Abstract
Objective
People living with HIV (PLWH) experience high rates of childhood maltreatment, which increases risk for posttraumatic stress disorder (PTSD). Thus, it is important to understand how HIV status interacts with childhood maltreatment to influence PTSD symptom severity and underlying psychophysiology.
Methods
The current study assessed whether HIV status interacts with childhood maltreatment to influence PTSD symptom severity and heart rate variability during a dark-enhanced startle (DES) task in 88 Black women with (n=30) and without HIV (n=58).
Results
HIV was associated with greater PTSD symptom severity only in women with low levels of childhood maltreatment (p=.024). Startle potentiation during DES was highest in women living without HIV and with high childhood maltreatment (p=.018). In women who had experienced low levels of childhood maltreatment, respiratory sinus arrhythmia (RSA) was lower during the dark phase of DES in women living without HIV than women living with HIV (WLWH), (p=.046). RSA during the light phase of DES was lower in WLWH than in women living without HIV (p=.042).
Conclusion
In the current sample of Black women, HIV status was associated with PTSD symptom severity in a manner dependent on level of childhood maltreatment, suggesting that HIV status may be an important factor to consider for behavioral and pharmacological treatment strategies for PTSD. Additionally, HIV status is associated with lower percent potentiation to darkness and lower RSA during the light phase of DES, suggesting physiological mechanisms by which HIV may contribute to PTSD symptoms in individuals exposed to low levels of childhood maltreatment.
Keywords:
Introduction
The HIV epidemic remains a major challenge despite waning public attention and media coverage. In 2020, approximately 1.5 million people worldwide were newly diagnosed with HIV (HIV.gov, 2021). Fortunately, the development of effective antiretroviral therapy (ART) for HIV infection has reduced HIV-related morbidity and mortality such that approximately 30 million people currently live with HIV worldwide (Hallett et al., Citation2014). However, there is a great deal of variability in individual response to ART in people living with HIV (PLWH) (Moniz et al., Citation2014), with poor adherence to treatment regimens being a primary factor for suboptimal ART response (Li et al., Citation2014). Exposure to stressful life events, including trauma exposure across the lifespan, was recently identified as a critical factor associated with decreased adherence to ART and increased odds of virologic failure (Mugavero et al., Citation2009).
Exposure to early life trauma and maltreatment during childhood and development of posttraumatic stress disorder (PTSD) are both associated with ART nonadherence in PLWH (Keuroghlian et al., Citation2011; Machtinger et al., Citation2012; Samuels et al., Citation2011; Whetten et al., Citation2013). PTSD is a severe psychiatric disorder that occurs after a traumatic life event and increases individual vulnerability to an array of adverse health outcomes (Dedert et al., Citation2010). PTSD presentation is heterogeneous, but is generally characterized by dysregulation of fear responses, presenting with symptoms across different domains, including hyperarousal, avoidance/numbing, and reexperiencing symptoms (Kessler et al., Citation1995; Redican et al., Citation2021). These PTSD symptoms, in turn, have an adverse influence on patient compliance with treatment of concurrent somatic illness (Hilerio et al., Citation2005; Kartha et al., Citation2008; Shemesh et al., Citation2001), and may independently influence progression and risk for somatic diseases and disorders in PLWH (Delahanty et al., Citation2004; Kubzansky et al., Citation2007). Furthermore, women living with HIV (WLWH) are more likely to develop PTSD compared to women living without HIV (Martinez et al., Citation2002). Thus, it is important to understand how HIV status and childhood maltreatment predict PTSD symptoms and biological pathways that may be responsible for increased risk for somatic disorders and diseases in PLWH.
One neurobiological system that is impacted by childhood maltreatment and HIV status and implicated in the pathophysiology of PTSD is the autonomic nervous system (ANS). The ANS is comprised of the sympathetic (SNS) and parasympathetic systems (PNS), which work together to regulate the activity of multiple organs in response to stress exposure (McCorry, Citation2007). Individuals with a history of repeated trauma and PTSD show dysregulation of the SNS. Chronic activation of the SNS results in sustained release of norepinephrine and epinephrine, and leads to maintenance of higher heart rates and blood pressure, as well as decreases in heart rate variability (HRV) (Azulay et al., Citation2022; Gordan et al., Citation2015). HRV is the variation in time between each heartbeat and a measure of the heart’s ability to adapt to stressors. High HRV is indicative of normal ANS function and the ability of the heart to adapt to stress. Reduced HRV reflects ANS dysregulation and impaired cardiac stress adaptation (Williams et al., Citation2019). HRV is quantified primarily using respiratory sinus arrhythmia (RSA), a measure of HRV in synchrony with respiration (Shaffer et al., Citation2014; Yasuma & Hayano, Citation2004).
Trauma and PTSD are both associated with lower HRV, which is linked with increased risk for cardiovascular disease (Dennis et al., Citation2017; Sloan et al., Citation1999). Similarly, a meta-analysis (k = 8) compared HRV between PLWH on ART and individuals without HIV and found evidence for a shift toward sympathetic dominance in PLWH on ART, with lower HRV (McIntosh, Citation2016). Additionally, the ANS regulates musculature involved in the acoustic startle reflex, an unconscious defensive response to unexpected loud sounds that results in contraction of skeletal musculature, which can be measured using electromyogram (EMG) recordings of the eye-blink muscle (Ramirez-Moreno & Sejnowski, Citation2012). In persons exposed to high levels of trauma and those with PTSD the startle response is exaggerated in response to both generally anxiogenic (e.g. darkness) and fear-conditioned stimuli (Grillon et al., Citation1998; Jovanovic et al., Citation2012; Jovanovic et al., Citation2009; Kamkwalala et al., Citation2012; Norrholm et al., Citation2015; Norrholm et al., Citation2014). Greater startle response during the dark-enhanced startle (DES) paradigm, a measure of startle potentiation in response to darkness, an anxiogenic, nonspecific stressor, is associated with PTSD and alterations in HRV (Kamkwalala et al., Citation2012). Taken together, these data suggest that HIV status and childhood maltreatment may influence both PTSD symptoms and their underlying neurobiology.
Critically, minoritized and under-resourced Black Americans living in urban environments are at greater risk for experiencing high levels of childhood maltreatment and PTSD (Alim et al., Citation2006; Breslau, Citation2001; Gillespie et al., Citation2009), as well as increased risk for HIV infection (Murrain & Barker, Citation1997; Ojikutu & Mayer, Citation2021). Understanding how HIV status impacts PTSD symptom severity and underlying psychophysiology is critical for informing behavioral and pharmacological treatment strategies for trauma exposed PLWH (Machtinger et al., Citation2012; O’Cleirigh et al., Citation2013). The overall goals of the current cross-sectional study were to characterize the effects of HIV and childhood maltreatment on PTSD symptom severity, DES responses, and HRV in trauma-exposed Black women. We hypothesized that greater PTSD symptom severity, greater DES, lower RSA would be present in women exposed to higher levels of childhood maltreatment and in women living with HIV (WLWH). We also explored the interaction of childhood maltreatment and HIV status on outcomes.
Methods
Participants
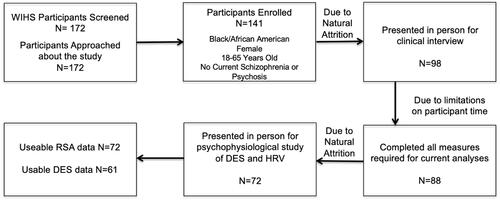
Participants were women recruited from individuals enrolled in wave four of the Women’s Interagency HIV Study (WIHS) in Atlanta, GA from 2013-2015 (Adimora et al., Citation2018; Barkan et al., Citation1998). Wave four of the WIHs study enrolled women from 23-60 years of age who were living with HIV (73%) or without HIV (27%) (Adimora et al., Citation2018; Barkan et al., Citation1998). The sample demographics of WLWH and without HIV in the WIHS are similar (Black 72%, White 11%, Hispanic 14% and Other 3%) and are generally representative of WLWH in the United States (Adimora et al., Citation2018; Barkan et al., Citation1998). Inclusion criteria for the current study included being a Black woman between the ages of 18 and 65 years who was not actively psychotic. Of the 141 participants consented to participate in the current study (81.9%), only 99 completed the in-person clinical interview (69.5%) (). Of those, 88 women [30 women without HIV (34% of sample) and 58 WLWH (66% of sample)] had usable data for PTSD symptom severity analysis, 66 for the RSA analysis, and 61 for the DES analysis (). Medical data regarding HIV status at time of enrollment in the current study were collected via the WIHS. All study procedures were approved by the Emory Institutional Review Board and the Grady Hospital Research Oversight Committee.
Figure 1. Consort diagram of our cross-sectional study. N = 172 participants were screened for eligibility in the study, with 172 meeting initial inclusion/exclusion criteria and approached for consent. N = 141 participants consented to participation (81.9%) with 99 returning for the in-person clinical interview (69.5%). N = 88 (89.8%) participants with usable data necessary for PTSD symptom severity analysis, N = 66 for the RSA analysis, and N = 61 for the DES analysis.
Clinical interview measures
All participants completed a clinical interview conducted by a clinician trained to administer all psychological assessment instruments. Participants were compensated $50 for completing these assessments, which in total lasted one to three hours. Demographic information was collected using a locally-developed demographics form to assess for age, self-identified race, education, and income (Gillespie et al., Citation2009).
The Traumatic Events Inventory (TEI) was administered to assess lifetime adult trauma exposure history (Gillespie et al., Citation2009; Mekawi et al., Citation2021). Previous work has demonstrated construct validity of this measure showing that higher levels of reported trauma using this measure is associated with more severe symptoms of relevant constructs such as PTSD symptoms (Mekawi et al., Citation2021). In the current study, total types of trauma exposure experienced and witnessed were summed across different types of trauma (including exposure to a life-threatening illness, such as HIV) to measure overall trauma exposure in one’s lifetime. Higher values in the summed TEI score indicate greater exposure to different types of traumas in adulthood.
The Childhood Trauma Questionnaire (CTQ) was used to assess childhood exposure to emotional, physical, and sexual abuse, as well as emotional and physical neglect (Bernstein et al., Citation2003). Per standard procedure (Bernstein et al., Citation2003), individual item responses on the CTQ were summed and a group variable was created for reported rates of none-to-low (referred to in this paper as “low”) and moderate-to-severe (referred to in this paper as “high”) emotional, physical, and sexual maltreatment. In the current sample, the CTQ had a Cronbach’s Alpha of .96.
The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) is an interviewer-administered psychometrically valid and standardized diagnostic instrument (Weathers et al., Citation2001). The CAPS assesses current PTSD symptoms based on DSM-5 criteria, and it yields both a categorical diagnosis and a dimensional score for total symptom severity and severity of each PTSD symptom cluster based on frequency and intensity of symptoms. For the current study, CAPS-5 was scored for the two most impactful trauma exposures, one in childhood and one in adulthood (or two in adulthood if no childhood criterion A trauma experienced). Then, PTSD severity measures were collapsed into total PTSD symptom severity based on the trauma that produced the highest level of PTSD symptoms. This approach was chosen for the study population due to the high level of chronic trauma exposure and the difficulty with choosing one index trauma and the risk of underdiagnosing PTSD symptoms. Extensive training of all CAPS interviewers for this study was completed over 1-2 months, and included in-person observations, weekly supervision, and tape review with a licensed clinical psychologist. Previously examined interrater reliability (IRR) has been high for the diagnosis of PTSD in a similar sample (κ=.83; (Powers et al., Citation2017)). Additionally, unpublished reliability of presence/absence of all PTSD symptoms on CAPS-5 was examined with 25 randomly chosen videos and results showed good IRR across all items (κ=.77).
Dark-enhanced startle and heart rate variability assessment
A sub-set of participants (n = 72; ) also completed a psychophysiology study visit that was conducted by a trained staff member as described below. Participants were compensated $50 for completing these assessments that lasted ∼ two hours.
The psychophysiological paradigm we leveraged in the current study has been used previously to describe the impact of trauma and PTSD on the startle response across species (Jovanovic et al., Citation2012; Kamkwalala et al., Citation2012). The startle paradigm method has been standardized and has reproducibility shown an exaggerated in response to generally anxiogenic (e.g. darkness) stimuli across several populations and age groups (Cao et al., Citation2017; Jovanovic et al., Citation2013; Jovanovic et al., Citation2011; Kamkwalala et al., Citation2012; Ressler et al., Citation2011). The startle session consisted of a two-minute acclimation period during which no auditory startle probes were delivered, a two-minute startle habituation period and a four-minute dark-enhanced startled phase that happened without breaks as previously described (Kamkwalala et al., Citation2012). During the habituation period two blocks with four startle probes in each block were conducted, totaling eight probe trials. These habituation probes were followed immediately by the dark-enhanced startle segment that also consisted of two blocks each with eight startle probes. In each of these blocks, four startle probes were delivered in the dark phase and four probes in the light phase. The lights in the startle booth were controlled by an automated timer that was synchronized with the presentation of the startle probes. The light and dark phases of the protocol were alternated every minute and the order of presentation of the light and dark phases were counterbalanced across study participants.
BIOPAC MP150 for Windows (BIOPAC Systems, Inc, Goleta, CA) was used to acquire psychophysiological data that were filtered and rectified using MindWare software (MindWare Technologies, Ltd, Gahanna, OH) as previously described (Michopoulos et al., Citation2017). Startle data were collected using the EMG module of the BIOPAC system by recording the eyeblink muscle contraction via two 5-mg Ag/AgCl electrodes that were placed on the orbicularis oculi muscle below the pupil with electrolyte gel. The startle probe was delivered binaurally through headphones and consisted of a 108 dB sound pressure level; 40-millisecond burst of broadband noise with near instantaneous rise time. Startle magnitude was measured as the maximum amplitude of the eyeblink muscle contraction 20 to 200 milliseconds after the presentation of the startle probe (Michopoulos et al., Citation2017). The primary outcome for the dark-enhanced psychophysiology was the percent startle potentiation in the dark phase relative to the light phase ((Dark-Light)/Light)*100) during each block.
The electrocardiogram (ECG) and respiration module of the BIOPAC system were used to acquire heart rate variability data. As previously described (Kamkwalala et al., Citation2012), ECG was recorded via three Ag/AgCl electrodes placed in the Lead II position. The ECG signal was amplified by a gain of 5000, filtered with a Hamming windowing function and with a 60-Hz notch filter. A chest band transducer was used to measure respiration, which was assessed as respiratory rate per minute used in the calculation of RSA (Grossman et al., Citation2004). In accordance with methods recommended by the Society for the Psychophysiological Research Committee on HRV (Berntson et al., Citation1997), RSA was measured during one-minute intervals during the DES paradigm via spectral analysis of the time-sampled interbeat interval series. RSA was sampled from 0.12 to 0.40 Hz.
Statistical analyses
Chi-square analysis was used to assess differences between women living with and without HIV on categorical variables (income and education) and correlations for continuous variables. An analysis of covariance (ANCOVA) with covariates for education and income were used to assess the effects of HIV status (two levels: HIV- and HIV + women), childhood maltreatment (two levels: low vs. high) and their interaction on PTSD symptom severity on the CAPS-5. An ANCOVA with covariates for education and income were used to assess the effects of HIV status, childhood maltreatment, block (first vs. second) and their interactions on startle potentiation. An ANCOVA with covariates for education and income were used to assess the effects of HIV status, childhood maltreatment, DES phase (light vs. dark), block (first vs. second), and their interaction on RSA. Data are summarized as the mean ± standard error of the mean (SEM). All data were analyzed using SPSS (v.29) and the alpha level was set at p≤.05.
Results
Sociodemographic and trauma exposure characteristics
While women living with and without HIV in the current study did not differ in age (p=.76; ), the two groups did differ with regards to education and income levels. More specifically, WLWH had higher levels of education (p=.029) and income (p =.028) compared to women without HIV in the current sample (). Overall rates of childhood maltreatment and adult trauma exposure were not significantly different between women with and without HIV (p’s>.05; ). Approximately eighty-six percent of WLWH in the current study were virally suppressed ().
Table 1. Demographic characteristics for overall study sample.
Effects of childhood maltreatment and HIV on PTSD symptom severity
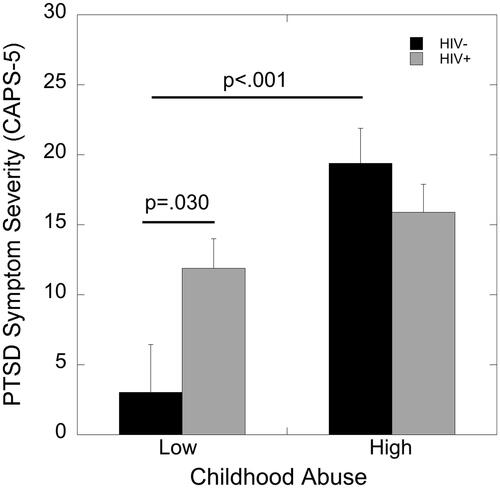
An ANCOVA with covariates for education and income showed a main effect of childhood maltreatment on PTSD symptom severity as determined by the CAPS-5 (F(1,82)=15.35, p<.001, partial eta2=0.16). Women who experienced high childhood maltreatment endorsed more severe PTSD symptoms than women who endorsed low childhood maltreatment. Importantly, this effect of childhood maltreatment was moderated by HIV status, as indicated by a significant childhood maltreatment by HIV status interaction (F(1,82)=5.84, p=.018, partial eta2=0.067). HIV was associated with greater PTSD symptom severity only in women with low levels of childhood maltreatment (p=.030; ). There was no association of HIV status with PTSD symptom severity in women exposed to high childhood maltreatment (p=.31; ). In women without HIV, high childhood maltreatment was also associated with greater PTSD symptom severity compared to the low maltreatment group (p<.001; ).
Figure 2. Effects of HIV status and childhood maltreatment on CAPS-5 PTSD symptom severity. HIV was associated with greater PTSD symptoms severity only in women with low levels of childhood maltreatment (p=.030). In women without HIV, high versus low childhood maltreatment was also associated with greater PTSD symptom severity (p<.001).
Effects of childhood maltreatment and HIV on DES
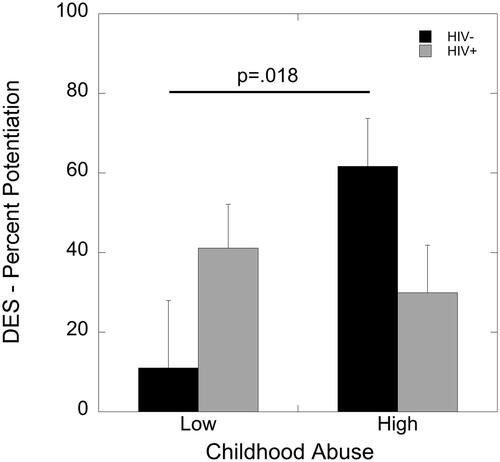
An ANCOVA with covariates for education and income revealed a main effect of block (F(1,55)=4.91, p=.031, partial eta2=0.082), such that startle potentiation was greater in the first block compared to the second. There were no main effects of HIV status or childhood maltreatment (p’s>.05). However, there was a significant interaction of HIV status and childhood maltreatment exposure on degree of startle potentiation (F(1,55)=5.62, p=.021, partial eta2=0.093). High childhood maltreatment was associated with greater startle potentiation compared to low maltreatment in women living without HIV (p=.018) but not in WLWH (p=.49; ).
Figure 3. Effects of HIV status and childhood maltreatment on percent potentiation due to darkness during the DES paradigm. Childhood maltreatment was associated with greater percent potentiation to darkness in women living without HIV (p=.018) but not in WLWH (p=.49).
Effects of childhood maltreatment and HIV on HRV
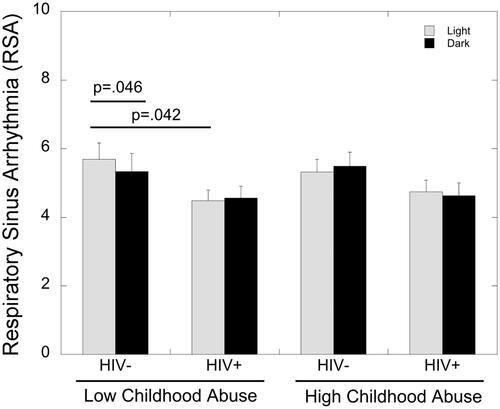
An ANCOVA with covariates for education and income showed a significant main effect of HIV status (F(1,60)=5.95, p=.05, partial eta2=0.062) on RSA, as RSA was significantly lower in WLWH compared to those living without HIV (4.6±.24 vs. 5.46±.33, respectively). There was also a significant three-way interaction of phase, HIV status and childhood maltreatment on RSA (F(1,60)=7.38, p=.009, partial eta2=0.11). In women without HIV who had experienced low levels of childhood maltreatment, RSA was significantly lower during the dark compared to the light phase of the DES task (p=.046; ). This difference in RSA between DES phases was not present in WLWH with low childhood maltreatment (p>.05). WLWH with low childhood maltreatment showed significantly lower RSA during the light phase of the DES compared to women without HIV (p=.042; ). There were no differences in RSA due to HIV status or DES phase in women with a history of high levels of childhood maltreatment (p’s>.05).
Figure 4. Effects of HIV status, childhood maltreatment, and phase of DES (light vs. dark) on RSA. RSA was significantly lower during the dark compared to the light phase of the DES task in women without HIV who had experienced low levels of childhood maltreatment (p=.046). WLWH with low childhood maltreatment showed significantly lower RSA during the light phase of the DES compared to women without HIV (p=.042).
Discussion
Prior research indicates that trauma and PTSD symptoms are negatively associated with poor patient outcomes for PLWH (Brief et al., Citation2004; Kalichman et al., Citation2002; Machtinger et al., Citation2012; Nightingale et al., Citation2011). While previous work has linked HIV status with increased PTSD symptom severity (Samuels et al., Citation2011), questions remain about the effect of HIV on PTSD- related psychophysiology in trauma-exposed individuals. We aimed to address these questions by collecting and analyzing information about trauma history, PTSD symptoms, and HIV status in a sample of Black women. Our main hypotheses were that PTSD symptom severity would be greater in women exposed to higher levels of childhood maltreatment and greater in women living with HIV. We also assessed the impact of childhood maltreatment and HIV status on underlying psychophysiology including startle potentiation to darkness and HRV. Finally, we also explored the interaction of childhood maltreatment and HIV status and its effect on PTSD symptoms, DES, and RSA HRV. We hypothesized that there would be higher startle potentiation to darkness and lower RSA in women exposed to higher levels of childhood maltreatment and in women living with HIV.
Although the current results did not show main effects of HIV status on PTSD symptoms, we found that childhood maltreatment severity moderated the effect of HIV. More specifically, PTSD symptom severity was greater in WLWH compared to women living without HIV, only among women who had experienced low levels of childhood maltreatment. This extends previous work that has linked HIV status with increased PTSD symptom severity (Samuels et al., Citation2011) by suggesting that childhood maltreatment is an important factor to consider, in that women with high levels of childhood maltreatment have heightened PTSD symptoms regardless of HIV status, indicating a ceiling effect. Additionally, our finding that PTSD symptoms in women who reported high levels of childhood maltreatment were not impacted by HIV status corroborate previous research that found that childhood trauma exposure is a critical risk factor for the development of chronic PTSD symptoms (McLaughlin et al., Citation2020). The effect of HIV status on PTSD symptoms in women with low levels of childhood maltreatment suggest that HIV may impact biological pathways that are implicated in the pathophysiology of PTSD, including but not limited to dysregulation of the HPA axis, neuroinflammation, and oxidative stress (Michopoulos et al., Citation2015). While future studies are necessary to assess the impacts of HIV on these biological measures, the current study did assess its impact on psychophysiological outcomes linked to PTSD, namely DES and HRV.
High levels of trauma exposure and PTSD are associated with an exaggerated psychophysiological response to both nonspecific (e.g. darkness) and specific threat stimuli (Grillon et al., Citation1998; Jovanovic et al., Citation2012; Jovanovic et al., Citation2009; Kamkwalala et al., Citation2012; Norrholm et al., Citation2015; Norrholm et al., Citation2014). In the current study we assessed potentiation of the acoustic startle response in response to darkness, an anxiogenic, nonspecific stressor that has previously been associated with PTSD and alterations in HRV (Kamkwalala et al., Citation2012). We found an interaction of HIV and childhood maltreatment, such that greater startle potentiation darkness was observed in women who had experienced high compared to low levels of childhood maltreatment only in those living without HIV. Childhood maltreatment did not influence DES in WLWH, indicating that HIV may also have long term effects on this psychophysiological process. Findings from our assessment of HRV during the DES paradigm suggest that this could be due to the impact of chronic HIV on ANS regulation by shifting it into SNS dominance over the PNS. Indeed, RSA was significantly lower in WLWH compared to those living without HIV, results that corroborate a meta-analysis reporting lower HRV and SNS dominance in PLWH on ART (McIntosh, Citation2016).
Interestingly, our analysis indicated that the effect of HIV status on RSA was impacted by childhood maltreatment and the phase of the DES paradigm in which it was collected. In women who had experienced low levels of childhood maltreatment, RSA was significantly lower during the dark (anxiogenic phase) compared to the light phase of the DES task in women without HIV. While a reduction in HRV and sympathetic dominance occur under anxiogenic and other stressful situations (McCorry, Citation2007; Williams et al., Citation2019), this difference in RSA was not present in WLWH with low levels of childhood maltreatment, likely due to the lower RSA during the light condition in WLWH compared to those living without HIV. These effects of HIV status on HRV were present only in women who had experienced low levels of childhood maltreatment and not in women who had experienced high levels of childhood maltreatment, similar to the results of PTSD symptom severity.
Taken together, these results indicate that HIV may increase risk for PTSD symptoms in trauma-exposed individuals by conferring ANS dysfunction via increased sympathetic dominance and decreased parasympathetic tone (). The current results suggest that WLWH may benefit from trauma-informed interventions focused on mitigating the impacts of stress on adverse physical and behavioral health outcomes that are linked to ANS dysfunction. These behavioral interventions that may restore ANS function may also have beneficial impacts on HIV viral reservoir, as ANS dysfunction has also been linked to differences in viral load (Sloan et al., Citation2006). Strengths of the current study include a sample of WLWH and without HIV generally representative representation of the Atlanta WIHS cohort, the use of the clinician-administered CAPS-5 for the assessment of PTSD, the inclusion of multiple index traumas our clinical assessment of PTSD that takes both psychological and medical consequences in the face of polytrauma into account, and the measurement of psychophysiological during a nonspecific stressor (DES). However, there were several limitations in the study: the cross-sectional design prohibits our ability to determine causality of childhood maltreatment and HIV status on the assessed outcomes. It is also important to note that the generalizability of the current findings may be limited because the current sample consisted only of Black women. However, this limitation can also be viewed as strength given the disproportionate burden and prevalence of both HIV, trauma exposure and childhood maltreatment, and PTSD among Black women (Gluck et al., Citation2021; Mekawi et al., Citation2021; Ojikutu & Mayer, Citation2021).
Table 2. Summary of study findings broken down by HIV status (HIV + vs HIV-) and childhood maltreatment (Low vs. High). Significant differences are denoted by comparisons and lack of differences by dashes.
Overall, our findings indicate that HIV impacts PTSD symptom severity and HRV in Black women depending on severity of childhood maltreatment and suggest that childhood trauma may be an important factor to consider for behavioral and pharmacological treatment strategies for WLWH and PTSD. Our findings also underscore the importance of accounting for life history and current psychological conditions in WLWH as these are risk factors for adverse neuropsychiatric and somatic outcomes, ART noncompliance, and engagement in risk behaviors, such as sexual transmission risk behaviors (Machtinger et al., Citation2012; O’Cleirigh et al., Citation2013).
Acknowledgements
This study would not have been possible without the research expertise and technical assistance of Angelo Brown and all the staff and volunteers of the Grady Trauma Project and the Women’s Interagency HIV Study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Vasiliki Michopoulos
Vasiliki Michopoulos, Ph.D., is an Associate Professor of Psychiatry & Behavioral Sciences and a Core Scientist at the Emory National primate Research Center. Dr. Michopoulos is co-director of the Grady Trauma Project and co-director for the TL1 Program of the Georgia Clinical and Translational Science Alliance (CTSA).
Mariana Rocha
Mariana Rocha is a Ph.D. candidate in Neuroscience at Emory University specializing in the psychophysiological consequences of trauma exposure.
Rebecca Hinrichs
Rebecca Hinrichs is the Program Director for the Grady Trauma Project and an expert in the psychophysiological consequences of trauma exposure.
Susie Turkson
Susie Turkson is a M.D./Ph.D. candidate in Neuroscience at Virginia Commonwealth University specializing in the cognitive effects of HIV in older women.
Samya Dyer
Samya Dyer, MPH, completed degrees in biology and psychology at Virginia Commonwealth University and holds a master’s in public health.
Paul Howell
Paul Howell, MS, is a Lab Technician at Virginia Commonwealth University and holds a master’s in biomedical engineering.
Elizabeth C. Heaton
Elizabeth C. Heaton completed a Ph.D. in Neuroscience at Emory University specializing in the effects of stressors on brain health.
Jakayla Hart
Jakayla Hart completed her undergraduate studies at Emory University and her M.D. at Meharry Medical College.
Abigail Powers
Abigail Powers, Ph.D., is an Associate Professor of Psychiatry & Behavioral Sciences and a co-director of the Grady Trauma Project.
Yara Mekawi
Yara Mekawi, Ph.D., is an Assistant Professor in the Department of Psychological and Brain Sciences at Louisville University and head of the Challenging Ongoing Legacies of Racism (COLOR) Lab.
Sierra Carter
Sierra Carter, Ph.D., is an Associate Professor of Psychology at Georgia State University.
Ighovwerha Ofotokun
Ighovwerha Ofotokun, M.D., is a Professor of Medicine at Emory University, Department of Medicine, Division of Infectious Diseases. He serves as the administrative PI of the MACS/WIHS Combined Cohort Study in Atlanta, the Co-Director of the Georgia CTSA KL2 Program, the administrative PI and Co-Director of Emory Building Interdisciplinary Research Careers in Women’s Health (BIRCWH).
Tanja Jovanovic
Tanja Jovanovic, Ph.D., is a Professor in the Department of Psychiatry and Behavioral Neurosciences and the David and Patricia Barron Chair for PTSD Neurobiology at Wayne State University. She is the director of the Detroit Trauma Project.
Gretchen N. Neigh
Gretchen N. Neigh, Ph.D., is a Professor of Anatomy & Neurobiology. Dr. Neigh is director of Translational Research for the VCU Institute of Women’s Health, co-director of research for BIRCWH, and co-director for the Clinical and Translational Sciences PhD Program at Virginia Commonwealth University.
References
- Adimora, A. A., Ramirez, C., Benning, L., Greenblatt, R. M., Kempf, M. C., Tien, P. C., Kassaye, S. G., Anastos, K., Cohen, M., Minkoff, H., Wingood, G., Ofotokun, I., Fischl, M. A., & Gange, S. (2018). Cohort profile: The women’s interagency HIV study (WIHS). International Journal of Epidemiology, 47(2), 1–9. https://doi.org/10.1093/ije/dyy021
- Alim, T. N., Graves, E., Mellman, T. A., Aigbogun, N., Gray, E., Lawson, W., & Charney, D. S. (2006). Trauma exposure, posttraumatic stress disorder and depression in an African-American primary care population. Journal of the National Medical Association, 98(10), 1630–1636. http://www.ncbi.nlm.nih.gov/pubmed/17052054
- Azulay, N., Olsen, R. B., Nielsen, C. S., Stubhaug, A., Jenssen, T. G., Schirmer, H., Frigessi, A., Rosseland, L. A., & Tronstad, C. (2022). Reduced heart rate variability is related to the number of metabolic syndrome components and manifest diabetes in the sixth Tromsø study 2007–2008. Scientific Reports, 12(1), 11998. https://doi.org/10.1038/s41598-022-15824-0
- Barkan, S. E., Melnick, S. L., Preston-Martin, S., Weber, K., Kalish, L. A., Miotti, P., Young, M., Greenblatt, R., Sacks, H., & Feldman, J. (1998). The women’s interagency HIV study. WIHS collaborative study group. Epidemiology, 9(2), 117–125. https://www.ncbi.nlm.nih.gov/pubmed/9504278 https://doi.org/10.1097/00001648-199803000-00004
- Berntson, G. G., Bigger, J. T., Jr., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., Nagaraja, H. N., Porges, S. W., Saul, J. P., Stone, P. H., & van der Molen, M. W. (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. https://doi.org/10.1111/j.1469-8986.1997.tb02140.x
- Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., Stokes, J., Handelsman, L., Medrano, M., Desmond, D., & Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl, 27(2), 169–190. https://doi.org/10.1016/s0145-2134(02)00541-0 [pii]
- Breslau, N. (2001). The epidemiology of posttraumatic stress disorder: What is the extent of the problem? The Journal of Clinical Psychiatry, 62 (Suppl. 17), 16–22. http://www.ncbi.nlm.nih.gov/pubmed/11495091
- Brief, D. J., Bollinger, A. R., Vielhauer, M. J., Berger-Greenstein, J. A., Morgan, E. E., Brady, S. M., Buondonno, L. M., & Keane, T. M. (2004). Understanding the interface of HIV, trauma, post-traumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care, 16(Suppl 1), S97–S120. https://doi.org/10.1080/09540120412301315259
- Cao, M., Powers, A., Cross, D., Bradley, B., & Jovanovic, T. (2017). Maternal emotion dysregulation, parenting stress, and child physiological anxiety during dark-enhanced startle. Developmental Psychobiology, 59(8), 1021–1030. https://doi.org/10.1002/dev.21574
- Dedert, E. A., Calhoun, P. S., Watkins, L. L., Sherwood, A., & Beckham, J. C. (2010). Posttraumatic stress disorder, cardiovascular, and metabolic disease: A review of the evidence. Annals of Behavioral Medicine: a Publication of the Society of Behavioral Medicine, 39(1), 61–78. https://doi.org/10.1007/s12160-010-9165-9
- Delahanty, D. L., Bogart, L. M., & Figler, J. L. (2004). Posttraumatic stress disorder symptoms, salivary cortisol, medication adherence, and CD4 levels in HIV-positive individuals. AIDS Care, 16(2), 247–260. https://doi.org/10.1080/09540120410001641084
- Dennis, P. A., Kimbrel, N. A., Sherwood, A., Calhoun, P. S., Watkins, L. L., Dennis, M. F., & Beckham, J. C. (2017). Trauma and autonomic dysregulation: Episodic–versus systemic–negative affect underlying cardiovascular risk in posttraumatic stress disorder. Psychosomatic Medicine, 79(5), 496–505. https://doi.org/10.1097/PSY.0000000000000438
- Gillespie, C. F., Bradley, B., Mercer, K., Smith, A. K., Conneely, K., Gapen, M., Weiss, T., Schwartz, A. C., Cubells, J. F., & Ressler, K. J. (2009). Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry, 31(6), 505–514. [pii] https://doi.org/10.1016/j.genhosppsych.2009.05.003
- Gluck, R. L., Hartzell, G. E., Dixon, H. D., Michopoulos, V., Powers, A., Stevens, J. S., Fani, N., Carter, S., Schwartz, A. C., Jovanovic, T., Ressler, K. J., Bradley, B., & Gillespie, C. F. (2021). Trauma exposure and stress-related disorders in a large, urban, predominantly African-American, female sample. Archives of Women’s Mental Health, 24(6), 893–901. https://doi.org/10.1007/s00737-021-01141-4
- Gordan, R., Gwathmey, J. K., & Xie, L.-H. (2015). Autonomic and endocrine control of cardiovascular function. World Journal of Cardiology, 7(4), 204–214. https://doi.org/10.4330/wjc.v7.i4.204
- Grillon, C., Morgan, C. A., III, Davis, M., & Southwick, S. M. (1998). Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry, 44(10), 1027–1036. https://doi.org/10.1016/s0006-3223(98)00034-1
- Grossman, P., Wilhelm, F. H., & Spoerle, M. (2004). Respiratory sinus arrhythmia, cardiac vagal control, and daily activity. American Journal of Physiology. Heart and Circulatory Physiology, 287(2), H728–734. https://doi.org/10.1152/ajpheart.00825.2003
- Hallett, T. B., Zaba, B., Stover, J., Brown, T., Slaymaker, E., Gregson, S., Wilson, D. P., & Case, K. K. (2014). Embracing different approaches to estimating HIV incidence, prevalence and mortality. AIDS, 28(Supplement 4), S523–S532. https://doi.org/10.1097/QAD.0000000000000488
- Hilerio, C. M., Martínez, J., Zorrilla, C. D., & Torres, R. (2005). Posttraumatic stress disorder symptoms and adherence among women living with HIV. Ethnicity & Disease, 15(4 Suppl 5), S5. https://www.ncbi.nlm.nih.gov/pubmed/16315382
- Gov, H. I. V. (2021). HIV basics- Overview: Data & trends: global statistics. Retrieved June 2, 2021, from https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics
- Jovanovic, T., Kazama, A., Bachevalier, J., & Davis, M. (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. https://doi.org/10.1016/j.neuropharm.2011.02.023
- Jovanovic, T., Norrholm, S. D., Davis, J., Mercer, K. B., Almli, L., Nelson, A., Cross, D., Smith, A., Ressler, K. J., & Bradley, B. (2013). PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Molecular Psychiatry, 18(7), 742–743. https://doi.org/10.1038/mp.2012.98
- Jovanovic, T., Norrholm, S. D., Fennell, J. E., Keyes, M., Fiallos, A. M., Myers, K. M., Davis, M., & Duncan, E. J. (2009). Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Research, 167(1-2), 151–160. https://doi.org/10.1016/j.psychres.2007.12.014
- Jovanovic, T., Smith, A., Kamkwalala, A., Poole, J., Samples, T., Norrholm, S. D., Ressler, K. J., & Bradley, B. (2011). Physiological markers of anxiety are increased in children of abused mothers. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52(8), 844–852. https://doi.org/10.1111/j.1469-7610.2011.02410.x
- Kalichman, S. C., Sikkema, K. J., DiFonzo, K., Luke, W., & Austin, J. (2002). Emotional adjustment in survivors of sexual assault living with HIV-AIDS. Journal of Traumatic Stress, 15(4), 289–296. https://doi.org/10.1023/A:1016247727498
- Kamkwalala, A., Norrholm, S. D., Poole, J. M., Brown, A., Donley, S., Duncan, E., Bradley, B., Ressler, K. J., & Jovanovic, T. (2012). Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: putative sex-specific correlates of posttraumatic stress disorder. Psychosomatic Medicine, 74(2), 153–159. https://doi.org/10.1097/PSY.0b013e318240803a
- Kartha, A., Brower, V., Saitz, R., Samet, J. H., Keane, T. M., & Liebschutz, J. (2008). The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Medical Care, 46(4), 388–393. https://doi.org/10.1097/MLR.0b013e31815dc5d2
- Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., & Nelson, C. B. (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52(12), 1048–1060. http://www.ncbi.nlm.nih.gov/pubmed/7492257 https://doi.org/10.1001/archpsyc.1995.03950240066012
- Keuroghlian, A. S., Kamen, C. S., Neri, E., Lee, S., Liu, R., & Gore-Felton, C. (2011). Trauma, dissociation, and antiretroviral adherence among persons living with HIV/AIDS. Journal of Psychiatric Research, 45(7), 942–948. https://doi.org/10.1016/j.jpsychires.2011.05.003
- Kubzansky, L. D., Koenen, K. C., Spiro, A., 3rd, Vokonas, P. S., & Sparrow, D. (2007). Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Archives of General Psychiatry. 64(1), 109–116. https://doi<?sch-permit JATS-0034-007?>.org/64/1/109[pii]
- Li, J. Z., Gallien, S., Ribaudo, H., Heisey, A., Bangsberg, D. R., & Kuritzkes, D. R. (2014). Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS (London, England), 28(2), 181–186. https://doi.org/10.1097/QAD.0000000000000123
- Machtinger, E. L., Wilson, T. C., Haberer, J. E., & Weiss, D. S. (2012). Psychological trauma and PTSD in HIV-positive women: A meta-analysis. AIDS and Behavior, 16(8), 2091–2100. https://doi.org/10.1007/s10461-011-0127-4
- Martinez, A., Israelski, D., Walker, C., & Koopman, C. (2002). Posttraumatic stress disorder in women attending human immunodeficiency virus outpatient clinics. AIDS Patient Care and STDs, 16(6), 283–291. https://doi.org/10.1089/10872910260066714
- McCorry, L. K. (2007). Physiology of the autonomic nervous system. American Journal of Pharmaceutical Education, 71(4), 78. https://doi.org/10.5688/aj710478
- McIntosh, R. C. (2016). A meta-analysis of HIV and heart rate variability in the era of antiretroviral therapy. Clinical Autonomic Research: official Journal of the Clinical Autonomic Research Society, 26(4), 287–294. https://doi.org/10.1007/s10286-016-0366-6
- McLaughlin, K. A., Colich, N. L., Rodman, A. M., & Weissman, D. G. (2020). Mechanisms linking childhood trauma exposure and psychopathology: A transdiagnostic model of risk and resilience. BMC Medicine, 18(1), 96. https://doi.org/10.1186/s12916-020-01561-6
- Mekawi, Y., Carter, S., Brown, B., Martinez de Andino, A., Fani, N., Michopoulos, V., & Powers, A. (2021). Interpersonal trauma and posttraumatic stress disorder among black women: Does racial discrimination matter? Journal of Trauma & Dissociation: The Official Journal of the International Society for the Study of Dissociation (ISSD), 22(2), 154–169. https://doi.org/10.1080/15299732.2020.1869098
- Mekawi, Y., Kuzyk, E., Dixon, H. D., McKenna, B., Camacho, L., de Andino, A. M., Stevens, J., Michopolous, V., & Powers, A. (2021). Characterizing typologies of polytraumatization: A replication and extension study examining internalizing and externalizing psychopathology in an urban population. Clinical Psychological Science: a Journal of the Association for Psychological Science, 9(6), 1144–1163. https://doi.org/10.1177/21677026211000723
- Michopoulos, V., Norrholm, S. D., & Jovanovic, T. (2015). Diagnostic biomarkers for posttraumatic stress disorder: Promising horizons from translational neuroscience research. Biological Psychiatry, 78(5), 344–353. https://doi.org/10.1016/j.biopsych.2015.01.005
- Michopoulos, V., Norrholm, S. D., Stevens, J. S., Glover, E. M., Rothbaum, B. O., Gillespie, C. F., Schwartz, A. C., Ressler, K. J., & Jovanovic, T. (2017). Dexamethasone facilitates fear extinction and safety discrimination in PTSD: A placebo-controlled, double-blind study. Psychoneuroendocrinology, 83, 65–71. https://doi.org/10.1016/j.psyneuen.2017.05.023
- Moniz, P., Alçada, F., Peres, S., Borges, F., Baptista, T., Miranda, A. C., Antunes, I., Aldir, I., Ventura, F., Nina, J., & Mansinho, K. (2014). Durability of first antiretroviral treatment in HIV chronically infected patients: why change and what are the outcomes? Journal of the International AIDS Society, 17(4 Suppl 3), 19797. https://doi.org/10.7448/IAS.17.4.19797
- Mugavero, M. J., Raper, J. L., Reif, S., Whetten, K., Leserman, J., Thielman, N. M., & Pence, B. W. (2009). Overload: impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multisite human immunodeficiency virus cohort study. Psychosomatic Medicine, 71(9), 920–926. https://doi.org/10.1097/PSY.0b013e3181bfe8d2
- Murrain, M., & Barker, T. (1997). Investigating the relationship between economic status and HIV risk. Journal of Health Care for the Poor and Underserved, 8(4), 416–423. http://www.ncbi.nlm.nih.gov/pubmed/9334534 https://doi.org/10.1353/hpu.2010.0032
- Nightingale, V. R., Sher, T. G., Mattson, M., Thilges, S., & Hansen, N. B. (2011). The effects of traumatic stressors and HIV-related trauma symptoms on health and health related quality of life. AIDS and Behavior, 15(8), 1870–1878. https://doi.org/10.1007/s10461-011-9980-4
- Norrholm, S. D., Glover, E. M., Stevens, J. S., Fani, N., Galatzer-Levy, I. R., Bradley, B., Ressler, K. J., & Jovanovic, T. (2015). Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. International Journal of Psychophysiology: official Journal of the International Organization of Psychophysiology, 98(2 Pt 2), 270–275. https://doi.org/10.1016/j.ijpsycho.2014.11.005
- Norrholm, S. D., Jovanovic, T., Briscione, M. A., Anderson, K. M., Kwon, C. K., Warren, V. T., Bosshardt, L., & Bradley, B. (2014). Generalization of fear-potentiated startle in the presence of auditory cues: A parametric analysis. Frontiers in Behavioral Neuroscience, 8, 361. https://doi.org/10.3389/fnbeh.2014.00361
- O’Cleirigh, C., Traeger, L., Mayer, K. H., Magidson, J. F., & Safren, S. A. (2013). Anxiety specific pathways to HIV sexual transmission risk behavior among young Gay and bisexual men. Journal of Gay & Lesbian Mental Health, 17(3), 314–326. https://doi.org/10.1080/19359705.2012.755142
- Ojikutu, B. O., & Mayer, K. (2021). HIV prevention among Black women in the US-time for multimodal integrated strategies. JAMA Network Open, 4(4), e215356. https://doi.org/10.1001/jamanetworkopen.2021.5356
- Powers, A., Fani, N., Carter, S., Cross, D., Cloitre, M., & Bradley, B. (2017). Differential predictors of DSM-5 PTSD and ICD-11 complex PTSD among African American women. European Journal of Psychotraumatology, 8(1), 1338914. https://doi.org/10.1080/20008198.2017.1338914
- Ramirez-Moreno, D. F., & Sejnowski, T. J. (2012). A computational model for the modulation of the prepulse inhibition of the acoustic startle reflex. Biological Cybernetics, 106(3), 169–176. https://doi.org/10.1007/s00422-012-0485-7
- Redican, E., Nolan, E., Hyland, P., Cloitre, M., McBride, O., Karatzias, T., Murphy, J., & Shevlin, M. (2021). A systematic literature review of factor analytic and mixture models of ICD-11 PTSD and CPTSD using the International Trauma Questionnaire. J Anxiety Disord, 79, 102381. https://doi.org/10.1016/j.janxdis.2021.102381
- Ressler, K. J., Mercer, K. B., Bradley, B., Jovanovic, T., Mahan, A., Kerley, K., Norrholm, S. D., Kilaru, V., Smith, A. K., Myers, A. J., Ramirez, M., Engel, A., Hammack, S. E., Toufexis, D., Braas, K. M., Binder, E. B., & May, V. (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature, 470(7335), 492–497. https://doi<?sch-permit JATS-0034-007?>.org/nature09856[pii]
- Samuels, E., Khalife, S., Alfonso, C. A., Alvarez, R., & Cohen, M. A. (2011). Early childhood trauma, posttraumatic stress disorder, and non-adherence in persons with AIDS: A psychodynamic perspective. The Journal of the American Academy of Psychoanalysis and Dynamic Psychiatry, 39(4), 633–650. https://doi.org/10.1521/jaap.2011.39.4.633
- Shaffer, F., McCraty, R., & Zerr, C. L. (2014). A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Frontiers in Psychology, 5, 1040. https://doi.org/10.3389/fpsyg.2014.01040
- Shemesh, E., Rudnick, A., Kaluski, E., Milovanov, O., Salah, A., Alon, D., Dinur, I., Blatt, A., Metzkor, M., Golik, A., Verd, Z., & Cotter, G. (2001). A prospective study of posttraumatic stress symptoms and nonadherence in survivors of a myocardial infarction (MI). General Hospital Psychiatry, 23(4), 215–222. https://doi.org/10.1016/s0163-8343(01)00150-5
- Sloan, E. K., Tarara, R. P., Capitanio, J. P., & Cole, S. W. (2006). Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. Journal of Virology, 80(9), 4326–4335. https://doi.org/10.1128/JVI.80.9.4326-4335.2006
- Sloan, R., Shapiro, P., Bagiella, E., Myers, M., & Gorman, J. (1999). Cardiac autonomic control buffers blood pressure variability responses to challenge: A psychophysiologic model of coronary artery disease. Psychosomatic Medicine, 61(1), 58–68. https://doi.org/10.1097/00006842-199901000-00010
- Weathers, F. W., Keane, T. M., & Davidson, J. R. (2001). Clinician-administered PTSD scale: A review of the first ten years of research. Depression and Anxiety, 13(3), 132–156. https://doi.org/10.1002/da.1029
- Whetten, K., Shirey, K., Pence, B. W., Yao, J., Thielman, N., Whetten, R., Adams, J., Agala, B., Ostermann, J., O’Donnell, K., Hobbie, A., Maro, V., Itemba, D., Reddy, E., & Team, C. R. (2013). Trauma history and depression predict incomplete adherence to antiretroviral therapies in a low income country. PLoS One. 8(10), e74771. https://doi.org/10.1371/journal.pone.0074771
- Williams, D. P., Koenig, J., Carnevali, L., Sgoifo, A., Jarczok, M. N., Sternberg, E. M., & Thayer, J. F. (2019). Heart rate variability and inflammation: A meta-analysis of human studies. Brain, Behavior, and Immunity, 80, 219–226. https://doi.org/10.1016/j.bbi.2019.03.009
- Yasuma, F., & Hayano, J-I (2004). Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm? Chest, 125(2), 683–690. https://doi.org/10.1378/chest.125.2.683

