low plasma concentrations of glucose and elevated concentrations of β-hydroxybutyrate (BHBA) (Athanasiou & Phillips, Citation1978; Gross et al., Citation2011). Beerda et al. (Citation2004) also found associations between the extent of NEB (which is linked to concentrations of glucose and BHBA) and adrenocortical reactivity (Beerda et al., Citation2004) in this stage. In mid-lactation dairy cows, Gross et al. (Citation2015) found that the manipulated decrease and increase in the plasma concentration of glucose and BHBA, respectively, could elevate cortisol secretion and decrease its peak length; at the same time, when these metabolites maintained dairy cows were challenged with intramammary inflammation, the pulsatile nature of cortisol diminished, and the baseline cortisol concentration was fond to be greater in BHBA and in euglycemic maintained cows than in hypoglycemic maintained cows (Gross et al., Citation2015). In ewes, insulin-induced hypoglycemia affected the release of cortisol (Dobson & Smith, Citation2000). These studies emphasize the regulatory role of metabolites on cortisol release in cows. Additionally, cortisol reacts endogenously when the mammary gland is challenged by inflammatory stress. Plasma cortisol and insulin increased in mid-lactating dairy cows after intramammary LPS stimulation on insulin or glucose and insulin treatment (Vernay et al., Citation2012); concentrations of total cortisol, corticosterone, and proportion of free cortisol to total cortisol also increased until 3.5 h after intramammary LPS challenge, while metabolic adaptation indicators of insulin, glucagon, and glucose increased 5 h after LPS challenge (Gross et al., Citation2021). In Escherichia coli-induced mastitis in cows, the serum cortisol response was prolonged in severely mastitic cows but then decreased after the second challenge (Hirvonen et al., Citation1999). However, the concentration of cortisol was reduced during acute stress in early lactating cows suffering from severe inflammatory status with ACTH challenge (Trevisi et al., Citation2013).
More importantly, not only in the mammary gland but also studies of the body as a whole when challenged by various stresses have illustrated the important role of cortisol in the stress response. Acute stress usually causes cortisol release and reduces milk yields, while chronic stress often affects animal welfare and is difficult to detect using circulating or salivary cortisol levels (Friend et al., Citation1985; Mormède et al., Citation2007). When the source of stress is chronic inflammation, ACTH plays primary roles in regulating adrenal function, while during chronic inflammatory stress, a study found that interleukin-4 (IL-4) could augment the ACTH-stimulated release of cortisol (Prodan et al., Citation2019). Due to this, parameters of hair cortisol have been verified as an indicator of chronic stress under different environments in Holstein cows (Shi et al., Citation2021; Grelet et al., Citation2022). These studies suggest that endocrine GC levels are usually enhanced or weakened by metabolic and inflammatory stress, and testing tissue or hair cortisol levels may help us understand the health condition of animals under different environmental stimuli.
4. Cross-talk of endocrine glucocorticoids with other hormones in the regulation of mastitis or milk yields
GCs are equally important for their regulatory effects due to their interactions with other hormones. Cortisol also stimulates hepatic gluconeogenesis and elevates GC concentrations to ensure the availability of nutrients (e.g. glucose), particularly in tissues (e.g. fat and muscle tissue) when insulin resistance occurs. During lipopolysaccharide-induced mastitis, cortisol release could lead to insulin resistance, and the immune system is guaranteed to support nutrients more preferentially. Cortisol degradation is associated with insulin sensitivity, and cortisol metabolism is high during insulin resistance (Tomlinson et al., Citation2008). However, homeostasis in vivo can be restored as quickly as possible by inducing insulin resistance despite excessive cortisol release due to lipopolysaccharide-induced mastitis (Gross et al., Citation2015; Kobayashi et al., Citation2017a). In a gram-negative mastitis study in cows, animals with lower thyroxine (T4) 2 days prepartum developed gram-negative mastitis, and the variance in T4 levels analyzed with multiregression was positively associated with triiodothyronine (T3) and IGF-1 but negatively associated with cortisol (Nikolić et al., Citation2003). Serum cortisol in Japanese Black cattle with haplotype C of the GH gene polymorphism tended to be higher when the animals suffered severe confinement in a race, which suggests that GH concentration may affect stress responses and cortisol release (Tachi et al., Citation2014). However, although GH affects milk production, administration of exogenous bovine GH to Holstein cows in early and late lactation could increase milk production but did not affect plasma concentrations of glucose, insulin, and cortisol (Peel et al., Citation1983). In goats, an immediate increase in cortisol induced by administration of ACTH did not affect IGF-1 release, milk yields, and cell proliferation rates, while cortisol addition in vitro increased gene expression of GH and IGF receptors (Bomfim et al., Citation2018). ACTH administration and cortisol release accelerated goat mammary involution during the early dry-off period (Manica et al., Citation2022). These reports demonstrated a complex interaction of GCs with other hormones in regulating mammary disease or milk yields.
5. Future work
In conclusion, GCs play a role in mammary gland development, affect milk production and milk components in response to long external stress, and could treat mastitis in ruminant animals (). To accelerate the development of the modern domestic animal industries in the next few years, we could take measures to prevent excessive stress to maintain the endocrine balance and inhibit the excess release of cortisol in the animal to ensure mammary gland development and lactation. We can also explore new artificial GCs and their appropriate administration methods to treat cow mastitis. However, the mechanism of GCs in mammary gland development and milk secretion has not been fully studied. Future research could focus on the mechanism of GCs in regulating MECs in vitro in regard to mammary gland development, lactation and mastitis with advanced technology such as high-throughput sequencing, gene editing, or single-cell sequencing. This review provides new ideas for the future use of GCs on milk production and mammary disease in ruminant animals.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Abdel Gadir Atif, E., Hildebrandt, G., Kleer, J. N., Molla, B., Kyule, M. N., & Baumann, M. P. (2006). Comparison of California Mastitis Test (CMT), Somatic Cell Counts (SCC) and bacteriological examinations for detection of camel (Camelus dromedarius) mastitis in Ethiopia. Berliner Und Munchener Tierarztliche Wochenschrift, 119(1–2), 1–9.
- Akhtar, M., Guo, S., Guo, Y. F., Zahoor, A., Shaukat, A., Chen, Y., Umar, T., Deng, P. G., & Guo, M. (2020). Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Tropica, 207, 105458. https://doi.org/10.1016/j.actatropica.2020.105458
- Arriza, J. L., Weinberger, C., Cerelli, G., Glaser, T. M., Handelin, B. L., Housman, D. E., & Evans, R. M. (1987). Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science (New York, N.Y.), 237(4812), 268–275. https://doi.org/10.1126/science.3037703
- Athanasiou, V. N., & Phillips, R. W. (1978). Stability of plasma metabolites and hormones in parturient dairy cows. American Journal of Veterinary Research, 39(6), 953–956.
- Barnes, P. J. (1998). Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clinical Science (London, England: 1979), 94(6), 557–572. https://doi.org/10.1042/cs0940557
- Baxter, E. M., Hall, S. A., Farish, M., Donbavand, J., Brims, M., Jack, M., Lawrence, A. B., & Camerlink, I. (2023). Piglets’ behaviour and performance in relation to sow characteristics. Animal : An International Journal of Animal Bioscience, 17(2), 100699. https://doi.org/10.1016/j.animal.2022.100699
- Beerda, B., Kornalijnslijper, J. E., van der Werf, J. T., Noordhuizen-Stassen, E. N., & Hopster, H. (2004). Effects of milk production capacity and metabolic status on HPA function in early postpartum dairy cows. Journal of Dairy Science, 87(7), 2094–2102. https://doi.org/10.3168/jds.S0022-0302(04)70027-2
- Bomfim, G. F., Merighe, G. K. F., de Oliveira, S. A., & Negrao, J. A. (2018). Effect of acute stressors, adrenocorticotropic hormone administration, and cortisol release on milk yield, the expression of key genes, proliferation, and apoptosis in goat mammary epithelial cells. Journal of Dairy Science, 101(7), 6486–6496. https://doi.org/10.3168/jds.2017-14123
- Bomfim, G. F., Merighe, G. K. F., de Oliveira, S. A., & Negrao, J. A. (2022). Acute and chronic effects of cortisol on milk yield, the expression of key receptors, and apoptosis of mammary epithelial cells in Saanen goats. Journal of Dairy Science, 105(1), 818–830. https://doi.org/10.3168/jds.2021-20364
- Borellini, F., & Oka, T. (1989). Growth control and differentiation in mammary epithelial cells. Environmental Health Perspectives, 80, 85–99. https://doi.org/10.1289/ehp.898085
- Caparros-Gonzalez, R. A., Romero-Gonzalez, B., Gonzalez-Perez, R., Lara-Cinisomo, S., Martin-Tortosa, P. L., Oliver-Roig, A., & Peralta-Ramirez, M. I. (2019). Maternal and neonatal hair cortisol levels and psychological stress are associated with onset of secretory activation of human milk production. Advances in Neonatal Care: Official Journal of the National Association of Neonatal Nurses, 19(6), E11–E20. https://doi.org/10.1097/ANC.0000000000000660
- Cowie, A. T., Tindal, J. S., & Yokoyama, A. (1966). The induction of mammary growth in the hypophysectomized goat. The Journal of Endocrinology, 34(2), 185–195. https://doi.org/10.1677/joe.0.0340185
- Dalanezi, F. M., Joaquim, S. F., Guimarães, F. F., Guerra, S. T., Lopes, B. C., Schmidt, E. M. S., Cerri, R. L. A., & Langoni, H. (2020). Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. Journal of Dairy Science, 103(4), 3648–3655. https://doi.org/10.3168/jds.2019-16841
- de Kloet, E. R., Meijer, O. C., de Nicola, A. F., de Rijk, R. H., & Joëls, M. (2018). Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Frontiers in Neuroendocrinology, 49, 124–145. https://doi.org/10.1016/j.yfrne.2018.02.003
- Díaz, J. R., Alejandro, M., Romero, G., Moya, F., & Peris, C. (2013). Variation in milk cortisol during lactation in Murciano-Granadina goats. Journal of Dairy Science, 96(2), 897–905. Research Support, Non-U S Gov’t) https://doi.org/10.3168/jds.2012-5614
- Dobson, H., & Smith, R. F. (2000). What is stress, and how does it affect reproduction? Animal Reproduction Science, 60-61, 743–752. https://doi.org/10.1016/s0378-4320(00)00080-4
- Ershun, Z., Yunhe, F., Zhengkai, W., Yongguo, C., Naisheng, Z., & Zhengtao, Y. (2014). Cepharanthine attenuates lipopolysaccharide-induced mice mastitis by suppressing the NF-κB signaling pathway. Inflammation, 37(2), 331–337. https://doi.org/10.1007/s10753-013-9744-6
- Formica, F. A., Barreto, G., & Zenobi-Wong, M. (2019). Cartilage-targeting dexamethasone prodrugs increase the efficacy of dexamethasone. Journal of Controlled Release: Official Journal of the Controlled Release Society, 295, 118–129. https://doi.org/10.1016/j.jconrel.2018.12.025
- Friend, T. H., Dellmeier, G. R., & Gbur, E. E. (1985). Comparison of four methods of calf confinement. I. Physiology. Journal of Animal Science, 60(5), 1095–1101. https://doi.org/10.2527/jas1985.6051095x
- Gabli, Z., Djerrou, Z., Gabli, A. E., & Bensalem, M. (2019). Prevalence of mastitis in dairy goat farms in Eastern Algeria. Veterinary World, 12(10), 1563–1572. https://doi.org/10.14202/vetworld.2019.1563-1572
- Gao, X., Fan, C., Zhang, Z., Li, S., Xu, C., Zhao, Y., Han, L., Zhang, D., & Liu, M. (2019). Enterococcal isolates from bovine subclinical and clinical mastitis: Antimicrobial resistance and integron-gene cassette distribution. Microbial Pathogenesis, 129, 82–87. https://doi.org/10.1016/j.micpath.2019.01.031
- Good, T. C., Harris, K. K., & Ihunnah, C. A. (2005). Corticosteroids as potential mechanism regulating variability in reproductive success in monogamous oldfield mice (Peromyscus polionotus). Physiology & Behavior, 86(1-2), 96–102. https://doi.org/10.1016/j.physbeh.2005.06.030
- Grelet, C., V. Vanden Dries, J. Leblois, J. Wavreille, L. Mirabito, H. Soyeurt, S. Franceschini, N. Gengler, Y. Brostaux, C. HappyMoo, and F. Dehareng. 2022. Identification of chronic stress biomarkers in dairy cows. Animal: An International Journal of Animal bioscience 16(5):100502. https://doi.org/10.1016/j.animal.2022.100502
- Gross, J. J., Schwinn, A. C., & Bruckmaier, R. M. (2021). Free and bound cortisol, corticosterone, and metabolic adaptations during the early inflammatory response to an intramammary lipopolysaccharide challenge in dairy cows. Domestic Animal Endocrinology, 74, 106554. https://doi.org/10.1016/j.domaniend.2020.106554
- Gross, J., van Dorland, H. A., Bruckmaier, R. M., & Schwarz, F. J. (2011). Performance and metabolic profile of dairy cows during a lactational and deliberately induced negative energy balance with subsequent realimentation. Journal of Dairy Science, 94(4), 1820–1830. https://doi.org/10.3168/jds.2010-3707
- Gross, J. J., Wellnitz, O., & Bruckmaier, R. M. (2015). Cortisol secretion in response to metabolic and inflammatory challenges in dairy cows. Journal of Animal Science, 93(7), 3395–3401. https://doi.org/10.2527/jas.2015-8903
- Hadef, L., Hamad, B., & Aggad, H. (2022). Risk factors associated with subclinical mastitis and its effect on physico-mineral features of camel milk. Tropical Animal Health and Production, 54(4), 224. https://doi.org/10.1007/s11250-022-03220-9
- Hirvonen, J., Eklund, K., Teppo, A. M., Huszenicza, G., Kulcsar, M., Saloniemi, H., & Pyörälä, S. (1999). Acute phase response in dairy cows with experimentally induced Escherichia coli mastitis. Acta Veterinaria Scandinavica, 40(1), 35–46. Research Support, Non-U S Gov’t) https://doi.org/10.1186/BF03547039
- Homer, H. C., Packan, D. R., & Sapolsky, R. M. (1990). Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology, 52(1), 57–64. https://doi.org/10.1159/000125539
- Hong, H., Lee, E., Lee, I. H., & Lee, S. R. (2019). Effects of transport stress on physiological responses and milk production in lactating dairy cows. Asian-Australasian Journal of Animal Sciences, 32(3), 442–451. https://doi.org/10.5713/ajas.18.0108
- Hooper, H. B., Dos Santos Silva, P., de Oliveira, S. A., Merighe, G. K. F., Titto, C. G., & Negrão, J. A. (2021). Long-term heat stress at final gestation: physiological and heat shock responses of Saanen goats. International Journal of Biometeorology, 65(12), 2123–2135. Randomized Controlled Trial, Veterinary) https://doi.org/10.1007/s00484-021-02175-0
- Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., Linsell, L., Staplin, N., Brightling, C., Ustianowski, A., Elmahi, E., Prudon, B., Green, C., Felton, T., Chadwick, D., Rege, K., Fegan, C., Chappell, L. C., Faust, S. N., Jaki, T., … Landray, M. J. (2021). Dexamethasone in hospitalized patients with Covid-19. The New England Journal of Medicine, 384(8), 693–704. https://doi.org/10.1056/NEJMoa2021436
- Horváth, K. M., Bánky, Z., Tóth, B. E., Halász, B., & Nagy, G. M. (2001). Effect of adrenalectomy and dexamethasone treatment on prolactin secretion of lactating rats. Brain Research Bulletin.
- Kendrick, S. F., Henderson, E., Palmer, J., Jones, D. E., & Day, C. P. (2010). Theophylline improves steroid sensitivity in acute alcoholic hepatitis. Hepatology (Baltimore, Md.), 52(1), 126–131. https://doi.org/10.1002/hep.23666
- Kingsley-Kallesen, M., Mukhopadhyay, S. S., Wyszomierski, S. L., Schanler, S., Schutz, G., & Rosen, J. M. (2002). The mineralocorticoid receptor may compensate for the loss of the glucocorticoid receptor at specific stages of mammary gland development. Molecular Endocrinology (Baltimore, Md.), 16(9), 2008–2018. https://doi.org/10.1210/me.2002-0103
- Kobayashi, K., Oyama, S., Kuki, C., Tsugami, Y., Matsunaga, K., Suzuki, T., & Nishimura, T. (2017a). Distinct roles of prolactin, epidermal growth factor, and glucocorticoids in beta-casein secretion pathway in lactating mammary epithelial cells. Molecular and Cellular Endocrinology, 440, 16–24. https://doi.org/10.1016/j.mce.2016.11.006
- Kobayashi, K., Oyama, S., Kuki, C., Tsugami, Y., Matsunaga, K., Suzuki, T., & Nishimura, T. (2017b). Distinct roles of prolactin, epidermal growth factor, and glucocorticoids in β-casein secretion pathway in lactating mammary epithelial cells. Molecular and Cellular Endocrinology, 440, 16–24. https://doi.org/10.1016/j.mce.2016.11.006
- Kobayashi, K., Tsugami, Y., Matsunaga, K., Oyama, S., Kuki, C., & Kumura, H. (2016). Prolactin and glucocorticoid signaling induces lactation-specific tight junctions concurrent with beta-casein expression in mammary epithelial cells. Biochimica et Biophysica Acta, 1863(8), 2006–2016. https://doi.org/10.1016/j.bbamcr.2016.04.023
- Koster, M. J., Crowson, C. S., Giblon, R. E., Jaquith, J. M., Duarte-García, A., Matteson, E. L., Weyand, C. M., & Warrington, K. J. (2022). Baricitinib for relapsing giant cell arteritis: a prospective open-label 52-week pilot study. Annals of the Rheumatic Diseases, 81(6), 861–867. https://doi.org/10.1136/annrheumdis-2021-221961
- Kung, M. H., Lee, Y. J., Hsu, J. T., Huang, M. C., & Ju, Y. T. (2015). A functional study of proximal goat β-casein promoter and intron 1 in immortalized goat mammary epithelial cells. Journal of Dairy Science, 98(6), 3859–3875. (Research Support, Non-U. S Gov’t) https://doi.org/10.3168/jds.2014-9054
- Lewis, J. G., Bagley, C. J., Elder, P. A., Bachmann, A. W., & Torpy, D. J. (2005). Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chimica Acta; International Journal of Clinical Chemistry, 359(1-2), 189–194. https://doi.org/10.1016/j.cccn.2005.03.044
- Liu, X., Wang, Y., Tian, Y., Yu, Y., Gao, M., Hu, G., Su, F., Pan, S., Luo, Y., Guo, Z., Quan, F., & Zhang, Y. (2014). Generation of mastitis resistance in cows by targeting human lysozyme gene to β-casein locus using zinc-finger nucleases. Proc Biol Sci, 281(1780), 7.
- Lohuis, J. A., Van Leeuwen, W., Verheijden, J. H., Van Miert, A. S., & Brand, A. (1988). Effect of dexamethasone on experimental Escherichia coli mastitis in the cow. Journal of Dairy Science, 71(10), 2782–2789. https://doi.org/10.3168/jds.S0022-0302(88)79872-0
- Maeda, Y., Tanaka, R., Ohtsuka, H., Matsuda, K., Tanabe, T., & Oikawa, M. (2011). Comparison of the immunosuppressive effects of dexamethasone, flunixin meglumine and meloxicam on the in vitro response of calf peripheral blood mononuclear cells. The Journal of Veterinary Medical Science, 73(7), 957–960. https://doi.org/10.1292/jvms.10-0422
- Manica, E., Silva, P. D. S., Merighe, G. K. F., de Oliveira, S. A., Bomfim, G. F., & Negrão, J. A. (2022). Effect of experimental stress and cortisol release induced by ACTH administration on expression of key genes related to milk synthesis and apoptosis during mammary involution of Saanen goats. The Journal of Dairy Research, 89(4), 404–409. https://doi.org/10.1017/s0022029922000735
- McNew, J. A. (2008). Regulation of SNARE-mediated membrane fusion during exocytosis. Chemical Reviews, 108(5), 1669–1686. https://doi.org/10.1021/cr0782325
- Mehdid, A., Martí-De Olives, A., Fernández, N., Rodríguez, M., & Peris, C. (2019). Effect of stress on somatic cell count and milk yield and composition in goats. Research in Veterinary Science, 125, 61–70. https://doi.org/10.1016/j.rvsc.2019.05.015
- Menge, C., & Dean-Nystrom, E. A. (2008). Dexamethasone depletes gammadelta T cells and alters the activation state and responsiveness of bovine peripheral blood lymphocyte subpopulations. Journal of Dairy Science, 91(6), 2284–2298. https://doi.org/10.3168/jds.2007-0937
- Merlot, E., Meunier-Salaun, M. C., Peuteman, B., Pere, M. C., Louveau, I., Perruchot, M. H., Prunier, A., Gardan-Salmon, D., Gondret, F., & Quesnel, H. (2022). Improving maternal welfare during gestation has positive outcomes on neonatal survival and modulates offspring immune response in pigs. Physiology & Behavior, 249, 113751. https://doi.org/10.1016/j.physbeh.2022.113751
- Moiré, N., Roy, O., & Gardey, L. (2002). Effects of dexamethasone on distribution and function of peripheral mononuclear blood cells in pneumonic calves. Veterinary Immunology and Immunopathology, 87(3-4), 459–466. https://doi.org/10.1016/s0165-2427(02)00074-0
- Mormède, P., Andanson, S., Aupérin, B., Beerda, B., Guémené, D., Malmkvist, J., Manteca, X., Manteuffel, G., Prunet, P., van Reenen, C. G., Richard, S., & Veissier, I. (2007). Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiology & Behavior, 92(3), 317–339. https://doi.org/10.1016/j.physbeh.2006.12.003
- Mostyn, A., Pearce, S., Budge, H., Elmes, M., Forhead, A. J., Fowden, A. L., Stephenson, T., & Symonds, M. E. (2003). Influence of cortisol on adipose tissue development in the fetal sheep during late gestation. The Journal of Endocrinology, 176(1), 23–30. https://doi.org/10.1677/joe.0.1760023
- Motil, K. J., Thotathuchery, M., Montandon, C. M., Hachey, D. L., Boutton, T. W., Klein, P. D., & Garza, C. (1994). Insulin, cortisol and thyroid hormones modulate maternal protein status and milk production and composition in humans. The Journal of Nutrition, 124(8), 1248–1257. https://doi.org/10.1093/jn/124.8.1248
- Neville, M. C., McFadden, T. B., & Forsyth, I. (2002). Hormonal regulation of mammary differentiation and milk secretion. Journal of Mammary Gland Biology and Neoplasia, 7(1), 49–66. https://doi.org/10.1023/a:1015770423167
- Nikolić, J. A., Kulcsár, M., Kátai, L., Nedić, O., Jánosi, S., & Huszenicza, G. (2003). Periparturient endocrine and metabolic changes in healthy cows and in cows affected by mastitis. Journal of Veterinary Medicine. A, Physiology, Pathology, Clinical Medicine, 50(1), 22–29. https://doi.org/10.1046/j.1439-0442.2003.00500.x
- Olivry, T., DeBoer, D. J., Favrot, C., Jackson, H. A., Mueller, R. S., Nuttall, T., & Prélaud, P. (2015). Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Veterinary Research, 11(1), 210. https://doi.org/10.1186/s12917-015-0514-6
- Ollier, S., Beaudoin, F., Vanacker, N., Blouin, R., & Lacasse, P. (2016). Effect of reducing milk production using a prolactin-release inhibitor or a glucocorticoid on metabolism and immune functions in cows subjected to acute nutritional stress. Journal of Dairy Science, 100(7), 5782–5791. https://doi.org/10.3168/jds.2016-11711
- Ono, M., & Oka, T. (1980a). alpha-Lactalbumin-casein induction in virgin mouse mammary explants: dose-dependent differential action of cortisol. Science (New York, N.Y.), 207(4437), 1367–1369. https://doi.org/10.1126/science.6986657
- Ono, M., & Oka, T. (1980b). The differential actions of cortisol on the accumulation of alpha-lactalbumin and casein in midpregnant mouse mammary gland in culture. Cell, 19(2), 473–480. https://doi.org/10.1016/0092-8674(80)90522-x
- Peel, C. J., Fronk, T. J., Bauman, D. E., & Gorewit, R. C. (1983). Effect of exogenous growth hormone in early and late lactation on lactational performance of dairy cows. Journal of Dairy Science, 66(4), 776–782. https://doi.org/10.3168/jds.S0022-0302(83)81857-8
- Plessers, E., Watteyn, A., Wyns, H., Pardon, B., De Backer, P., & Croubels, S. (2015). Study of the immunomodulatory properties of gamithromycin and dexamethasone in a lipopolysaccharide inflammation model in calves. Research in Veterinary Science, 103, 218–223. https://doi.org/10.1016/j.rvsc.2015.10.014
- Ponchon, B., Zhao, X., Ollier, S., & Lacasse, P. (2017). Relationship between glucocorticoids and prolactin during mammary gland stimulation in dairy cows. Journal of Dairy Science, 100(2), 1521–1534. https://doi.org/10.3168/jds.2016-11490
- Prodan, N., Breisch, J., Hoopmann, M., Abele, H., Wagner, P., & Kagan, K. O. (2019). Dosing interval between mifepristone and misoprostol in second and third trimester termination. Archives of Gynecology and Obstetrics, 299(3), 675–679. https://doi.org/10.1007/s00404-018-5017-9
- Purba, F. Y., Nii, T., Yoshimura, Y., & Isobe, N. (2020). Translocation of intrauterine-infused bacterial lipopolysaccharides to the mammary gland in dexamethasone-treated goats. Reproduction in Domestic Animals = Zuchthygiene, 55(12), 1688–1697. https://doi.org/10.1111/rda.13820
- Reichardt, H. M., Horsch, K., Gröne, H. J., Kolbus, A., Beug, H., Hynes, N., & Schütz, G. (2001). Mammary gland development and lactation are controlled by different glucocorticoid receptor activities. European Journal of Endocrinology, 145(4), 519–527. https://doi.org/10.1530/eje.0.1450519
- Reiske, L., Schmucker, S., Pfaffinger, B., Weiler, U., Steuber, J., & Stefanski, V. (2020). Intravenous infusion of cortisol, adrenaline, or noradrenaline alters porcine immune cell numbers and promotes innate over adaptive immune functionality. Journal of Immunology (Baltimore, Md.: 1950), 204(12), 3205–3216. https://doi.org/10.4049/jimmunol.2000269
- Reul, J. M., & de Kloet, E. R. (1985). Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology, 117(6), 2505–2511. https://doi.org/10.1210/endo-117-6-2505
- Reul, J. M., Gesing, A., Droste, S., Stec, I. S., Weber, A., Bachmann, C., Bilang-Bleuel, A., Holsboer, F., & Linthorst, A. C. (2000). The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. European Journal of Pharmacology, 405(1-3), 235–249. https://doi.org/10.1016/s0014-2999(00)00677-4
- Romero, G., Restrepo, I., Muelas, R., Bueso-Ródenas, J., Roca, A., & Díaz, J. R. (2015). Within-day variation and effect of acute stress on plasma and milk cortisol in lactating goats. Journal of Dairy Science, 98(2), 832–839. (Research Support, Non-U. S Gov’t) https://doi.org/10.3168/jds.2014-8052
- Rosen, J. M., Wyszomierski, S. L., & Hadsell, D. (1999). Regulation of milk protein gene expression. Annual Review of Nutrition, 19(1), 407–436. https://doi.org/10.1146/annurev.nutr.19.1.407
- Rothman, J. E. (1994). Mechanisms of intracellular protein transport. Nature, 372(6501), 55–63. Review) https://doi.org/10.1038/372055a0
- Runciman, D. J., Malmo, J., & Deighton, M. (2010). The use of an internal teat sealant in combination with cloxacillin dry cow therapy for the prevention of clinical and subclinical mastitis in seasonal calving dairy cows. Journal of Dairy Science, 93(10), 4582–4591. https://doi.org/10.3168/jds.2009-2956
- Sangild, P. T., Diernaes, L., Christiansen, I. J., & Skadhauge, E. (1993). Intestinal transport of sodium, glucose and immunoglobulin in neonatal pigs. Effect of glucocorticoids. Experimental Physiology, 78(4), 485–497. https://doi.org/10.1113/expphysiol.1993.sp003700
- Sasano, H., Fukushima, K., Sasaki, I., Matsuno, S., Nagura, H., & Krozowski, Z. S. (1992). Immunolocalization of mineralocorticoid receptor in human kidney, pancreas, salivary, mammary and sweat glands: a light and electron microscopic immunohistochemical study. The Journal of Endocrinology, 132(2), 305–310. https://doi.org/10.1677/joe.0.1320305
- Shi, R., Dou, J., Liu, J., Sammad, A., Luo, H., Wang, Y., Guo, G., & Wang, Y. (2021). Genetic parameters of hair cortisol as an indicator of chronic stress under different environments in Holstein cows. Journal of Dairy Science, 104(6), 6985–6999. https://doi.org/10.3168/jds.2019-17856
- Shimba, A., & Ikuta, K. (2020). Control of immunity by glucocorticoids in health and disease. Seminars in Immunopathology, 42(6), 669–680. https://doi.org/10.1007/s00281-020-00827-8
- Sipka, A., Gurjar, A., Klaessig, S., Duhamel, G. E., Skidmore, A., Swinkels, J., Cox, P., & Schukken, Y. (2013). Prednisolone and cefapirin act synergistically in resolving experimental Escherichia coli mastitis. Journal of Dairy Science, 96(7), 4406–4418. https://doi.org/10.3168/jds.2012-6455
- Spies, C. M., Gaber, T., Hahne, M., Naumann, L., Tripmacher, R., Schellmann, S., Stahn, C., Burmester, G. R., Radbruch, A., & Buttgereit, F. (2010). Rimexolone inhibits proliferation, cytokine expression and signal transduction of human CD4+ T-cells. Immunology Letters, 131(1), 24–32. Research Support, Non-U S Gov’t) https://doi.org/10.1016/j.imlet.2010.03.009
- Stahn, C., Löwenberg, M., Hommes, D. W., & Buttgereit, F. (2007). Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Molecular and Cellular Endocrinology, 275(1-2), 71–78. https://doi.org/10.1016/j.mce.2007.05.019
- Stein, T. J., Pellin, M., Steinberg, H., & Chun, R. (2010). Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. Journal of the American Animal Hospital Association, 46(6), 413–417. https://doi.org/10.5326/0460413
- Stewart, H. J., & Thompson, G. E. (1984). Adrenocorticotrophic hormone stimulation of mammarysecretion in lactating goats independent of increased mammary uptake of glucose. J. Endocr.
- Svennersten-Sjaunja, K., & Olsson, K. (2005). Endocrinology of milk production. Domestic Animal Endocrinology, 29(2), 241–258. https://doi.org/10.1016/j.domaniend.2005.03.006
- Tachi, N., Tanaka, S., Ardiyanti, A., Katoh, K., & Sato, S. (2014). Bovine growth hormone gene polymorphism affects stress response in Japanese Black cattle. Animal Science Journal = Nihon Chikusan Gakkaiho, 85(6), 722–728. https://doi.org/10.1111/asj.12212
- Thatcher, W. W., & Tucker, H. A. (1970). Lactational performance of rats injected with oxytocin, cortisol-21-acetate, prolactin and growth hormone during prolonged lactation. Endocrinology, 86(2), 237–240. https://doi.org/10.1210/endo-86-2-237
- Thayer, Z. M., Agustin Bechayda, S., & Kuzawa, C. W. (2018). Circadian cortisol dynamics across reproductive stages and in relation to breastfeeding in the Philippines. American Journal of Human Biology : The Official Journal of the Human Biology Council, 30(4), e23115. https://doi.org/10.1002/ajhb.23115
- Tomlinson, J. W., Finney, J., Hughes, B. A., Hughes, S. V., & Stewart, P. M. (2008). Reduced glucocorticoid production rate, decreased 5alpha-reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes, 57(6), 1536–1543. https://doi.org/10.2337/db08-0094
- Topper, Y. J., & Freeman, C. S. (1980). Multiple hormone interactions in the developmental biology of the mammary gland. Physiological Reviews, 60(4), 1049–1106. https://doi.org/10.1152/physrev.1980.60.4.1049
- Trevisi, E., Bertoni, G., Lombardelli, R., & Minuti, A. (2013). Relation of inflammation and liver function with the plasma cortisol response to adrenocorticotropin in early lactating dairy cows. Journal of Dairy Science, 96(9), 5712–5722. https://doi.org/10.3168/jds.2012-6375
- Tuncel, U., Turan, A., Bayraktar, M. A., Erkorkmaz, U., & Kostakoglu, N. (2013). Efficacy of dexamethasone with controlled hypotension on intraoperative bleeding, postoperative oedema and ecchymosis in rhinoplasty. Journal of Cranio-Maxillo-Facial Surgery : official Publication of the European Association for Cranio-Maxillo-Facial Surgery, 41(2), 124–128. https://doi.org/10.1016/j.jcms.2012.06.003
- van der Kolk, J. H. (1990). The bovine pituitary-adrenocortical axis and milk yield. The Veterinary Quarterly, 12(2), 114–120. https://doi.org/10.1080/01652176.1990.9694253
- Vernay, M. C., Wellnitz, O., Kreipe, L., van Dorland, H. A., & Bruckmaier, R. M. (2012). Local and systemic response to intramammary lipopolysaccharide challenge during long-term manipulated plasma glucose and insulin concentrations in dairy cows. Journal of Dairy Science, 95(5), 2540–2549. Research Support, Non-U S Gov’t) https://doi.org/10.3168/jds.2011-5188
- VirginJr, C. E., Ha, T. P. T., Packan, D. R., Tombaugh, G. C., Yang, S. H., Homer, H. C., & Sapolsky, R. M. (1991). Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. Journal of Neurochemistry, 57(4), 1422–1428. https://doi.org/10.1111/j.1471-4159.1991.tb08309.x
- Wagner, S. A., & Apley, M. D. (2004). Effects of two anti-inflammatory drugs on physiologic variables and milk production in cows with endotoxin-induced mastitis. American Journal of Veterinary Research, 65(1), 64–68. Comparative Study https://doi.org/10.2460/ajvr.2004.65.64Research Support, Non-U S Gov’t)
- Wall, S. K., Hernandez-Castellano, L. E., Ahmadpour, A., Bruckmaier, R. M., & Wellnitz, O. (2016). Differential glucocorticoid-induced closure of the blood-milk barrier during lipopolysaccharide- and lipoteichoic acid-induced mastitis in dairy cows. Journal of Dairy Science, 99(9), 7544–7553. https://doi.org/10.3168/jds.2016-11093
- Wellnitz, O., & Bruckmaier, R. M. (2021). Invited review: The role of the blood-milk barrier and its manipulation for the efficacy of the mammary immune response and milk production. Journal of Dairy Science, 104(6), 6376–6388. https://doi.org/10.3168/jds.2020-20029
- Wolfgang Doppler, B. G. & Ball, R. K. (1989). Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat 13-casein gene promoter constructs in a mammary epithelial cell line. Biochemistry, 86(1), 104–108. https://doi.org/10.1073/pnas.86.1.104
- Yang, F., Ma, Q., Liu, Z., Li, W., Tan, Y., Jin, C., Ma, W., Hu, Y., Shen, J., Ohgi, K. A., Telese, F., Liu, W., & Rosenfeld, M. G. (2017). Glucocorticoid Receptor:MegaTrans Switching Mediates the Repression of an ERα-Regulated Transcriptional Program. Molecular Cell, 66(3), 321–331.e6. https://doi.org/10.1016/j.molcel.2017.03.019
- Zhu, Z., Jiang, W., & Thompson, H. J. (1998). Effect of corticosterone administration on mammary gland development and p27 expression and their relationship to the effects of energy restriction on mammary carcinogenesis. Carcinogenesis, 19(12), 2101–2106. (Research Support, U S Gov’t, P H S. https://doi.org/10.1093/carcin/19.12.2101
- Ziv, G., Shem-Tov, M., & Ascher, F. (1998). Combined effect of ampicillin, colistin and dexamethasone administered intramuscularly to dairy cows on the clinico-pathological course of E. coli-endotoxin mastitis. Veterinary Research, 29(1), 89–98.