Abstract
Studies show that prenatal maternal stress (PNMS) is related to risk for child autism, and to atypical amygdala functional connectivity in the autistic child. Yet, it remains unclear whether amygdala functional connectivity mediates the association between PNMS and autistic traits, particularly in young adult offspring. We recruited women who were pregnant during, or within 3 months of, the 1998 Quebec ice storm crisis, and assessed three aspects of PNMS: objective hardship (events experienced during the ice storm), subjective distress (post-traumatic stress symptoms experienced as a result of the ice storm) and cognitive appraisal. At age 19, 32 young adults (21 females) self-reported their autistic-like traits (i.e., aloof personality, pragmatic language impairment and rigid personality), and underwent structural MRI and resting-state functional MRI scans. Seed-to-voxel analyses were conducted to map the amygdala functional connectivity network. Mediation analyses were implemented with bootstrapping of 20,000 resamplings. We found that greater maternal objective hardship was associated with weaker functional connectivity between the left amygdala and the right postcentral gyrus, which was then associated with more pragmatic language impairment. Greater maternal subjective distress was associated with weaker functional connectivity between the right amygdala and the left precentral gyrus, which was then associated with more aloof personality. Our results demonstrate that the long-lasting effect of PNMS on offspring autistic-like traits may be mediated by decreased amygdala-sensorimotor circuits. The differences between amygdala-sensory and amygdala-motor pathways mediating different aspects of PNMS on different autism phenotypes need to be studied further.
Introduction
Prenatal environmental exposures have long-lasting effects on offspring neurodevelopment (Lautarescu et al., Citation2020; van den Bergh et al., Citation2018). For example, childhood autism spectrum disorder (ASD) is associated with different types of prenatal maternal stress (PNMS): depression (Gao et al., Citation2015; Say et al., Citation2016; Zhang et al., Citation2010), bereavement (Class et al., Citation2014), life events (Beversdorf et al., Citation2005; Rai et al., Citation2012; Varcin et al., Citation2017), and hurricanes and tropical storms (Kinney et al., Citation2008).
The association between PNMS and child ASD may be the result of altered amygdala activity. The dysregulation of the fetal hypothalamic-pituitary-adrenal (HPA) axis may be one possible mechanism by which prenatal stress influences offspring physiological and psychological outcomes (Howland et al., Citation2017). The amygdala has been implicated in the regulation of the HPA axis in the following ways. During stress, corticotropin-releasing hormone (CRH) is synthesized in the paraventricular nucleus of the hypothalamus and released into the hypophyseal portal blood CRH binds to its receptors on pituitary corticotropes, stimulating the release of adrenocorticotrophic hormone that binds to its receptors in the adrenal cortex and that stimulates the release of glucocorticoids (Howland et al., Citation2017). CRH-producing neurons in the paraventricular nucleus of the hypothalamus are largely innervated by excitatory projections from the amygdala (Herman et al., Citation2005). It could also be plausible that prenatal stress operates directly on fetal neurodevelopment; stress stimulates the release of maternal glucocorticoids, and the elevated maternal glucocorticoid levels attenuate 11β-hydroxysteroid dehydrogenase type-2 expression and cause elevated fetal glucocorticoid load (Mandy & Nyirenda, Citation2018). The fetal amygdala is particularly sensitive to glucocorticoids given its high concentration of glucocorticoid receptors (Lautarescu et al., Citation2020).
It is evident that the amygdala is involved in the stress response and the regulation of stress memory (Roozendaal et al., Citation2009). Studies utilizing resting-state functional MRI (rs-fMRI) have shown the vulnerability of the offspring amygdala to PNMS exposure: prenatal maternal depression (Posner et al., Citation2016) and prenatal maternal life events (Humphreys et al., Citation2020) are associated with weaker resting-state functional connectivity (rs-FC) from the amygdala to the prefrontal cortex (PFC) in neonates.
Altered amygdala rs-FC has then been observed in individuals with ASD. Given social impairment as the core symptomatology of ASD, attention has been paid to the amygdala that plays a critical role in social information processing (Fishman et al., Citation2018). Studies using rs-fMRI have reported abnormalities in rs-FC from the amygdala to subcortical and cortical regions in individuals with ASD compared to controls, with lower rs-FC from the amygdala to the medial PFC, bilateral temporal lobes, striatum, thalamus, cingulate cortex, and cerebellum in preschool-aged children with ASD (Shen et al., Citation2016), with lower rs-FC from the amygdala to the thalamus and putamen in adolescents with ASD (Guo et al., Citation2016), and with lower amygdala-occipital rs-FC and higher amygdala-pericentral rs-FC in adolescents with ASD (Fishman et al., Citation2018). In addition, among the adolescents with ASD, the lower amygdala-thalamus/putamen rs-FC has been associated with more severe social deficits (Guo et al., Citation2016).
Unlike naturally-occurring life events (e.g., job loss or divorce), disasters tend to have sudden onsets and to be independent of the control of individuals (King & Laplante, Citation2015). Prenatal exposure to a natural disaster is a type of PNMS that is independent of potential confounders (e.g., maternal personality and socioeconomic status). In the case of the Project Ice Storm, the family socioeconomic status is uncorrelated with the degree of objective hardship (r = −0.03) and is only weakly correlated with the women’s subjective distress (r = 0.22) (Laplante et al., Citation2007).
In January 1998, three separate weather systems passed through southern Quebec producing five continuous days of freezing rain and leaving up to 100 mm of ice on the region. The weight of the ice toppled electrical poles and high-tension power lines and pylons leaving more than 1.5 million households without power for as long as 45 days in the coldest month of the year. With $1.5 billion in damages and more than 27 deaths, the 1998 ice storm is one of the worst disasters in Canadian history. In June 1998, we launched Project Ice Storm, in which we recruited women who gave birth in 1998, assessed three aspects of their stress caused by the ice storm, and prospectively followed up their children development. We found that prenatal maternal objective hardship and subjective distress were associated with autistic-like traits at age 6½ years as rated by their mothers, especially when exposure was in early pregnancy (Walder et al., Citation2014). At age 19, the children self-reported their symptoms of the broad autism phenotype (BAP) including aloof personality, pragmatic language impairment and rigid personality, and we found that different aspects of PNMS explain different phenotypes, with maternal objective hardship and cognitive appraisal related to aloof personality and rigid personality, and maternal subjective distress related to pragmatic language impairment (Li et al., Citation2023).
Given the vulnerability of the amygdala to PNMS, and given its possible involvement in ASD, atypical amygdala rs-FC may mediate the association between PNMS and ASD symptoms, although this has never been demonstrated. Here, we aimed to determine (1) amygdala rs-FC network in the young adults of mothers exposed to disaster-related PNMS; and (2) the extent to which the association between disaster-related PNMS and BAP is mediated by atypical amygdala rs-FC.
Methods
Participants
Project Ice Storm: Women with due dates between January 9, 1998 and December 31, 1998 were identified by their physicians who mailed the questionnaire to them on June 1, 1998. The initial Project Ice Storm cohort consisted of 176 women who responded to the first questionnaire and who agreed to further contact. These mother-child dyads were followed up, and the children’s development was assessed, periodically between the ages of 6 months and 19 years. At age 19, 33 youths completed the Broad Autism Phenotype Questionnaire (BAPQ); of those, 32 underwent structural MRI and rs-fMRI scans. The most common structural MRI sequence is the T1-weighted (T1w) scan, a 3D image of the entire brain that is represented in a volume of a given matrix. The most common functional MRI technique uses fluctuations of blood-oxygen-level-dependent (BOLD) contrast based on the magnetic susceptibility of hemoglobin. During rest (absence of tasks), participants were instructed to relax and not to think of anything in particular, and their level of spontaneous brain activity was measured, which was referred to as rs-fMRI (Glover, Citation2011).
Ethical approval
All phases of this study were approved by the Research Ethics Board of Douglas Hospital Research Center in Montreal, Canada. All participants gave written informed consent.
Prenatal maternal stress
In June 1998, three aspects of PNMS were measured by the postal questionnaire: objective hardship (i.e., events experienced during the ice storm), subjective distress (i.e., post-traumatic stress symptoms experienced as a result of the ice storm), and cognitive appraisal (i.e., judging the consequences of the ice storm). These three measures were only weakly correlated with each other (|r|s = 0.211 − 0.372). The severity of objective hardship experienced by pregnant women during January 1998 ice storm was assessed based on four dimensions of exposure: Threat (e.g., injuries), Loss (e.g., loss of personal income), Scope (e.g., duration without electricity), and Change (e.g., temporary shelter) (Bromet & Dew, Citation1995). There were eight points for each dimension, and a total score (Storm32) was calculated by summing scores across all four dimensions (Laplante et al., Citation2007). Storm32 has shown predictive validity by correlating significantly with a number of child outcomes including functional play development (Laplante et al., Citation2007) and cognitive and language development (King & Laplante, Citation2005) at 2 years of age.
Women’s subjective distress at the time of recruitment was assessed using a validated French version (Brunet et al., Citation2003) of the Impact of Event Scale—Revised (IES-R) (Weiss et al., Citation1997). This 22-item scale has been widely used for assessing the severity of post-traumatic stress disorder symptoms in the preceding week including three categories: Intrusive Thoughts, Hyperarousal, and Avoidance. The IES-R has also predicted a number of child behaviors including autistic-like traits at 6½ years (Walder et al., Citation2014) and externalizing behaviors via enlarged amygdala volume at 11½ years (Jones et al., Citation2019). In the current study, we used log-transformed values of the total score due to the skewed distribution.
Women’s cognitive appraisal of the ice storm was assessed using the following question: “Overall, what were the consequences of the ice storm on you and your family?”; the five response options were coded into three levels: “negative” (“-1”), “neutral” (“0”), and “positive” (“1”). Women’s negative cognitive appraisal has altered several child outcomes (e.g., BMI and C-peptide) via DNA methylation at 13½ years (Cao-Lei et al., Citation2016, Citation2018).
Broad autism phenotype
The BAPQ was specifically designed to assess autistic-like traits in non-clinical populations, and this self-report tool has good validity in clinical assessment of the broad autism phenotype (Sasson et al., Citation2013). The BAPQ includes 36 items assessing three domains: Aloof Personality (12 items), Pragmatic Language (12 items), and Rigid Personality (12 items). Each item is rated using a 6-point Likert scale ranging from “very rarely” (1) to “very often” (6) (Sasson et al., Citation2013). Cronbach’s alpha coefficient was 0.94 for the Aloof Personality subscale, 0.91 for the Rigid Personality subscale, 0.85 for the Pragmatic Language subscale and 0.95 across all 36 items. Aloof personality is defined as a lack of interest in, or enjoyment of, social interactions (corresponding to social deficits of autism). Pragmatic language impairment refers to deficits in the social aspects of language, resulting in difficulties communicating effectively or in holding a fluid, reciprocal conversation (corresponding to communication deficits of autism). Rigid personality is defined as little interest in change or difficulty adjusting to change (corresponding to restricted and repetitive behaviors of autism) (Hurley et al., Citation2007).
MRI data acquisition
MR images were acquired using a 3 T Siemens MAGNETOM Trio TIM Syngo MRI scanner, with a 12-channel head coil. Anatomical images were obtained using a T1w Magnetization Prepared Rapid Gradient Echo sequence (192 slices; TR = 2400 ms; TE = 2.43 ms; slice thickness = 1 mm; flip angle = 8°; matrix = 256 × 256). Resting-sate functional images were acquired using a T2*-weighted echo-planar imaging sequence (42 slices; TR = 2600 ms; TE = 30 ms; flip angle = 90°; slice thickness = 3.4 mm; FoV = 218 mm, matrix = 64 × 64). Throughout the 5:01 min resting state scan, participants were instructed to lie still with their eyes open.
MRI data preprocessing
fMRIPrep 1.5.7 (Esteban et al., Citation2019) was used for preprocessing. T1w images were corrected for intensity non-uniformity with N4BiasFieldCorrection (Tustison et al., Citation2010), distributed with ANTs 2.2.0 (Avants et al., Citation2008), and used as T1w-reference throughout the workflow. The T1w-reference was then skull-stripped with a Nipype implementation of the antsBrainExtraction.sh workflow (from ANTs), using OASIS30ANTs as target template. Brain tissue segmentation of gray matter, white matter, and cerebrospinal fluid was performed on the brain-extracted T1w using fast FSL 5.0.9 (Zhang et al., Citation2001). Volume-based spatial normalization to the Montreal Neurological Institute (MNI) space was performed through nonlinear registration with antsRegistration (ANTs 2.2.0), using brain-extracted versions of both T1w reference and the T1w template. For each of the average BOLD runs found per subject, the following preprocessing was performed. First, a reference volume and its skull-stripped version were generated using a custom methodology of fMRIPrep. A deformation field to correct for susceptibility distortions was estimated based on fMRIPrep’s fieldmap-less approach. The deformation field is that resulting from co-registering the BOLD reference to the same-subject T1w-reference with its intensity inverted (Huntenburg, Citation2014; Wang et al., Citation2017). Registration is performed with antsRegistration (ANTs 2.2.0), and the process regularized by constraining deformation to be nonzero only along the phase-encoding direction, and modulated with an average fieldmap template (Treiber et al., Citation2016). Based on the estimated susceptibility distortion, a corrected echo-planar imaging reference was calculated for a more accurate co-registration with the anatomical reference. The BOLD reference was then co-registered to the T1w reference using flirt FSL 5.0.9 (Jenkinson & Smith, Citation2001) with the boundary-based registration (Greve & Fischl, Citation2009) cost-function. Co-registration was configured with nine degrees of freedom to account for distortions remaining in the BOLD reference. Head-motion parameters with respect to the BOLD reference (transformation matrices, and six corresponding rotation and translation parameters) are estimated before any spatiotemporal filtering using mcflirt FSL 5.0.9 (Jenkinson et al., Citation2002). The BOLD time-series were resampled onto their original, native space by applying a single, composite transform to correct for head-motion and susceptibility distortions. The BOLD time-series were then resampled into the MNI space. Automatic removal of motion artifacts using independent component analysis (ICA-AROMA) (Pruim et al., Citation2015) was performed on the spatially-normalized, preprocessed BOLD on MNI space time-series after removal of non-steady state volumes and spatial smoothing with an isotropic, Gaussian kernel of 6 mm full width at half maximum. In addition, we removed signals of white matter and cerebrospinal fluid from the BOLD time series and temporally bandpass filtering (> 0.01 Hz).
Resting-state functional connectivity analysis
Functional connectivity analyses were conducted using CONN functional connectivity toolbox 19b (Whitfield-Gabrieli & Nieto-Castanon, Citation2012). For amygdala (left and right) masks, we used the Harvard-Oxford structural atlas. In the individual level, seed-to-voxel analyses were implemented to examine the amygdala rs-FC network: Pearson’s correlation coefficients were calculated between BOLD time series extracted from each amygdala mask (left and right, separately) and the time courses of all voxels across the brain, for each participant. The resultant correlation coefficients were converted to normally distributed z-values. Group-level one-sample t tests were used to map amygdala rs-FC network. Positive mean z-values indicate positive functional connectivity between BOLD signals in the amygdala and a cluster (i.e., the two regions are likely to be active at the same time), while negative mean z-values indicate negative functional connectivity between BOLD signals in the amygdala and a cluster (i.e., when one region is active, the other one is not active and vice versa). Thresholds for all significant results were set to voxel-level uncorrected p < 0.001 for voxels, cluster-corrected p < 0.05. We extracted mean z-values between the amygdala and the clusters.
Mediation analysis
We extracted mean z-values between the left amygdala and the 23 clusters, and between the right amygdala and the 13 clusters (shown in Supplemental Table S1). Mediation analyses were conducted to determine the extent to which amygdala rs-FC network (left and right, separately) mediates the association between PNMS and BAPQ scores (i.e., total and three domain scores). The SPSS 26 PROCESS macro (Hayes, Citation2017) was used to calculate the mediation model, and the mediation Model 4 template is presented in Supplemental Figure S1. Given the small sample size, bootstrapping with 20,000 resamplings was used to determine the significance of the mediation effect. As shown in Supplemental Figure S2, PROCESS Model 4 included BAPQ scores as the outcome, PNMS as the predictor, and amygdala rs-FC (i.e., the mean z-values) as the mediator. A mediation effect was considered significant (p < 0.05) if 0 was not included in the bootstrap confidence interval.
Results
Sample description
The characteristics of the mother-child dyads are presented in . Regarding parental socioeconomic status at 1998 recruitment, 28.10% (9/32) were middle class; 46.90% (15/32) were upper middle class; and 25.00% (8/32) were upper class. None of the families were lower or lower middle class. On January 9th, 1998 (the date at which the storm peaked), 25.00% (8/32) of the mothers were exposed within the 3-month preconception period; 25.00% (8/32) were exposed in the 1st trimester of pregnancy; 28.10% (9/32) were exposed in the 2nd trimester; and 21.90% (7/32) were exposed in the 3rd trimester.
Table 1. Demographic characteristics of 32 mother-child dyads.
The young adult offspring (21 females; 11 males) were aged from 18.10 to 19.62 years. The Full-Scale intelligence quotient was above 90. Regarding the BAPQ, 15.63% (5/32) of the young adults met total score cutoffs; 43.75% (14/32) met aloof personality cutoffs; 21.88% (7/32) met pragmatic language impairment cutoffs; and 46.88% (15/32) met rigid personality cutoffs. Although these rates seem high, they are not dissimilar from those (25.30% met BAPQ total score cutoffs) found in a sample of 170 college students who completed the BAPQ (Dovgan & Villanti, Citation2021).
Amygdala functional connectivity network
The functional connectivity analysis results are presented in and and Supplemental Table S1. In total, we found that left and right amygdala functionally connected with multiple brain regions (shown in ). Supplemental Table S1 presents the details that the left amygdala connected with 23 clusters and that the right amygdala connected with 13 clusters. summarizes the results by grouping the clusters according to different functional networks including the emotional regulation network (bilateral insula and prefrontal cortices), the sensory and perceptual network (motor-sensory cortices, basal ganglia, parietal, temporal and occipital cortices, and cerebellum), and the emotional memory network (bilateral hippocampus, parahippocampus and temporal pole).
Figure 1. Amygdala functional connectivity network.
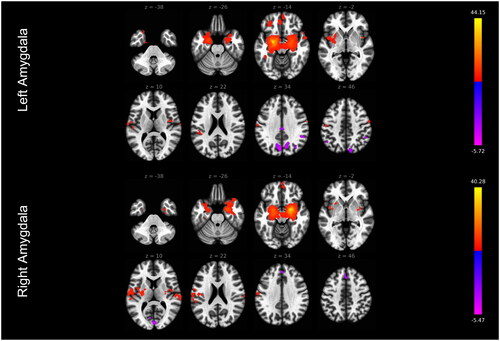
Table 2. The amygdala functional connectivity network.
Following up on the mean z-values from the 23 clusters for the left amygdala and from the 13 clusters for the right amygdala, 23 simple mediations were tested for the left amygdala rs-FC and 13 mediations were conducted for the right amygdala rs-FC. The significant mediations are presented below.
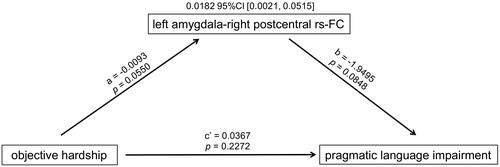
Decreased amygdala-somatosensory functional connectivity mediates the association between objective hardship and pragmatic language impairment
As illustrated in , higher maternal objective hardship was marginally significantly associated with weaker rs-FC from the left amygdala to the right postcentral gyrus. The weaker rs-FC in that connection was in turn marginally significantly associated with greater pragmatic language impairment (reflecting communication deficits). The mediation effect was significant (index of mediation = 0.0182 and bootstrap 95% CI = [0.0021, 0.0515]): the positive association between maternal objective hardship and pragmatic language impairment was mediated by decreased rs-FC from the left amygdala to the right postcentral gyrus.
Figure 2. Mediation effect of left amygdala-right postcentral rs-FC.
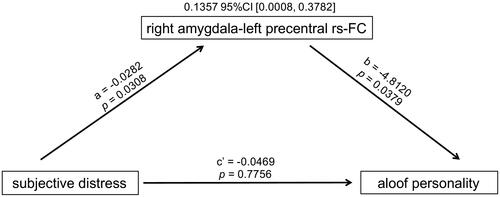
Decreased amygdala-somatomotor functional connectivity mediates the association between subjective distress and aloof personality
As shown in , higher maternal subjective distress was significantly associated with weaker rs-FC from the right amygdala to the left precentral gyrus. The weaker rs-FC in that connection was in turn significantly associated with greater aloof personality (reflecting social deficits). The mediation effect was significant (index of mediation = 0.1357 and bootstrap 95% CI = [0.0008, 0.3782]): the positive association between maternal subjective distress and aloof personality was mediated by decreased rs-FC from the right amygdala to the left precentral gyrus.
Figure 3. Mediation effect of right amygdala-left precentral rs-FC.
Discussion
Using the 1998 Quebec ice storm as a source of PNMS, the current study indicates the mediator role of amygdala rs-FC on the association between PNMS and autistic-like traits in young adults, such that weaker amygdala rs-FC with the sensorimotor regions, including the right postcentral gyrus and the left precentral gyrus, mediated associations between greater maternal objective hardship and subjective distress and more severe communication and social deficits in young adults.
First, we found that the amygdala was functionally connected to networks associated with emotional regulation, sensation and perception, and emotional memory in the young adults of mothers exposed to PNMS. These findings are consistent with the afferent and efferent anatomical connections of the amygdala observed in rats, cats and monkeys (Sah et al., Citation2003). These functional connections of the amygdala were also seen in a previous study of 6-month-old infants whose mothers had prenatal depressive symptoms (Qiu et al., Citation2015). Our results suggest that these positive functional connections of the amygdala, observed in the context of PNMS, may persist from infancy into adulthood.
Our results also raise a discussion on hemispheric differences of the amygdala contributing to the emotional regulation network and the sensory and perceptual network. Indeed, several hypotheses about putative differences between the left and right amygdala have been suggested (Baas et al., Citation2004). For example, one hypothesis suggests that the right amygdala is more active when the emotional property of the stimulus is visual in nature, whereas the left amygdala may be more active when the emotional property of a stimulus is learned through verbal communication (Phelps et al., Citation2001). In our study, the right amygdala, not the left amygdala, was more functionally connected to the visual cortex including the left/right intracalcarine cortex, right occipital pole and left lingual gyrus, which can be partly supported by this hypothesis. In addition, the laterality of the amygdala is supported by functional neuroimaging studies showing the left amygdala is more often activated than the right amygdala in top-down emotion generation, whereas both the left and right amygdala are activated in bottom-up emotion generation (Ochsner et al., Citation2009). Inconsistently, we found that the left and right amygdala were functionally connected to the frontal medial cortex and left/right paracingulate gyrus, respectively; these regions are involved in top-down emotion generation. Although our results cannot be thoroughly explained, the hypotheses extend our understanding of the neural basis of emotion processing.
The amygdala plays a critical role in both bottom-up and top-down emotion generation processes (McRae et al., Citation2012; Ochsner et al., Citation2009). The bottom-up process activates the amygdala and the parietal and occipital cortices implicated in detecting affectively arousing stimuli and in modulating their encoding into memory. The top-down process activates the amygdala, PFC and temporal cortices implicated in working memory and in the retrieval of information from semantic memory (Ochsner et al., Citation2009). In the current study, the amygdala functionally connected with sensory (i.e., somatosensory, visual, and auditory modalities) cortices and temporal cortices, forming the circuit for bottom-up emotion generation. In addition, we found that the amygdala was positively functionally connected with the PFC, forming the top-down emotion generation circuit.
Relevant to the bottom-up process of the amygdala, we found that, for Path a, maternal objective hardship and subjective distress were associated with decreased amygdala-somatosensory rs-FC and decreased amygdala-somatomotor rs-FC in young adult offspring. Neuroimaging research has shown that the amygdala and somatosensory cortex co-activate when processing emotional stimuli (Toschi et al., Citation2017; Van den Stock et al., Citation2011). Decreased amygdala-sensorimotor rs-FC has been observed in post-traumatic stress disorder (Belleau et al., Citation2020; Liu et al., Citation2021; Simmons et al., Citation2011), indicating reduced integration of the sensorimotor input to the amygdala circuits of stress regulation. For Path b, the decreased amygdala-somatosensory rs-FC and decreased amygdala-somatomotor rs-FC were associated with pragmatic language impairment and aloof personality, consistent with a previous study showing decreased amygdala-sensorimotor rs-FC associated with language and social deficits in adolescents and young adults with ASD (Fishman et al., Citation2018). Our finding that pragmatic language impairment and aloof personality were associated with the decreased amygdala-somatosensory rs-FC, supports the current consensus that atypical responsiveness to sensory stimuli is associated with core ASD symptoms (i.e., communication and social difficulties) (Mikkelsen et al., Citation2018). This is not surprising because atypical response to sensory stimuli may underlie difficulties understanding and communicating with other people who respond “typically” to the same stimuli (Hilton et al., Citation2010). It has been reported that approximately 90% of children with ASD experience atypical sensory responsiveness; as such, “hypo- or hyper-reactivity to sensory input” was added to the diagnostic criteria of ASD in the DSM-5 (American Psychiatric Association [APA], 2013; Marco et al., Citation2011).
Of note, we found that decreased amygdala-somatosensory rs-FC mediated the association between maternal objective hardship and pragmatic language impairment in young adult offspring, and that decreased amygdala-somatomotor rs-FC mediated the association between maternal subjective distress and aloof personality in the offspring. These findings suggest that different aspects of PNMS may have distinct correlates with respect to amygdala circuits and autistic-like phenotypes in young adulthood. Future studies with larger sample sizes are needed to clarify the differences between amygdala-somatosensory and amygdala-somatomotor pathways involved in the etiology of different autistic-like phenotypes.
Relevant to the top-down process of the amygdala, the current study found no associations between maternal objective hardship or subjective distress and amygdala-PFC rs-FC in the young adult offspring, which is inconsistent with a prior study showing that higher levels of prenatal maternal depressive symptoms were associated with greater amygdala-medial PFC functional connectivity in the infant offspring (Qiu et al., Citation2015). The discrepancy may be attributed to the different type of stress (objective hardship vs. depression) and offspring age (19 years old vs. 6 months). Different types of stress could have different and even opposing effects on offspring neurodevelopment; for example, unlike greater prenatal depressive symptoms associated with stronger amygdala-medial PFC rs-FC in infants (Qiu et al., Citation2015), in another study, more prenatal stressful events were associated with weaker amygdala-medial PFC rs-FC in infants (Humphreys et al., Citation2020).
Some limitations should be considered. First, after long-term follow-up in Project Ice Storm, attrition led to a small sample size at age 19 years, thus constraining statistical power; we used bootstrapping of 20,000 resamples to minimize this limitation. Second, to preserve statistical power, we did not correct the results for multiple comparisons, which included three aspects of PNMS, BAPQ scores (i.e., total and three domain scores), 23 clusters for the left amygdala rs-FC, and 13 clusters for the right amygdala rs-FC. Finally, the sample at age 19 was of higher socioeconomic status relative to the recruitment sample, which may limit the generalizability of the findings to lower socioeconomic status. Our study also had strengths. First, because we examined a sudden-onset natural disaster as the source of PNMS, exposure to objective hardship was independent of maternal characteristics. Second, we measured three different aspects of PNMS soon after the ice storm, and identified distinct associations with three different BAP domains. Finally, because the ice storm had a known and sudden onset, we were able to accurately measure timing of exposure to the ice storm.
Conclusions
Our study highlights amygdala-sensorimotor rs-FC as a mediator between disaster-related PNMS and autistic-like traits in young adulthood, suggesting long-lasting fetal programming of the transmission of maternal stress to the offspring. Our results also suggest that the differences between amygdala-somatosensory and amygdala-somatomotor circuits may explain associations between different aspects of PNMS and different autistic-like phenotypes.
Supplemental Material
Download MS Word (146 KB)Acknowledgements
We would like to thank all Project Ice Storm participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Xinyuan Li
Xinyuan Li, M.D., Ph.D. , completed her PhD in Neuroscience at McGill University in Montreal, specializing in the effect of prenatal maternal stress on offspring neurodevelopment. She is currently a postdoctoral fellow at Alberta Children's Hospital in Calgary, Canada.
Muhammad Naveed Iqbal Qureshi
Muhammad Naveed Iqbal Qureshi, Ph.D. (Biomedical Engineering, Computational Neuroscience, and Artificial Intelligence) completed post-doctoral fellowships at the Department of Psychiatry and Neurosurgery at McGill University, and at the Department of Neuroscience at The Hospital for Sick Children, University of Toronto, Canada.
David P. Laplante
David P. Laplante, Ph.D. is a Senior Research Associate at McGill University’s Lady Davis Institute-Jewish General Hospital in Montreal. His interest lies in examining the interplay between prenatal exposure to maternal adversity (mood, disaster stress), child characteristics, early environmental influences, and youth mental health.
Guillaume Elgbeili
Guillaume Elgbeili, M.Sc. is a statistician working at the Douglas Research Center in Montreal specialized in modeling concurrent and longitudinal associations between parental and child physical and mental health, perinatal and early life environment, and genetics and epigenetics.
Vincent Paquin
Vincent Paquin, M.D. is a Psychiatry Resident and candidate in the Clinician Investigator Program at McGill University, Canada. His research focuses on socio-environmental determinants of mental health in youth.
Sherri Lee Jones
Sherri Lee Jones, Ph.D. (Psychology, Behavioral Neuroscience) completed post-doctoral fellowships at the Department of Psychiatry at McGill University, specializing in the roles of parental factors (e.g. stress, mental health) in offspring neuroendocrine and behavioral development. She is currently a Research Associate at the Douglas Research Center in Montreal, Canada.
Suzanne King
Suzanne King, Ph.D. is Professor Emerita (retired, active) in the Department of Psychiatry at McGill University, and Principal Investigator at the Douglas Hospital Research Centre. To increase understanding about prenatal stress in humans as risk factor for child development her research program focuses on women pregnant during natural disasters (www.mcgill.ca/spiral).
Pedro Rosa-Neto
Pedro Rosa-Neto, M.D., Ph.D. is a Professor of Neurology and neurosurgery and Psychiatry. Dr. Rosa-Neto directs the Translational Neuroimaging Laboratory (TNL) at McGill. University.
References
- American Psychiatric Association (APA). (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub.
- Avants, B. B., Epstein, C. L., Grossman, M., & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12(1), 1–10. https://doi.org/10.1016/j.media.2007.06.004
- Baas, D., Aleman, A., & Kahn, R. S. (2004). Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research. Brain Research Reviews, 45(2), 96–103. https://doi.org/10.1016/j.brainresrev.2004.02.004
- Belleau, E. L., Ehret, L. E., Hanson, J. L., Brasel, K. J., Larson, C. L., & deRoon-Cassini, T. A. (2020). Amygdala functional connectivity in the acute aftermath of trauma prospectively predicts severity of posttraumatic stress symptoms. Neurobiology of Stress, 12, 100217. https://doi.org/10.1016/j.ynstr.2020.100217
- Beversdorf, D. Q., Manning, S. E., Hillier, A., Anderson, S. L., Nordgren, R. E., Walters, S. E., Nagaraja, H. N., Cooley, W. C., Gaelic, S. E., & Bauman, M. L. (2005). Timing of prenatal stressors and autism. Journal of Autism and Developmental Disorders, 35(4), 471–478. https://doi.org/10.1007/s10803-005-5037-8
- Bromet, E., & Dew, M. A. (1995). Review of psychiatric epidemiologic research on disasters. Epidemiologic Reviews, 17(1), 113–119. https://doi.org/10.1093/oxfordjournals.epirev.a036166
- Brunet, A., St-Hilaire, A., Jehel, L., & King, S. (2003). Validation of a French version of the impact of event scale-revised. Canadian Journal of Psychiatry. Revue canadienne de psychiatrie, 48(1), 56–61. https://doi.org/10.1177/070674370304800111
- Cao-Lei, L., Dancause, K. N., Elgbeili, G., Laplante, D. P., Szyf, M., & King, S. (2016). Pregnant women’s cognitive appraisal of a natural disaster affects their children’s BMI and central adiposity via DNA methylation: Project Ice Storm. Early Human Development, 103, 189–192. https://doi.org/10.1016/j.earlhumdev.2016.09.013
- Cao-Lei, L., Dancause, K. N., Elgbeili, G., Laplante, D. P., Szyf, M., & King, S. (2018). DNA methylation mediates the effect of maternal cognitive appraisal of a disaster in pregnancy on the child’s C-peptide secretion in adolescence: Project Ice Storm. PloS One, 13(2), e0192199. https://doi.org/10.1371/journal.pone.0192199
- Class, Q. A., Abel, K. M., Khashan, A. S., Rickert, M. E., Dalman, C., Larsson, H., Hultman, C. M., Långström, N., Lichtenstein, P., & D’Onofrio, B. M. (2014). Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychological Medicine, 44(1), 71–84. https://doi.org/10.1017/s0033291713000780
- Dovgan, K. N., & Villanti, K. M. (2021). The prevalence of broad autism phenotype in young adults: The roles of genetic relationship to autism, gender, and academic major. The Journal of Genetic Psychology, 182(3), 174–181. https://doi.org/10.1080/00221325.2021.1904817
- Esteban, O., Markiewicz, C. J., Blair, R. W., Moodie, C. A., Isik, A. I., Erramuzpe, A., Kent, J. D., Goncalves, M., DuPre, E., Snyder, M., Oya, H., Ghosh, S. S., Wright, J., Durnez, J., Poldrack, R. A., & Gorgolewski, K. J. (2019). fMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16(1), 111–116. https://doi.org/10.1038/s41592-018-0235-4
- Fishman, I., Linke, A. C., Hau, J., Carper, R. A., & Müller, R. A. (2018). Atypical functional connectivity of amygdala related to reduced symptom severity in children with autism. Journal of the American Academy of Child and Adolescent Psychiatry, 57(10), 764–774.e763. https://doi.org/10.1016/j.jaac.2018.06.015
- Gao, L., Xi, Q. Q., Wu, J., Han, Y., Dai, W., Su, Y. Y., & Zhang, X. (2015). Association between prenatal environmental factors and child autism: A case control study in Tianjin, China. Biomedical and Environmental Sciences. 28(9), 642–650. https://doi.org/10.3967/bes2015.090
- Glover, G. H. (2011). Overview of functional magnetic resonance imaging. Neurosurgery Clinics of North America, 22(2), 133–139, vii. https://doi.org/10.1016/j.nec.2010.11.001
- Greve, D. N., & Fischl, B. (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1), 63–72. https://doi.org/10.1016/j.neuroimage.2009.06.060
- Guo, X., Duan, X., Long, Z., Chen, H., Wang, Y., Zheng, J., Zhang, Y., Li, R., & Chen, H. (2016). Decreased amygdala functional connectivity in adolescents with autism: A resting-state fMRI study. Psychiatry Research. Neuroimaging, 257, 47–56. https://doi.org/10.1016/j.pscychresns.2016.10.005
- Hayes, A. F. (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications.
- Herman, J. P., Ostrander, M. M., Mueller, N. K., & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 29(8), 1201–1213. https://doi.org/10.1016/j.pnpbp.2005.08.006
- Hilton, C. L., Harper, J. D., Kueker, R. H., Lang, A. R., Abbacchi, A. M., Todorov, A., & LaVesser, P. D. (2010). Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(8), 937–945. https://doi.org/10.1007/s10803-010-0944-8
- Howland, M. A., Sandman, C. A., & Glynn, L. M. (2017). Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Review of Endocrinology & Metabolism, 12(5), 321–339. https://doi.org/10.1080/17446651.2017.1356222
- Humphreys, K. L., Camacho, M. C., Roth, M. C., & Estes, E. C. (2020). Prenatal stress exposure and multimodal assessment of amygdala-medial prefrontal cortex connectivity in infants. Developmental Cognitive Neuroscience, 46, 100877. https://doi.org/10.1016/j.dcn.2020.100877
- Huntenburg, J. M. (2014). Evaluating nonlinear coregistration of BOLD EPI and T1w images. Freie Universität Berlin.
- Hurley, R. S., Losh, M., Parlier, M., Reznick, J. S., & Piven, J. (2007). The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders, 37(9), 1679–1690. https://doi.org/10.1007/s10803-006-0299-3
- Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. https://doi.org/10.1016/s1053-8119(02)91132-8
- Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. https://doi.org/10.1016/s1361-8415(01)00036-6
- Jones, S. L., Dufoix, R., Laplante, D. P., Elgbeili, G., Patel, R., Chakravarty, M. M., King, S., & Pruessner, J. C. (2019). Larger amygdala volume mediates the association between prenatal maternal stress and higher levels of externalizing behaviors: Sex specific effects in project ice storm. Frontiers in Human Neuroscience, 13, 144. https://doi.org/10.3389/fnhum.2019.00144
- King, S., & Laplante, D. P. (2005). The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress (Amsterdam, Netherlands), 8(1), 35–45. https://doi.org/10.1080/10253890500108391
- King, S., & Laplante, D. P. (2015). Using natural disasters to study prenatal maternal stress in humans. Adv Neurobiol, 10, 285–313. https://doi.org/10.1007/978-1-4939-1372-5_14
- Kinney, D. K., Miller, A. M., Crowley, D. J., Huang, E., & Gerber, E. (2008). Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. Journal of Autism and Developmental Disorders, 38(3), 481–488. https://doi.org/10.1007/s10803-007-0414-0
- Laplante, D. P., Zelazo, P. R., Brunei, A., & King, S. (2007). Functional play at 2 years of age: effects of prenatal maternal stress. Infancy: The Official Journal of the International Society on Infant Studies, 12(1), 69–93. https://doi.org/10.1111/j.1532-7078.2007.tb00234.x
- Lautarescu, A., Craig, M. C., & Glover, V. (2020). Prenatal stress: Effects on fetal and child brain development. International Review of Neurobiology, 150, 17–40. https://doi.org/10.1016/bs.irn.2019.11.002
- Li, X., Laplante, D. P., Elgbeili, G., & King, S. (2023). Preconception and prenatal maternal stress are associated with broad autism phenotype in young adults: Project Ice Storm. Journal of Developmental Origins of Health and Disease, 14(4), 481–489. https://doi.org/10.1017/s2040174423000156
- Liu, T., Ke, J., Qi, R., Zhang, L., Zhang, Z., Xu, Q., Zhong, Y., Lu, G., & Chen, F. (2021). Altered functional connectivity of the amygdala and its subregions in typhoon-related post-traumatic stress disorder. Brain and Behavior, 11(1), e01952. https://doi.org/10.1002/brb3.1952
- Mandy, M., & Nyirenda, M. (2018). Developmental Origins of Health and Disease: The relevance to developing nations. International Health, 10(2), 66–70. https://doi.org/10.1093/inthealth/ihy006
- Marco, E. J., Hinkley, L. B., Hill, S. S., & Nagarajan, S. S. (2011). Sensory processing in autism: a review of neurophysiologic findings. Pediatric Research, 69(5 Pt 2), 48r–54r. https://doi.org/10.1203/PDR.0b013e3182130c54
- McRae, K., Misra, S., Prasad, A. K., Pereira, S. C., & Gross, J. J. (2012). Bottom-up and top-down emotion generation: Implications for emotion regulation. Social Cognitive and Affective Neuroscience, 7(3), 253–262. https://doi.org/10.1093/scan/nsq103
- Mikkelsen, M., Wodka, E. L., Mostofsky, S. H., & Puts, N. A. J. (2018). Autism spectrum disorder in the scope of tactile processing. Developmental Cognitive Neuroscience, 29, 140–150. https://doi.org/10.1016/j.dcn.2016.12.005
- Ochsner, K. N., Ray, R. R., Hughes, B., McRae, K., Cooper, J. C., Weber, J., Gabrieli, J. D., & Gross, J. J. (2009). Bottom-up and top-down processes in emotion generation: Common and distinct neural mechanisms. Psychological Science, 20(11), 1322–1331. https://doi.org/10.1111/j.1467-9280.2009.02459.x
- Phelps, E. A., O’Connor, K. J., Gatenby, J. C., Gore, J. C., Grillon, C., & Davis, M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4(4), 437–441. https://doi.org/10.1038/86110
- Posner, J., Cha, J., Roy, A. K., Peterson, B. S., Bansal, R., Gustafsson, H. C., Raffanello, E., Gingrich, J., & Monk, C. (2016). Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Translational Psychiatry, 6(11), e935–e935. https://doi.org/10.1038/tp.2016.146
- Pruim, R. H. R., Mennes, M., van Rooij, D., Llera, A., Buitelaar, J. K., & Beckmann, C. F. (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. https://doi.org/10.1016/j.neuroimage.2015.02.064
- Qiu, A., Anh, T. T., Li, Y., Chen, H., Rifkin-Graboi, A., Broekman, B. F., Kwek, K., Saw, S. M., Chong, Y. S., Gluckman, P. D., Fortier, M. V., & Meaney, M. J. (2015). Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry, 5(2), e508–e508. https://doi.org/10.1038/tp.2015.3
- Rai, D., Golding, J., Magnusson, C., Steer, C., Lewis, G., & Dalman, C. (2012). Prenatal and early life exposure to stressful life events and risk of autism spectrum disorders: Population-based studies in Sweden and England. PloS One, 7(6), e38893. https://doi.org/10.1371/journal.pone.0038893
- Roozendaal, B., McEwen, B. S., & Chattarji, S. (2009). Stress, memory and the amygdala. Nature Reviews. Neuroscience, 10(6), 423–433. https://doi.org/10.1038/nrn2651
- Sah, P., Faber, E. S., Lopez De Armentia, M., & Power, J. (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews, 83(3), 803–834. https://doi.org/10.1152/physrev.00002.2003
- Sasson, N. J., Lam, K. S., Childress, D., Parlier, M., Daniels, J. L., & Piven, J. (2013). The broad autism phenotype questionnaire: Prevalence and diagnostic classification. Autism Research: Official Journal of the International Society for Autism Research, 6(2), 134–143. https://doi.org/10.1002/aur.1272
- Say, G. N., Karabekiroğlu, K., Babadağı, Z., & Yüce, M. (2016). Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatrics International: Official Journal of the Japan Pediatric Society, 58(4), 265–269. https://doi.org/10.1111/ped.12822
- Shen, M. D., Li, D. D., Keown, C. L., Lee, A., Johnson, R. T., Angkustsiri, K., Rogers, S. J., Müller, R. A., Amaral, D. G., & Nordahl, C. W. (2016). Functional connectivity of the amygdala is disrupted in preschool-aged children with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 55(9), 817–824. https://doi.org/10.1016/j.jaac.2016.05.020
- Simmons, A. N., Matthews, S. C., Strigo, I. A., Baker, D. G., Donovan, H. K., Motezadi, A., Stein, M. B., & Paulus, M. P. (2011). Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biology of Mood & Anxiety Disorders, 1(1), 6. https://doi.org/10.1186/2045-5380-1-6
- Toschi, N., Duggento, A., & Passamonti, L. (2017). Functional connectivity in amygdalar-sensory/(pre)motor networks at rest: New evidence from the Human Connectome Project. The European Journal of Neuroscience, 45(9), 1224–1229. https://doi.org/10.1111/ejn.13544
- Treiber, J. M., White, N. S., Steed, T. C., Bartsch, H., Holland, D., Farid, N., McDonald, C. R., Carter, B. S., Dale, A. M., & Chen, C. C. (2016). Characterization and correction of geometric distortions in 814 diffusion weighted images. PloS One, 11(3), e0152472. https://doi.org/10.1371/journal.pone.0152472
- Tustison, N. J., Avants, B. B., Cook, P. A., Zheng, Y., Egan, A., Yushkevich, P. A., & Gee, J. C. (2010). N4ITK: improved N3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. https://doi.org/10.1109/tmi.2010.2046908
- van den Bergh, B. R. H., Dahnke, R., & Mennes, M. (2018). Prenatal stress and the developing brain: Risks for neurodevelopmental disorders. Development and Psychopathology, 30(3), 743–762. https://doi.org/10.1017/s0954579418000342
- Van den Stock, J., Tamietto, M., Sorger, B., Pichon, S., Grézes, J., & de Gelder, B. (2011). Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Proceedings of the National Academy of Sciences of the United States of America, 108(39), 16188–16193. https://doi.org/10.1073/pnas.1107214108
- Varcin, K. J., Alvares, G. A., Uljarević, M., & Whitehouse, A. J. O. (2017). Prenatal maternal stress events and phenotypic outcomes in Autism Spectrum Disorder. Autism Research: official Journal of the International Society for Autism Research, 10(11), 1866–1877. https://doi.org/10.1002/aur.1830
- Walder, D. J., Laplante, D. P., Sousa-Pires, A., Veru, F., Brunet, A., & King, S. (2014). Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Research, 219(2), 353–360. https://doi.org/10.1016/j.psychres.2014.04.034
- Wang, S., Peterson, D. J., Gatenby, J. C., Li, W., Grabowski, T. J., & Madhyastha, T. M. (2017). Evaluation of field map and nonlinear registration methods for correction of susceptibility artifacts in diffusion MRI. Frontiers in Neuroinformatics, 11, 17. https://doi.org/10.3389/fninf.2017.00017
- Weiss, D. S., Marmar, C., Wilson, J. P., & Keane, T. (1997). Assessing psychological trauma and PTSD. Guilford Press.
- Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. https://doi.org/10.1089/brain.2012.0073
- Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. https://doi.org/10.1109/42.906424
- Zhang, X., Lv, C. C., Tian, J., Miao, R. J., Xi, W., Hertz-Picciotto, I., & Qi, L. (2010). Prenatal and perinatal risk factors for autism in China. Journal of Autism and Developmental Disorders, 40(11), 1311–1321. https://doi.org/10.1007/s10803-010-0992-0

