Abstract
Rhythmicity is a intrinsic feature of biological systems, including the hypothalamic-pituitary-adrenal axis, a mammalian neurohormonal system crucial both in daily life and as a network that responds to stressful stimuli. Circadian and ultradian rhythmicity underlie HPA activity in rodents and in humans, regulating gene expression, metabolism and behavior, and adverse consequences occur when rhythms are disturbed. In the assessment of human disease, the complexity of HPA rhythmicity is rarely acknowledged or understood, and is currently a limitation to better diagnosis and treatment. However, the recent emergence of ambulatory, high frequency and blood-free hormone sampling techniques has the promise to substantially change our understanding of the function of HPA axis in healthy normal life, and provide new opportunities for the diagnosis and treatment of disease.
Mary Dallman was the most extraordinary person. She was a caring human being and an original, inquisitive, and influential scientist. SL benefited personally from her wisdom as a nurturing and thought-provoking mentor and is proud to be a member of the great family of ‘Dallmanites’.
Mary made massive contributions to our understanding of the neural control of ACTH secretion and the function of the adrenocortical system in the overall physiology of mammals. Her earliest contributions to the literature were the beautifully designed studies in which she, together with Mortyn Jones, described the critical time domains involved in glucocorticoid negative feedback. These were followed by further classic studies of hypothalamic-pituitary-adrenal (HPA) axis dynamics and diurnal changes in HPA axis regulation. It is Mary’s interest in the different time domains of HPA regulation that became the principal driver for both our basic science and clinical studies of HPA axis function.
Oscillatory activity is a characteristic feature of both inorganic and biological systems. In 1905, Albert Einstein demonstrated how the constant movement of particles explained Brownian motion, while in much more complex systems seen in biology, oscillatory stochastic interactions and deterministic processes occur over multiple timescales, providing the ability for organisms to adapt to changes in both internal and external environments.
These inorganic and organic processes interact in a world where the physical movement of our planet provides a 24 hour light:dark cycle. This cycle acts as a powerful zeitgeber to multiple time-sensitive endogenous systems. One of these was Mary’s domain – the life-critical neurohormonal system, the HPA axis. This anticipatory hormonal system ensures energy supplies are available prior to awakening and the start of daily activities (in the morning in humans and in the evening in the nocturnal rodent). It is also a system able to rapidly respond to perceived stressors at any time during the day.
At the time of Mary’s studies it was widely accepted that a circadian rhythm regulates the HPA axis through output of the suprachiasmatic nucleus (the body clock in the hypothalamus) acting on corticotrophs (in the paraventricular nucleus). However, what was less well recognized was that this daily rhythm results from changes in pulse amplitude of a much faster ultradian rhythm of CORT (cortisol in humans or corticosterone in the rodent). We now know that this ultradian rhythm emerges as a natural consequence of the interaction between adrenocorticotropic hormone (ACTH)-secreting cells of the anterior pituitary and CORT secreting cells of the adrenal zona fasciculata. This feedforward (ACTH stimulating CORT release): feedback (CORT inhibiting ACTH secretion) system has a delay, ensuring that the system must oscillate. Furthermore the adrenal gland cannot store CORT, and thus new CORT must be synthesized following activation by ACTH, resulting in an approximate 20-minute delay before the adrenal gland response (Walker et al., Citation2010; Citation2012),.
The large oscillations of total CORT (the sum of CORT bound to corticosteroid binding globulin and albumin, plus free CORT) in the plasma, is also reflected in similar large oscillations of active free CORT in the brain and subcutaneous tissue (Qian et al., Citation2011) indicating that both glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) will be exposed to oscillating levels of this ligand. We have shown that each endogenous pulse of CORT results in a rapid increase in activated GR and cyclical changes in GR chromatin association profiles on regulatory elements of CORT regulated gene promotors (Conway-Campbell et al., Citation2007; Stavreva et al., Citation2009).
It is now becoming increasingly clear that both ultradian pulsatility and circadian rhythms not only have major effects on gene transcription, but also effects on behavior. Glucocorticoid pulsatility has specific effects both on miniature excitatory postsynaptic current frequency in different brain areas (Karst et al., Citation2010) and AMPA receptor signalling (Sarabdjitsingh et al., Citation2014). Also, adrenalectomized rats replaced with constant CORT infusions show blunted ACTH and behavioral responses to noise stress in comparison to rats receiving the same dose of CORT but in a pulsatile manner (Sarabdjitsingh et al., Citation2010). Parallel studies in humans also show that the presence of pulsatility is important for memory and emotional state (Kalafatakis et al., Citation2018, Citation2021) and that optimal pulsatile CORT replacement therapy changes neural processing and some aspects of the psychological profile of patients with adrenocortical insufficiency, compared to standard, suboptimal treatment (Russell et al., Citation2023). Studies on a major metabolic tissue- the liver - have also shown that there is major CORT pattern-dependent glucocorticoid receptor binding and transcriptional regulation, with important effects on carbohydrate, cholesterol, glucose and fat metabolic pathways as well as on inflammation associated pathways (Flynn et al., Citation2021).
Circadian oscillations of CORT also have major effects on many cognitive, metabolic, cardiovascular, and immunological systems. With respect to cognitive function, Birnie et al. (Birnie et al., Citation2023) have shown that in rodents, loss of the circadian pattern of plasma CORT resulting from oral synthetic glucocorticoid administration results in changes in the hippocampal transcriptome. Furthermore, the alteration in the hippocampal transcriptome was associated with a loss of circadian change in hippocampal long-term potentiation (LTP), and a deficit in hippocampal dependent memory.
Studies aiming to examine rhythmicity of hormones and other metabolites in humans have always been bedeviled by the difficulty of obtaining samples over a full circadian cycle (24 hours or more) outside of a clinical investigation facility. Single blood sample estimates, which are frequently used as decision support information in clinical settings, may frequently be inaccurate or even misleading when used to measure systems that are inherently rhythmic and reactive, such as the HPA axis. Outpatient methods of sample collection, such as saliva sampling, can potentially provide crude estimates of a daily rhythm but crucially, lack the fidelity of in-laboratory high frequency systems necessary to discern detail, assess dynamic responses, and detect ultradian rhythmicity, particularly during the period of sleep, where major fluxes in hormone systems occur.
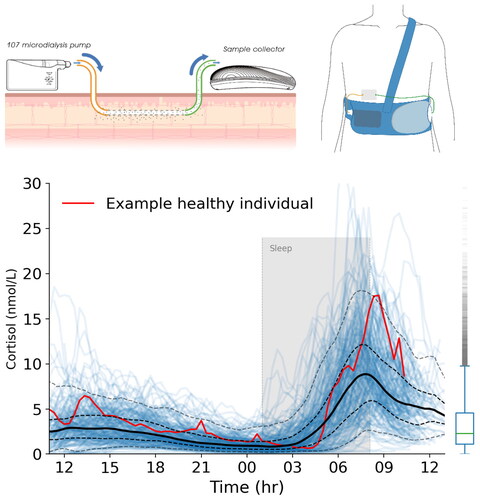
Recently, we have developed a methodology for outpatient, ambulatory bio-sampling that has capability of measuring dynamical hormones, such as cortisol, at high frequency, but without either the need for intervention by the subject, removal of any blood, or hospitalisation (Bhake, Citation2015; Bhake et al., Citation2019, Citation2020) (). The system, which is based on continuous sampling of the subcutaneous interstitial fluid, utilizes microdialysis, and permits measurement of non-protein bound hormone in tissue. Using this technology it has now been possible to repeatedly sample and therefore assess dynamic responses of the HPA axis to stressful insults such as cardiac surgery in neonates for example, which would otherwise be impossible, due to the low blood volume of very young children (Fudulu et al., Citation2020). In a major milestone, our group has now published 24-hour tissue profiles of multiple adrenal steroids, including cortisol, cortisone and aldosterone, sampled every 20 minutes, in a cohort of 214 healthy adult volunteers aged 18–68 (Upton et al., Citation2023), in real-world ambulatory settings. Using mathematical and computational methods, interindividual variability, often substantial, was quantified across different times of the day. Since multiple hormones can be measured at the same time in the same sample, we were able to examine the rhythms of relatively novel steroid metabolites such as 18-hydroxycortisol (Lenders et al., Citation2018), and the complexity that surrounds the regulation and secretion of aldosterone and cortisol in response to ACTH (Daidoh et al., Citation1995). Most importantly, this data will serve as potential reference range for comparison with disease states, in particular endocrine disorders such as Cushing’s (Nieman et al., Citation2008) and primary aldosteronism (Funder et al., Citation2016), where disturbance of normal hormonal rhythmicity is a pathogenic feature.
Figure 1. The U-RHYTHM microdialysis system and example data. Top left: Samples are collected using a linear microdialysis catheter placed superficially in abdominal subcutaneous tissue. The samples are fractionated and stored in the sample collector. Top right: the system is body worn and allows most normal activities to continue during the sampling period. Bottom: Data from a single volunteer (red line) and their sleep period (grey shaded area) are shown against the background variation of 214 healthy controls (blue lines) with interquartile range (bold is 50th centile, dashed lines 5th to 95th).
In conclusion, dynamical systems are an intrinsic part of the natural world, and an inherent feature of biological systems such as the HPA axis in mammals. Daily and ultradian rhythms are important for preparation and adaptation to a constantly changing environment, and disturbance of those rhythms has consequences. New technologies that can measure and assess in detail rhythms of hormones in humans have the potential to substantially increase not only our understanding of normal and abnormal states, but also the degree and impact of interindividual variation that exists because of every person’s unique genes and personal experiences of life.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Stafford Lightman
Stafford Lightman is Professor of Medicine at the University of Bristol. He is a past president of the British Neuroscience Association, and a co-founder of Dynamic Therapeutics.
Thomas Upton
Thomas Upton MBChB PhD is currently completing his first post-doctoral fellowship in Translational Health Sciences at University of Bristol Medical School, UK. He is a specialist in the use of ambulatory human microdialysis and its applications particularly in endocrinology and chronobiology.
References
- Bhake, R. C. (2015). Free Cortisol in Healthy Individuals - Combining Microdialysis and a Novel Portable Collection Device for Continuous Ambulatory Sampling. University of Bristol.
- Bhake, R. C., Kluckner, V., Stassen, H., Russell, G. M., Leendertz, J., Stevens, K., Linthorst, A. C. E., & Lightman, S. L. (2019). Continuous free cortisol profiles—circadian rhythms in healthy men. The Journal of Clinical Endocrinology and Metabolism, 104(12), 1–4. https://doi.org/10.1210/jc.2019-00449
- Bhake, R., Russell, G. M., Kershaw, Y., Stevens, K., Zaccardi, F., Warburton, V. E. C., Linthorst, A. C. E., & Lightman, S. L. (2020). Continuous free cortisol profiles in healthy men. The Journal of Clinical Endocrinology & Metabolism, 105(4), e1749–e1761. https://doi.org/10.1210/clinem/dgz002
- Birnie, M. T., Claydon, M. D. B., Troy, O., Flynn, B. P., Yoshimura, M., Kershaw, Y. M., Zhao, Z., Demski-Allen, R. C. R., Barker, G. R. I., Warburton, E. C., Bortolotto, Z. A., Lightman, S. L., & Conway-Campbell, B. L. (2023). Circadian regulation of hippocampal function is disrupted with corticosteroid treatment. Proceedings of the National Academy of Sciences of the United States of America, 120(15), e2211996120. https://doi.org/10.1073/pnas.2211996120
- Conway-Campbell, B. L., McKenna, M. A., Wiles, C. C., Atkinson, H. C., de Kloet, E. R., & Lightman, S. L. (2007). Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology, 148(11), 5470–5477. https://doi.org/10.1210/en.2007-0585
- Daidoh, H., Morita, H., Mune, T., Murayama, M., Hanafusa, J., Ni, H., Shibata, H., & Yasuda, K. (1995). Responses of plasma adrenocortical steroids to low dose ACTH in normal subjects. Clinical Endocrinology, 43(3), 311–315. https://doi.org/10.1111/j.1365-2265.1995.tb02037.x
- Flynn, B. P., Birnie, M. T., Kershaw, Y. M., Pauza, A. G., Kim, S., Baek, S., Rogers, M. F., Paterson, A. R., Stavreva, D. A., Murphy, D., Hager, G. L., Lightman, S. L., & Conway-Campbell, B. L. (2021). Corticosterone pattern-dependent glucocorticoid receptor binding and transcriptional regulation within the liver. PLoS Genetics, 17(8), e1009737. https://doi.org/10.1371/journal.pgen.1009737
- Fudulu, D. P., Angelini, G. D., Papadopoulou, F. F., Evans, J., Walker-Smith, T., Kema, I., van Faassen, M., Stoica, S., Caputo, M., Lightman, S., & Gibbison, B. (2020). The Peacock study: Feasibility of the dynamic characterisation of the paediatric hypothalamic-pituitary-adrenal function during and after cardiac surgery. BMC Cardiovascular Disorders, 20(1), 245. https://doi.org/10.1186/s12872-020-01516-y
- Funder, J. W., Carey, R. M., Mantero, F., Murad, M. H., Reincke, M., Shibata, H., Stowasser, M., & Young, W. F. (2016). The management of primary aldosteronism: case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 101(5), 1889–1916. https://doi.org/10.1210/jc.2015-4061
- Kalafatakis, K., Russell, G. M., Ferguson, S. G., Grabski, M., Harmer, C. J., Munafò, M. R., Marchant, N., Wilson, A., Brooks, J. C., Thakrar, J., Murphy, P., Thai, N. J., & Lightman, S. L. (2021). Glucocorticoid ultradian rhythmicity differentially regulates mood and resting state networks in the human brain: A randomised controlled clinical trial. Psychoneuroendocrinology, 124, 105096. https://doi.org/10.1016/j.psyneuen.2020.105096
- Kalafatakis, K., Russell, G. M., Harmer, C. J., Munafo, M. R., Marchant, N., Wilson, A., Brooks, J. C., Durant, C., Thakrar, J., Murphy, P., Thai, N. J., & Lightman, S. L. (2018). Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proceedings of the National Academy of Sciences of the United States of America, 115(17), E4091–E4100. https://doi.org/10.1073/pnas.1714239115
- Karst, H., Berger, S., Erdmann, G., Schütz, G., & Joëls, M. (2010). Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences of the United States of America, 107(32), 14449–14454. https://doi.org/10.1073/pnas.0914381107
- Lenders, J. W. M., Williams, T. A., Reincke, M., & Gomez-Sanchez, C. E. (2018). Diagnosis of endocrine disease: 18-Oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids? European Journal of Endocrinology, 178(1), R1–R9. https://doi.org/10.1530/EJE-17-0563
- Nieman, L. K., Biller, B. M. K., Findling, J. W., Newell-Price, J., Savage, M. O., Stewart, P. M., & Montori, V. M. (2008). The diagnosis of cushing’s syndrome: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 93(5), 1526–1540. https://doi.org/10.1210/jc.2008-0125
- Qian, X., Droste, S. K., Gutièrrez-Mecinas, M., Collins, A., Kersanté, F., Reul, J. M. H. M., & Linthorst, A. C. E. (2011). A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology, 152(10), 3738–3748. https://doi.org/10.1210/en.2011-1008
- Russell, G., Kalafatakis, K., Durant, C., Marchant, N., Thakrar, J., Thirard, R., King, J., Bowles, J., Upton, T., Thai, N. J., Brooks, J. C. W., Wilson, A., Phillips, K., Ferguson, S., Grabski, M., Rogers, C. A., Lampros, T., Wilson, S., Harmer, C., Munafo, M., & Lightman, S. L. (2023). Ultradian hydrocortisone replacement alters neuronal processing, emotional ambiguity, affect and fatigue in adrenal insufficiency: The PULSES trial. Journal of Internal Medicine, 295(1), 51–67. https://doi.org/10.1111/joim.13721
- Sarabdjitsingh, R. A., Conway-Campbell, B. L., Leggett, J. D., Waite, E. J., Meijer, O. C., de Kloet, E. R., & Lightman, S. L. (2010). Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain-region-specific manner. Endocrinology, 151(11), 5369–5379. https://doi.org/10.1210/en.2010-0832
- Sarabdjitsingh, R. A., Jezequel, J., Pasricha, N., Mikasova, L., Kerkhofs, A., Karst, H., Groc, L., & Joëls, M. (2014). Ultradian corticosterone pulses balance glutamatergic transmission and synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14265–14270. https://doi.org/10.1073/pnas.1411216111
- Stavreva, D. A., Wiench, M., John, S., Conway-Campbell, B. L., McKenna, M. A., Pooley, J. R., Johnson, T. A., Voss, T. C., Lightman, S. L., & Hager, G. L. (2009). Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nature Cell Biology, 11(9), 1093–1102. https://doi.org/10.1038/ncb1922
- Upton, T. J., Zavala, E., Methlie, P., Kämpe, O., Tsagarakis, S., Øksnes, M., Bensing, S., Vassiliadi, D. A., Grytaas, M. A., Botusan, I. R., Ueland, G., Berinder, K., Simunkova, K., Balomenaki, M., Margaritopoulos, D., Henne, N., Crossley, R., Russell, G., Husebye, E. S., & Lightman, S. L. (2023). High-resolution daily profiles of tissue adrenal steroids by portable automated collection. Science Translational Medicine, 15(701), eadg8464. https://doi.org/10.1126/scitranslmed.adg8464
- Walker, J. J., Spiga, F., Waite, E., Zhao, Z., Kershaw, Y., Terry, J. R., & Lightman, S. L. (2012). The origin of glucocorticoid hormone oscillations. PLoS Biology, 10(6), e1001341-e1001341. https://doi.org/10.1371/journal.pbio.1001341
- Walker, J. J., Terry, J. R., & Lightman, S. L. (2010). Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proceedings. Biological Sciences, 277(1688), 1627–1633. https://doi.org/10.1098/rspb.2009.2148
