Abstract
Exposure to social adversity has been associated with cortisol dysregulation during pregnancy and in later childhood; less is known about how prenatal exposure to social stressors affects postnatal cortisol of infants. In a secondary analysis of data from a longitudinal study, we tested whether a pregnant woman’s reports of social adversity during the third trimester were associated with their infant’s resting cortisol at 1, 6, and 12 months postnatal. Our hypothesis was that prenatal exposure to social adversity would be associated with elevation of infants’ cortisol. Measures included prenatal survey reports of social stressors and economic hardship, and resting cortisol levels determined from infant saliva samples acquired at each postnatal timepoint. Data were analyzed using linear mixed effects models. The final sample included 189 women and their infants (46.56% assigned female sex at birth). Prenatal economic hardship was significantly associated with infant cortisol at 6 months postnatal; reports of social stressors were not significantly associated with cortisol at any time point. Factors associated with hardship, such as psychological distress or nutritional deficiencies, may alter fetal HPA axis development, resulting in elevated infant cortisol levels. Developmental changes unique to 6 months of age may explain effects at this timepoint. More work is needed to better comprehend the complex pre- and post-natal physiologic and behavioral factors that affect infant HPA axis development and function, and the modifying role of environmental exposures.
Introduction
Children who are exposed to early life social adversity are at disproportionately higher risk for chronic health conditions later in life, such as obesity and metabolic disease (Fleischer et al., Citation2021; Nelson et al., Citation2020) and mental health challenges or psychopathologies (Nelson et al., Citation2020; Wesarg et al., Citation2020). Research suggests that alterations to the hypothalamic-pituitary-adrenal (HPA) axis may be part of the complex interplay of structural and biologic influences that link social adversity and health (Nelson et al., Citation2020; Wesarg et al., Citation2020). Chronic exposure to social stressors has been associated with cortisol elevation or reduction in children (Koss & Gunnar, Citation2018), demonstrating that social adversity may contribute to an altered and ultimately pathophysiologic stress response. Excessive HPA axis activation can disrupt the ability to regulate stress effectively (Nelson et al., Citation2020), stimulate alterations to energy metabolism and increased fat deposition (Incollingo Rodriguez et al., Citation2015), or negatively impact the hippocampus and pre-frontal cortex, impacting learning and mood regulation (Johnson et al., Citation2016).
Much research in this area has examined associations between children’s exposures to adversity and their HPA axis function during childhood. However, growing evidence suggests that prenatal exposures may impact fetal HPA axis development and function, with effects persisting after birth (Moisiadis & Matthews, Citation2014; Barrero-Castillero et al., Citation2019). Specifically, studies indicate that fetal exposure to elevated glucocorticoids released by the pregnant woman/birthing person during stress is associated with infant HPA axis dysregulation in the immediate postnatal period (Moisiadis & Matthews, Citation2014; Engel & Gunnar, Citation2020), and in some cases extends into later infancy (Niwa et al., Citation2020) and childhood (Ilg et al., Citation2019). The type and direction of HPA axis dysregulation found in studies varies, and may depend on expected developmental variations in cortisol secretion and reactivity throughout childhood. For example, cortisol reactivity in infants exposed to prenatal stress may be abnormally dampened in the early postnatal period when hyperarousal is typically expected, whereas cortisol secretion is elevated in later infancy and childhood when levels are expected to be lower (Moisiadis & Matthews, Citation2014; Engel & Gunnar, Citation2020). In either case, there is concern that these “programming” effects on fetal physiology could prolong impact on the infant’s stress response and regulation, and thus compromise later health. Social adversity can be a significant source of stress during pregnancy (Mitchell & Christian, Citation2017; Schreier et al., Citation2016), and, therefore, its association with HPA axis function in infancy warrants further investigation.
Gaps exist in knowledge about the trajectory of potential adversity-related alterations in HPA axis activity throughout infancy, including whether effects in the neonatal period persist across the first year of life. In addition, few studies have examined the impact of social adversity on HPA axis function in infants from diverse ethnic backgrounds or gestational ages. A better understanding of prenatal influences on infant stress physiology among representative groups of children is essential for the development of effective perinatal clinical and policy interventions that may prevent or disrupt biosocial pathways from adversity to disease.
Aims of this study
The purpose of our study was to understand how prenatal social adversity is associated with physiologic stress in infancy in a demographically diverse sample of infants. The primary aim of this study was to examine whether pregnant women’s reports of social adversity during the third trimester were associated with their infant’s resting cortisol level at 1, 6, and 12 months of age. Our hypothesis was that reports of prenatal exposure to social adversity would be associated with elevation of infants’ resting cortisol.
Methods
Design and sample
This was a secondary analysis of data collected in a longitudinal study of various risk factors during pregnancy, birth outcomes, and the stress regulation of infants in the first year of life (Weiss et al., Citation2023). In the original study, pregnant women 18 years of age or older were recruited from one of two university-affiliated obstetric clinics or a county infant health program in San Francisco, California. Inclusion criteria were English or Spanish fluency, between 24 and 34 weeks gestation, and identified by their obstetric clinician as being at increased risk for preterm labor based on health status and medical history. Participants who delivered at term (55%) were retained in the original study along with those who delivered prematurely (45%). Pregnant women who had ongoing steroid use or a history of endocrine conditions, those who smoked, and those with serious medical problems or cognitive impairment were excluded. Additionally, infants with chromosomal and genetic anomalies, chronic lung disease, congenital heart disease, or other major neonatal illness were excluded. The total number of participant dyads in the original study was 235. This study further limited the sample to those who had complete cortisol data for at least one of the three postnatal timepoints, for a total N of 189 dyads.
Protection of human subjects
As this was a secondary analysis of de-identified data, this specific analysis was exempt from review by the Institutional Review Board (IRB). In the original study, a member of the research team obtained informed consent from eligible and interested participants. The pregnant participants also provided proxy consent for their infants to participate. The original study was approved by the investigator’s IRB to ensure protection of human subjects (IRB #: 14-13516, 299254).
Measures
Data analyzed for this study were collected at 4 timepoints: prenatally – during the 3rd trimester of pregnancy, and postnatally – at 1, 6, and 12 months after birth (). Survey data were collected at all four timepoints; electronic health record (EHR) data were collected after delivery; and infant biospecimens were collected at all three postnatal timepoints.
Table 1. Measures and timing of data collection.
Outcome: infant cortisol
Resting cortisol levels were measured from infant saliva samples acquired at 1, 6, and 12 months and available as ug/dL values. Samples were collected by a research assistant in the participant’s home using a strict protocol. At the 1 month of age data collection point, if the infant was still hospitalized (14% of the sample), the specimen was collected by the nurse in the NICU using the same protocol. Infants were not fed within 30 minutes prior to specimen collection. No procedures or unusual social stimulation occurred with the infant for 5 minutes while they rested. After 5 minutes of rest, the end of a SalivaBio infant swab was placed in the infant’s mouth between the cheek and bottom gum and left in place for 5 minutes to assure that the swab was saturated. Biospecimens were stored at 20° Centigrade until they were sent to Salimetrics biochemical laboratory for analysis. At the lab, they were thawed to room temperature, vortexed, and centrifuged at 3000 RPM for 15 minutes prior to assay. Assays were performed with the Salimetric Salivary Cortisol Assay Kit, and high sensitivity salivary cortisol enzyme immunoassay (ELISA) used to analyze samples in duplicate (Weiss et al., Citation2023).
Predictors: prenatal social adversity
Prenatal experiences of social adversity were identified during the third trimester of pregnancy, at the time of enrollment in the original study. Two measures were used to identify experiences of social adversity in the prenatal period: 1) total number of social stressors over the past year, and 2) presence of economic hardship at the time of study enrollment.
Number of prenatal social stressors. Participants completed the Crisis in the Family Systems – Revised (CRISYS-R) Questionnaire, a 63-item written survey that evaluates the experience of various environmental and socio-economic stressors in the previous 12 months, in 11 possible domains (Berry et al., Citation2001). Respondents indicated “yes” or “no” to the question: “Has this event happened to you in the past 12 months?” This tool has demonstrated validity and reliability with diverse populations (Berry et al., Citation2001) and Spanish-speakers (Berry et al., Citation2006). In this study, we used the total third-trimester CRISYS-R events score to represent the total number of prenatal social stressors, where a higher number indicates exposure to a greater number of stressors.
Prenatal economic hardship. The presence of economic hardship was determined at the time of enrollment in the original study by participants’ response to the item: “Please check all types of government assistance you or your children receive.” Response options included up to seven different government assistance programs including food, housing, employment, or disability assistance, Medicaid health insurance, or other types of assistance not listed. Qualification for and enrollment in these programs represent material hardships that have been shown in the literature to impact health independent of income poverty, particularly in areas with a high cost of living (Neckerman et al., Citation2016; Rodems & Shaefer, Citation2020). Due to variability in cell counts across responses, we dichotomized responses into “yes” vs. “none,” where “yes” indicated the selection of any of the assistance options. Therefore, economic hardship was treated as a categorical binary variable in the final analyses.
Covariates
Covariates were chosen for their known and theoretical associations with social adversity or HPA axis function and development (Barrero-Castillero et al., Citation2019; Kortesluoma et al., Citation2022; Sutherland & Brunwasser, Citation2018), and included: age of the mother, infant’s gestational age and sex, obstetric and neonatal medical risks and complications, and depression symptoms or perceived stress reported by the mother. Participants self-reported their age and race in the original study through a sociodemographic questionnaire. Although we included participant self-reported race data in descriptive analyses to reflect the diversity of our sample, we did not include this variable as a covariate in the regression models. Self-reported race is a social construct that is tied to several complex societal issues – such as experiences of discrimination and racism – that impact health and are worthy of exploration in relation to infant cortisol (Paradies et al., Citation2015; Berger & Sarnyai, Citation2015). However, self-reported race alone has been considered too imprecise to be a proxy measure of such complex experiences (Flanagin et al., Citation2021; Adkins-Jackson et al., Citation2021); future research should include more specific and validated measures to best evaluate these associations (Adkins-Jackson et al., Citation2021).
Infant’s gestational age and sex assigned at birth were identified through review of the EHR. Data from the EHR regarding risks and complications related to pregnancy (e.g. placenta previa, polyhydramnios, substance use, and anemia) were extracted and scored to determine obstetric risk using a validated and reliable index with excellent predictive validity for adverse birth outcomes (Lobel et al., Citation2008). Similarly, the Morbidity Assessment Index for Newborns (MAIN) was used to measure severity of an infant’s health problems based on data extracted from the EHR (Verma et al., Citation1999). It contains 47 binary items representing 24 attributes of early neonatal morbidity, such as complications from fetal drug exposure or intraventricular hemorrhage. Weights are assigned to items reflecting overall morbidity based on predictive analyses with a sample of approximately 3000 newborns. A final score is computed by aggregating scale values of all items in the inventory from birth to 7 d of life. The MAIN has demonstrated substantial validity and reliability in a variety of studies (Verma et al., Citation2005).
Participants’ depression symptoms were measured via the Patient Health Questionnaire-9 (PHQ-9), which assesses how frequently respondents experienced depressive symptoms over the past two weeks (scores range from 0 to 24, with higher scores indicating greater severity of depressive symptoms) (Kroenke et al., Citation2001). The PHQ-9 has been used in pregnant populations (Sidebottom et al., Citation2012) and shown validity and reliability in diverse populations, including Spanish-speaking (Zhong et al., Citation2014). Perceived stress was examined through completion of the Perceived Stress Scale (PSS-10) (Cohen et al., Citation1983). The PSS-10 measures the degree to which situations in one’s life are appraised as stressful over the last month. This scale also has been used in pregnant populations (Katus et al., Citation2022) and has demonstrated validity and reliability in diverse populations, including Spanish-speakers (Baik et al., Citation2017). Responses are summed in a final score (10–50), with higher scores reflecting greater perceived stress. The CRISYS-R tool (described above) was also administered during the 6- and 12-month postnatal timepoints, and the total events score from each timepoint was used as a covariate in respective analyses to better isolate the effects of adversity specific to the prenatal period.
Community engagement
Principles of research equity and justice emphasize the importance of partnership throughout the investigative process with patients and other stakeholders affected by a research study (Brooks & Fields, Citation2021). As this study was a secondary analysis of previously collected data, we sought to engage the voice of community members with lived experience similar to that of the original study population, including experiences with social adversity during pregnancy. A local community advisory board (CAB) affiliated with the principal investigator’s research institution initially reviewed and provided written and verbal input on this study proposal that was incorporated prior to submission for IRB approval. The CAB consisted of members who identify as Black, Indigenous, and People of Color (BIPOC) and from historically and currently minoritized and marginalized populations whose expertise included lived experiences relevant to the context of this study. Following the data analysis phase, a smaller group of community research consultants was recruited to the research team, all of whom identify as women of color and mothers with lived experiences relevant for this study. These consultants engaged in discussions with the principal investigator around the interpretation and implication of results and plans for dissemination, including participation in the co-authorship of this manuscript.
Data analysis
Descriptive statistics were calculated for the sample characteristics (as well as all outcome and predictor variables); specifically, frequency and percent for categorical variables and median and interquartile range (IQR) for continuous variables. Spearman correlations were used in preliminary analyses of relationships between the outcomes and covariates. Between group comparisons of sample characteristics were conducted using chi-square, t-test, or Wilcoxon rank-sum tests as appropriate for variable type and distribution. We log-transformed variables to normality as needed. These analyses were conducted using Stata version 17 (StataCorp LLC 2021).
To test for an association between the outcome and predictor variables, and to account for the repeated measurements, data were analyzed using linear mixed models (LMM) using lme4 version 1.1.31 (Bates et al., Citation2015, Madison, WI, USA) with lmerTest version 3.1.3 (Kuznetsova et al., Citation2017, Lyngby, Denmark) in R version 4.2 (R Foundation for Statistical Computing, Citation2021). To handle missing covariate values, we used multiple imputation by chained equations (1000 multiple imputations) using mice version 3.15.0 (van Buuren & Groothuis-Oudshoorn, Citation2011, Leiden, The Netherlands) with utility functions from broom.mixed version 0.2.9.4 (Bolker & Robinson, Citation2022, Hamilton, Ontario, Canada). We built a backwards stepwise regression model considering terms with univariable p < 0.2, removing the least significant term when any p < 0.05. Plots of marginal means with 95% confidence intervals (CIs) were created using a nonparametric bootstrap with 1000 iterations.
Power and sample size
Design details for the original study, including power and sample size, are published elsewhere (Weiss et al., Citation2023). For this secondary data analysis, to ensure the sample size would be adequate to assess our hypothesis, we calculated the minimum detectable effect size (MDE) in our cohort. We ran 500 simulations for each scenario, solving for the MDE. We assumed 121 participants (number of individuals in our dataset with complete cortisol at 1 month postnatal and prenatal CRISYS-R data), α = 0.05, power = 0.8, a continuous outcome at 3 timepoints with a within subject correlation of 0.3, and a continuous predictor (covariate of interest). We computed power using LMM for standard normal distributions in R (R Foundation for Statistical Computing, Citation2021) with lme4 (Bates et al., Citation2015). The MDE was 0.20 (based on standard normal distributions) for a main effect difference, corresponding to ω2 = 0.081, roughly a medium effect size (Murphy & Myors, Citation2004).
Results
Sample characteristics
Characteristics for the sample are summarized in . The median age of mothers was 34 years (IQR: 31, 38) and median gestational age of infants was 37.1 weeks (IQR: 34.8, 39.15). Almost half of the infants (46.56%) were assigned female sex at birth. Diverse racial and ethnic groups were represented in the sample, including Asian (15.87%), Black/African-American (18.52%), Hispanic/Latinx (25.4%), White/European, non-Latinx (34.39%), and a few who identified as American Indian/Alaskan native (0.53%) or Hawaiian/Pacific Islander (1.59%). Average obstetric risk and neonatal morbidity were both low, indicating minimal complications, or health problems for participants overall. Prenatal symptoms of depression were mild for the full sample (median: 4; IQR: 2, 9), as was perceived stress (median: 14.5; IQR: 11, 20).
Table 2. Demographic and health characteristics of the sample (N = 189).
Infant resting cortisol was slightly higher at 1 month postnatal (median: 0.32 µg/dL; IQR: 0.19, 0.56) than at 6 months (median: 0.23 µg/dL; IQR: 0.15, 0.42) and 12 months (median: 0.2 µg/dL; IQR: 0.11, 0.49). The median number of prenatal social stressors was 5 (IQR: 3, 8), and just under half of the sample experienced prenatal economic hardship (49.04%).
Preliminary assessment of univariate effects
Only two covariates were significantly associated with infant cortisol in preliminary univariate correlations conducted separately for each timepoint (see Supplemental Table). Maternal age was found to be negatively correlated (r = −0.27) and neonatal morbidity was positively correlated (r = 0.33) with infant cortisol at 12 months postnatal (p < 0.05). No other significant correlations were noted at any time point.
Results of the unadjusted regressions for the effects of individual measures of prenatal social adversity on infant resting cortisol levels at each of the three postnatal timepoints are shown in . Prenatal economic hardship was significantly associated with infant cortisol at all postnatal timepoints, with a negative association at the 1 month (ß = −0.46; 95% CI: −0.88, −0.04; p = 0.03), and positive associations at the 6 months (ß = 0.96; 95% CI: 0.45, 1.49; p = 0.0003) and 12 months (ß = 0.76; 95% CI: 0.15, 1.37; p = 0.01) timepoints. Conversely, the total number of prenatal stressors reported on the CRISYS-R survey was not a significant predictor of infant cortisol at the 1 month (ß = −0.09; 95% CI: −0.33, 0.14; p = 0.43), 6 months (ß = −0.008; 95% CI: −0.42, 0.40; p = 0.97), or 12 months (ß = 0.03; 95% CI: −0.53, 0.60; p = 0.90) postnatal timepoints.
Table 3. Univariate analysis of effects of prenatal social adversity on infant resting cortisol (N = 189).
Linear mixed model
Multivariable regression results from the mixed model are presented in . The number of prenatal stressors had no significant relationship with infant cortisol at any timepoint in univariate analyses and was therefore not included in the final linear mixed model. In preliminary adjusted models, neonatal morbidity no longer had a significant relationship with cortisol. The only covariate that maintained a significant relationship with cortisol in the multivariable analyses was the mother’s age, and, therefore, it was the only covariate included in the final mixed model. Prenatal economic hardship was significantly associated with infant cortisol at 6 months postnatal compared to baseline (ß = 0.98; 95% CI: 0.35, 1.60; p = 0.0027), but not at 12 months compared to baseline (ß = 0.17; 95% CI: −0.51, 0.85; p = 0.63). shows a comparison of the marginal means of infant cortisol at each timepoint and highlights the significantly higher cortisol levels of infants at 6 months of age who had been exposed to prenatal economic hardship compared with those who were not exposed.
Figure 1. Marginal means of infant cortisol at 1, 6, and 12 months postnatal, by economic hardship exposure. Error bars represent 95% confidence intervals.
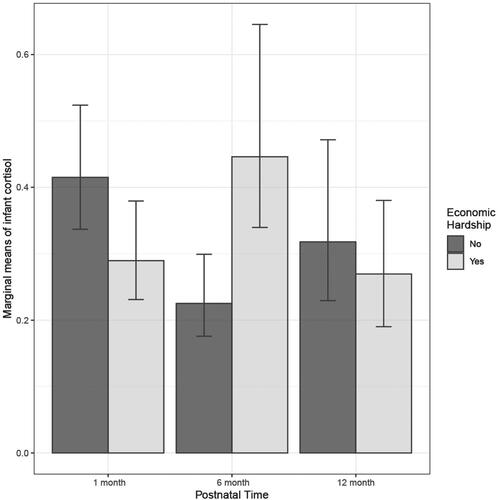
Table 4. Linear mixed model effects of prenatal exposure to economic hardship on infant resting cortisol over first postnatal year (N = 189).
Associations of sample characteristics with economic hardship
After finding a significant association between prenatal economic hardship and infant cortisol, we explored whether there were any significant differences in sample characteristics between participants who reported prenatal economic hardship and those who did not (). Participants who reported economic hardship were significantly more likely to be younger (median 31 vs. 36 years; p < 0.001) and their infants were less likely to be assigned female sex at birth (41.56 vs. 60%; p = 0.02). Participants with hardship were significantly more likely to identify their race as Black/African-American (38.96 vs. 2.5%) or Hispanic/Latinx (40.26 vs. 3.75%), while those who did not report hardship were significantly more likely to identify as Asian (27.5 vs. 5.19%) or White/European (63.75 vs. 6.49%; p < 0.001). Those who experienced prenatal economic hardship were also significantly more likely to report greater depressive symptoms (median 7.5 vs. 3; p < 0.001), higher perceived stress (median 17 vs. 13; p < 0.001), and more prenatal social stressors (median 8 vs. 4; p < 0.001).
Table 5. Characteristics of dyads by prenatal exposure to economic hardship (N = 157).
Discussion
In this study of a diverse sample of pregnant women and their infants, we found that prenatal economic hardship (i.e. reliance on government assistance with food, housing, health care, or other essential needs) was associated with higher levels of cortisol in infants at 6 months postnatal. This supports previous evidence demonstrating a link between prenatal social adversity and infant cortisol in the early neonatal period (Keenan et al., Citation2007), and contributes new knowledge around these associations in later infancy. Our findings should be interpreted with caution given that they are based on a single cross-sectional measurement of cortisol at each timepoint; however, they shed light on important potential pathways between prenatal economic hardship and elevated infant cortisol that warrant future investigation.
One possible mechanism is that psychological distress related to hardship experienced during pregnancy results in fetal exposure to elevated intrauterine levels of glucocorticoids, negatively altering fetal HPA axis development (Barrero-Castillero et al., Citation2019; Engel & Gunnar, Citation2020). While physiologic mechanisms exist to protect the fetus from the natural elevation of maternal glucocorticoid levels that occurs in the third trimester, evidence suggests that excess elevation due to stress, anxiety, or depression may impact these mechanisms in ways that leave the fetus vulnerable to programming of HPA axis dysregulation (Moisiadis & Matthews, Citation2014; Engel & Gunnar, Citation2020; Reynolds, Citation2013; Musillo et al., Citation2022). However, despite the fact that participants with economic hardship in this sample were significantly more likely to have higher depression symptoms and perceived stress, neither factor turned out to be significantly associated with infant cortisol. The PHQ-9 and PSS-10 measured participants’ recent symptoms (over the past two weeks and months, respectively). It is possible that these timeframes do not reflect the exact window of prenatal exposure to maternal psychological distress that is most critical to fetal HPA axis development. Associations between prenatal psychological distress and fetal cortisol are known to be complex and multifactorial (Moisiadis & Matthews, Citation2014; McGowan & Matthews, Citation2018), and these findings may have been impacted by other unmeasured maternal exposures including those which may have occurred during earlier trimesters.
Alternatively, this could suggest that other mechanisms are responsible for elevated infant cortisol. For example, undernutrition during pregnancy may also increase intrauterine HPA axis activity (Engel & Gunnar, Citation2020), therefore limited access to healthy foods resulting from economic hardship could contribute to greater fetal cortisol exposure. More studies are needed to better elucidate physiologic pathways between prenatal economic hardship and infant cortisol that may be connected to or independent of glucocorticoid elevation, including epigenetic changes, alterations to the microbiome, or inflammation (Barrero-Castillero et al., Citation2019; McGowan & Matthews, Citation2018). Lack of access to prenatal care has been associated with obstetric complications that could be another mechanism to influence fetal stress physiology (Keenan et al., Citation2007), although we did not detect these associations in our study. In our sample, there were significantly more males in the group exposed to economic hardship, and sex-dependent effects are also worthy of consideration. Evidence suggests that sex may modify the effects of prenatal stress on infant cortisol (Engel & Gunnar, Citation2020; Kortesluoma et al., Citation2022; Sutherland & Brunwasser, Citation2018); future studies with larger sample sizes are needed to explore such effects.
While we found a significant association between prenatal economic hardship and infant cortisol at all postnatal timepoints in unadjusted analyses, the association was only significant at the 6-month timepoint in the final mixed effects model and, therefore, did not demonstrate persistence across time. This may be explained by developmental factors that influence inter- and intra-individual variability in stress regulation across time during infancy. For example, prenatal stress may have altered the development of the HPA axis circadian rhythm in a way that is manifested at 6 months but not later as the rhythm continues to mature (de Weerth et al., Citation2003; Ivars et al., Citation2015).
Caregivers also play a major role in early infant emotion regulation and usually provide a buffer against stress (Engel & Gunnar, Citation2020). As the infant progresses developmentally, cortisol levels may be impacted by a number of factors including the child’s temperament (Bolten et al., Citation2013), level of attachment to caregivers (Berlin et al., Citation2019), or caregiving behaviors (Wu & Feng, Citation2020). At 6 months postnatal, infants become more engaged with the environment around them as they begin to explore and increase awareness of the world beyond their primary caregiver, including becoming more independent in mobility as well as transitioning to different types of nutritional intake (Scharf et al., Citation2016). This augmented interaction with the surrounding environment may increase their exposure to the ongoing scarcity of daily food, warm clothing, or other challenges that economic hardship can bring. By 12 months postnatal, the infant has progressed developmentally in the ability to use self-soothing, disengagement of attention, and other strategies to regulate emotional distress (Gennis et al., Citation2022). Advances in these skills may have concomitant effects on infant cortisol levels, enabling greater resilience to economic hardship. More research is needed to see whether developmental factors moderate associations between prenatal socioeconomic adversity and cortisol in later infancy with the hopes of identifying ways to support infant resilience.
Chronic elevation of cortisol has been linked to adverse metabolic, psychiatric, and cardiovascular health outcomes (Nelson et al., Citation2020; Incollingo Rodriguez et al., Citation2015; Barrero-Castillero et al., Citation2019). The combination of psychosocial and metabolic stressors during pregnancy – which may occur more frequently among populations experiencing economic hardship – may compound the impact of prenatal adversity on fetal dysregulation through placental signaling pathways (Musillo et al., Citation2022). The potential for fetal programming of persistent HPA axis hyperactivity therefore has significant clinical implications (Moisiadis & Matthews, Citation2014; Engel & Gunnar, Citation2020). Evidence from clinical studies demonstrates that cortisol regulation may be modifiable through psychosocial interventions in childhood (Berlin et al., Citation2019; Slopen et al., Citation2014), but the optimal time for intervention remains unclear. Stress reduction interventions during pregnancy have also been shown to reduce cortisol levels in the pregnant woman/birthing person (Urizar et al., Citation2019), but little is known about their postnatal effects on infant cortisol. Mounting evidence of fetal programming suggests that the study of prenatal interventions to support healthy fetal HPA axis development in the context of hardship or stress is a critical area for future research (Musillo et al., Citation2022).
Our findings also highlight important policy implications related to economic hardship during pregnancy. Almost half of the study sample reported prenatal economic hardship, and a national study found that almost one-quarter of peripartum women in the U.S. report unmet health care needs, with lack of affordability of care as one of the most common needs (Taylor et al., Citation2021). Prenatal economic hardship has been linked to adverse birth outcomes including low birthweight and preterm birth (Leifheit et al., Citation2020). Fetal cortisol has been proposed as an important mediator in such relationships (Baibazarova et al., Citation2013). Our findings suggest that specific alterations of the fetal HPA axis warrant further research to better understand any potential programming effects. It is also noteworthy that participants with economic hardship in our study were more likely to be people of color, which may reflect structural factors, such as systemic racism and xenophobia that are known to contribute to economic disparities and inequities in birth and infant health outcomes (Burris & Hacker, Citation2017). Future studies that include measures of structural racism may help to elucidate these associations (Adkins-Jackson et al., Citation2021).
Even without a clear understanding of the mechanisms at play, results from our study add to a growing body of evidence that supports policy and structural changes as the highest priority to create healthier and more equitable social conditions for all pregnant women/birthing people. In addition to further justifying the importance of social determinants, such as equitable housing and food access that have known benefits to perinatal health, our findings also support the need for policies that help with stress reduction during pregnancy, such as better work accommodations or medical leave benefits for pregnant women/birthing people. Support for caregivers about ways to help their infants manage distress when exposed to adversity is also warranted. Promising structural interventions to address economic inequality for pregnant women/birthing people are being implemented, such as the “Abundant Birth Project” in San Francisco, CA, which provides pregnancy income supplements in communities experiencing disproportionately high rates of adverse birth outcomes (Malawa et al., Citation2021). Evaluation of such programs offers new and exciting opportunities to advance the field of stress research by exploring how social care interventions influence stress physiology for both pregnant women/birthing people and their infants.
Limitations and strengths
There are three limitations to consider. First, secondary analysis is inherently limited by the measures and variables available to address questions that were not included in the original study’s aims. As such, our findings may be influenced by other variables that were not available for analysis (e.g. experiences with structural racism or the mother’s body mass index and prenatal cortisol level (Barrero-Castillero et al., Citation2019; Berger & Sarnyai, Citation2015; Volqvartz et al., Citation2023)). Second, our secondary analysis of infants’ cortisol from only one specimen at each time point limited our ability to account for dynamic variations in resting cortisol levels throughout the day; multiple assessments would have increased reliability of this measure. Third, the use of survey data to measure exposures to social adversity could have been affected by response bias related to the sensitive nature of the stressors (Tourangeau & Yan, Citation2007) measured in the CRYSIS-R instrument, or by “survey fatigue” related to the number of items in the questionnaire (Le et al., Citation2021). These factors could have led to higher completion rates among individuals with fewer stressors.
This study is strengthened by its diverse sample, which increases the generalizability of results to similar populations in the U.S., and the use of longitudinal data which allowed us to explore associations across multiple ages in infancy. Our use of enrollment in governmental assistance programs as an indicator of economic hardship strengthened our ability to capture a wider range of financial strain that income or education variables may have missed in our study population (Neckerman et al., Citation2016; Rodems & Shaefer, Citation2020). Our study was enriched by engagement with community stakeholders, although it is important to acknowledge that they were included during some, but not all, stages of the research process. Future biosocial research would be enhanced by frameworks, such as Community Partnered Participatory Research (CPPR), in which equitable collaboration and partnership with community stakeholders occurs during all study activities from conceptualization to dissemination (Brooks & Fields, Citation2021). Investigators choosing to conduct secondary analyses should be encouraged to collaborate with stakeholders during critical phases of the process such as identifying the research problem and analyzing data, and also prioritize the use of datasets from studies in which the original data collection process incorporated community partnership and engagement.
Conclusion
Results from our study suggest that prenatal exposure to economic hardship may influence infant stress physiology, although the effects do not appear to be persistent across infancy. More work is needed to better comprehend the complex pre- and postnatal physiologic and behavioral factors that affect infant HPA axis development and function, and the modifying role of environmental exposures. Increased understanding of these relationships might help explain how social factors get “under the skin” and affect the stress physiology of pregnant women/birthing people and their babies. It can also inform when and how to address adversity and stress during the perinatal period.
Availability of data and material
Data used in this study are available on reasonable request.
Supplemental Material
Download MS Word (12.3 KB)Acknowledgments
We would like to acknowledge the contributions of other members of our research team, Sandra Niemann and Nina Ahlers, as well as the original study participants without whom this work would not have been possible.
Disclosure statement
The authors have no conflicts of interest to declare that are relevant to the content of this article. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Additional information
Funding
Notes on contributors
Victoria F. Keeton
Victoria F. Keeton is Assistant Professor of Research at the University of California, Davis School of Nursing. Her research focuses on stress as a physiologic contributor to metabolic disease and emotional dysregulation in children who experience social adversity.
Thomas J. Hoffmann
Thomas J. Hoffmann is Professor of Epidemiology and Biostatistics at the University of California, San Francisco. His applied work in statistics and computer science encompasses a wide variety of genetic association studies related to human health.
Kalisha Moneé Goodwin
Kalisha Moneé Goodwin is an independent community research consultant focused on supporting birth equity and justice for women and birthing people of color.
Bree Powell
Bree Powell is an independent community research consultant focused on supporting birth equity and justice for women and birthing people of color.
Sophia Tupuola
Sophia Tupuola is an independent community research consultant focused on supporting birth equity and justice for women and birthing people of color.
Sandra J. Weiss
Sandra J. Weiss is Professor and Robert C. and Delphine Wentland Eschbach Chair in Mental Health at the University of California, San Francisco, School of Nursing, and Director of the Weiss Stress and Depression lab. She investigates neuroendocrine and other physiologic vulnerabilities and interactions with adverse events during pregnancy and infancy.
References
- Adkins-Jackson, P. B., Chantarat, T., Bailey, Z. D., & Ponce, N. A. (2021). Measuring structural racism: A guide for epidemiologists and other health researchers. American Journal of Epidemiology, 191(4), 1–11. https://doi.org/10.1093/aje/kwab239
- Baibazarova, E., van de Beek, C., Cohen-Kettenis, P. T., Buitelaar, J., Shelton, K. H., & van Goozen, S. H. (2013). Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology, 38(6), 907–915. https://doi.org/10.1016/j.psyneuen.2012.09.015
- Baik, S. H., Fox, R. S., Mills, S. D., Roesch, S. C., Sadler, G. R., Klonoff, E. A., & Malcarne, V. L. (2017). Reliability and validity of the Perceived Stress Scale-10 in Hispanic Americans with English or Spanish language preference. Journal of Health Psychology, 24(5), 628–639. https://doi.org/10.1177/1359105316684938
- Barrero-Castillero, A., Morton, S. U., Nelson, C. A.3rd. & Smith, V. C. (2019). Psychosocial stress and adversity: Effects from the perinatal period to adulthood. NeoReviews, 20(12), e686–e696. https://doi.org/10.1542/neo.20-12-e686
- Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
- Berger, M., & Sarnyai, Z. (2015). More than skin deep": Stress neurobiology and mental health consequences of racial discrimination. Stress (Amsterdam, Netherlands), 18(1), 1–10. https://doi.org/10.3109/10253890.2014.989204
- Berlin, L. J., Martoccio, T. L., Bryce, C. I., & Jones Harden, B. (2019). Improving infants’ stress-induced cortisol regulation through attachment-based intervention: A randomized controlled trial. Psychoneuroendocrinology, 103, 225–232. https://doi.org/10.1016/j.psyneuen.2019.01.005
- Berry, C. A., Quinn, K. A., Portillo, N., & Shalowitz, M. U. (2006). Reliability and validity of the Spanish version of the crisis in family systems-revised. Psychological Reports, 98(1), 123–132. https://doi.org/10.2466/pr0.98.1.123-132
- Berry, C., Shalowitz, M., Quinn, K., & Wolf, R. (2001). Validation of the Crisis in Family Systems-Revised, a contemporary measure of life stressors. Psychological Reports, 88(3 Pt 1), 713–724. https://doi.org/10.2466/pr0.2001.88.3.713
- Bolker, B., & Robinson, D. (2022). broom.mixed: Tidying methods for mixed models. Version R package version 0.2.9.4. https://CRAN.R-project.org/package=broom.mixed
- Bolten, M., Nast, I., Skrundz, M., Stadler, C., Hellhammer, D. H., & Meinlschmidt, G. (2013). Prenatal programming of emotion regulation: Neonatal reactivity as a differential susceptibility factor moderating the outcome of prenatal cortisol levels. Journal of Psychosomatic Research, 75(4), 351–357. https://doi.org/10.1016/j.jpsychores.2013.04.014
- Brooks, M., & Fields, E. (2021). Community partnered participatory research methods as tools for racial justice and health equity. Perspectives in Public Health, 141(5), 261–262. https://doi.org/10.1177/1757913921989377
- Burris, H. H., & Hacker, M. R. (2017). Birth outcome racial disparities: A result of intersecting social and environmental factors. Seminars in Perinatology, 41(6), 360–366. https://doi.org/10.1053/j.semperi.2017.07.002
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- de Weerth, C., Zijl, R. H., & Buitelaar, J. K. (2003). Development of cortisol circadian rhythm in infancy. Early Human Development, 73(1–2), 39–52. https://doi.org/10.1016/s0378-3782(03)00074-4
- Engel, M. L., & Gunnar, M. R. (2020). The development of stress reactivity and regulation during human development. International Review of Neurobiology, 150, 41–76. https://doi.org/10.1016/bs.irn.2019.11.003
- Flanagin, A., Frey, T., & Christiansen, S. L. (2021). Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA, 326(7), 621–627. https://doi.org/10.1001/jama.2021.13304
- Fleischer, T., Ulke, C., Beutel, M., Binder, H., Brähler, E., Johar, H., Atasoy, S., Kruse, J., Otten, D., Tibubos, A. N., Zöller, D., Speerforck, S., Grabe, H. J., Ladwig, K.-H., & Schomerus, G. (2021). The relation between childhood adversity and adult obesity in a population-based study in women and men. Scientific Reports, 11(1), 14068. https://doi.org/10.1038/s41598-021-93242-4
- Gennis, H. G., Bucsea, O., Badovinac, S. D., Costa, S., McMurtry, C. M., Flora, D. B., & Pillai Riddell, R. (2022). Child distress expression and regulation behaviors: A systematic review and meta-analysis. Children (Basel, Switzerland), 9(2), 174. https://doi.org/10.3390/children9020174
- Ilg, L., Kirschbaum, C., Li, S. C., Rosenlöcher, F., Miller, R., & Alexander, N. (2019). Persistent effects of antenatal synthetic glucocorticoids on endocrine stress reactivity from childhood to adolescence. The Journal of Clinical Endocrinology and Metabolism, 104(3), 827–834. https://doi.org/10.1210/jc.2018-01566
- Incollingo Rodriguez, A. C., Epel, E. S., White, M. L., Standen, E. C., Seckl, J. R., & Tomiyama, A. J. (2015). Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology, 62, 301–318. https://doi.org/10.1016/j.psyneuen.2015.08.014
- Ivars, K., Nelson, N., Theodorsson, A., Theodorsson, E., Ström, J. O., & Mörelius, E. (2015). Development of salivary cortisol circadian rhythm and reference intervals in full-term infants. PLoS One, 10(6), e0129502. https://doi.org/10.1371/journal.pone.0129502
- Johnson, S. B., Riis, J. L., & Noble, K. G. (2016). State of the art review: Poverty and the developing brain. Pediatrics, 137(4), e20153075. https://doi.org/10.1542/peds.2015-3075
- Katus, L., Foley, S., Murray, A. L., Luong-Thanh, B. Y., Taut, D., Baban, A., Madrid, B., Fernando, A. D., Sikander, S., Ward, C. L., Osafo, J., Marlow, M., Du Toit, S., Walker, S., Van Vo, T., Fearon, P., Valdebenito, S., Eisner, M. P., & Hughes, C. (2022). Perceived stress during the prenatal period: Assessing measurement invariance of the Perceived Stress Scale (PSS-10) across cultures and birth parity. Archives of Women’s Mental Health, 25(3), 633–640. https://doi.org/10.1007/s00737-022-01229-5
- Keenan, K., Gunthorpe, D., & Grace, D. (2007). Parsing the relations between SES and stress reactivity: Examining individual differences in neonatal stress response. Infant Behavior & Development, 30(1), 134–145. https://doi.org/10.1016/j.infbeh.2006.08.001
- Kortesluoma, S., Korhonen, L. S., Pelto, J., Tuulari, J. J., Karlsson, L., & Karlsson, H. (2022). Age and sex differences in the cortisol stress reactivity and recovery among infants exposed to prenatal psychological distress. Psychoneuroendocrinology, 135, 105580. https://doi.org/10.1016/j.psyneuen.2021.105580
- Koss, K. J., & Gunnar, M. R. (2018). Annual research review: Early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 59(4), 327–346. https://doi.org/10.1111/jcpp.12784
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
- Kuznetsova, A., Brockhoff, P. B., & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. https://doi.org/10.18637/jss.v082.i13
- Le, A., Han, B. H., & Palamar, J. J. (2021). When national drug surveys "take too long": An examination of who is at risk for survey fatigue. Drug and Alcohol Dependence, 225, 108769. https://doi.org/10.1016/j.drugalcdep.2021.108769
- Leifheit, K. M., Schwartz, G. L., Pollack, C. E., Edin, K. J., Black, M. M., Jennings, J. M., & Althoff, K. N. (2020). Severe housing insecurity during pregnancy: Association with adverse birth and infant outcomes. International Journal of Environmental Research and Public Health, 17(22), 8659. https://doi.org/10.3390/ijerph17228659
- Lobel, M., Cannella, D. L., Graham, J. E., DeVincent, C., Schneider, J., & Meyer, B. A. (2008). Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology, 27(5), 604–615. https://doi.org/10.1037/a0013242
- Malawa, Z., De La Cruz, M., & Karasek, D. (2021). Abundant Birth Project: A monthly cash supplement during pregnancy and the post-natal period provided to Black mothers (and other Black pregnant people) to address the persistent racial disparities in birth outcomes. Racism and the Economy: Focus on Health. Accessed September 12, 2023. https://www.minneapolisfed.org/-/media/assets/events/2021/racism-and-the-economy-focus-on-health/malawa-proposal.pdf
- McGowan, P. O., & Matthews, S. G. (2018). Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology, 159(1), 69–82. https://doi.org/10.1210/en.2017-00896
- Mitchell, A. M., & Christian, L. M. (2017). Financial strain and birth weight: The mediating role of psychological distress. Archives of Women’s Mental Health, 20(1), 201–208. https://doi.org/10.1007/s00737-016-0696-3
- Moisiadis, V. G., & Matthews, S. G. (2014). Glucocorticoids and fetal programming part 1: Outcomes. Nature Reviews. Endocrinology, 10(7), 391–402. https://doi.org/10.1038/nrendo.2014.73
- Murphy, K. R., & Myors, B. (2004). Statistical power analysis: A simple and general model for traditional and modern hypothesis tests (2nd ed., pp. 160-ix, 160). Lawrence Erlbaum Associates Publishers.
- Musillo, C., Berry, A., & Cirulli, F. (2022). Prenatal psychological or metabolic stress increases the risk for psychiatric disorders: the “funnel effect” model. Neuroscience and Biobehavioral Reviews, 136, 104624. https://doi.org/10.1016/j.neubiorev.2022.104624
- Neckerman, K. M., Garfinkel, I., Teitler, J. O., Waldfogel, J., & Wimer, C. (2016). Beyond income poverty: Measuring disadvantage in terms of material hardship and health. Academic Pediatrics, 16(3), S52–S9. https://doi.org/10.1016/j.acap.2016.01.015
- Nelson, C. A., Scott, R. D., Bhutta, Z. A., Harris, N. B., Danese, A., & Samara, M. (2020). Adversity in childhood is linked to mental and physical health throughout life. BMJ, 371, m3048. https://doi.org/10.1136/bmj.m3048
- Niwa, F., Kawai, M., Kanazawa, H., Okanoya, K., & Myowa, M. (2020). The development of the hypothalamus-pituitary-adrenal axis during infancy may be affected by antenatal glucocorticoid therapy. Journal of Neonatal-Perinatal Medicine, 13(1), 55–61. https://doi.org/10.3233/npm-180040
- Paradies, Y., Ben, J., Denson, N., Elias, A., Priest, N., Pieterse, A., Gupta, A., Kelaher, M., & Gee, G. (2015). Racism as a determinant of health: A systematic review and meta-analysis. PLoS One, 10(9), e0138511. https://doi.org/10.1371/journal.pone.0138511
- R Foundation for Statistical Computing. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
- Reynolds, R. M. (2013). Programming effects of glucocorticoids. Clinical Obstetrics and Gynecology, 56(3), 602–609. https://doi.org/10.1097/GRF.0b013e31829939f7
- Rodems, R., & Shaefer, H. L. (2020). Many of the kids are not alright: Material hardship among children in the United States. Children and Youth Services Review, 112, 104767. https://doi.org/10.1016/j.childyouth.2020.104767
- Scharf, R. J., Scharf, G. J., & Stroustrup, A. (2016). Developmental milestones. Pediatrics in Review, 37(1), 25–37; quiz 38, 47. https://doi.org/10.1542/pir.2014-0103
- Schreier, H. M. C., Enlow, M. B., Ritz, T., Coull, B. A., Gennings, C., Wright, R. O., & Wright, R. J. (2016). Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress (Amsterdam, Netherlands), 19(1), 45–52. https://doi.org/10.3109/10253890.2015.1117447
- Sidebottom, A. C., Harrison, P. A., Godecker, A., & Kim, H. (2012). Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Archives of Women’s Mental Health, 15(5), 367–374. https://doi.org/10.1007/s00737-012-0295-x
- Slopen, N., McLaughlin, K. A., & Shonkoff, J. P. (2014). Interventions to improve cortisol regulation in children: A systematic review. Pediatrics, 133(2), 312–326. https://doi.org/10.1542/peds.2013-1632
- StataCorp LLC. (2021). Stata statistical software: Release 17. StataCorp LLC.
- Sutherland, S., & Brunwasser, S. M. (2018). Sex differences in vulnerability to prenatal stress: A review of the recent literature. Current Psychiatry Reports, 20(11), 102. https://doi.org/10.1007/s11920-018-0961-4
- Taylor, K., Compton, S., Kolenic, G. E., Scott, J., Becker, N., Dalton, V. K., & Moniz, M. H. (2021). Financial hardship among pregnant and postpartum women in the United States, 2013 to 2018. JAMA Network Open, 4(10), e2132103. https://doi.org/10.1001/jamanetworkopen.2021.32103
- Tourangeau, R., & Yan, T. (2007). Sensitive questions in surveys. Psychological Bulletin, 133(5), 859–883. https://doi.org/10.1037/0033-2909.133.5.859
- Urizar, G. G., Jr., Yim, I. S., Rodriguez, A., & Schetter, C. D. (2019). The SMART moms program: A randomized trial of the impact of stress management on perceived stress and cortisol in low-income pregnant women. Psychoneuroendocrinology, 104, 174–184. https://doi.org/10.1016/j.psyneuen.2019.02.022
- van Buuren, S., & Groothuis-Oudshoorn, K. (2011). Mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 45(3), 1–67. https://doi.org/10.18637/jss.v045.i03
- Verma, A., Okun, N. B., Maguire, T. O., & Mitchell, B. F. (1999). Morbidity assessment index for newborns: A composite tool for measuring newborn health. American Journal of Obstetrics and Gynecology, 181(3), 701–708. https://doi.org/10.1016/s0002-9378(99)70516-8
- Verma, A., Weir, A., Drummond, J., & Mitchell, B. F. (2005). Performance profile of an outcome measure: Morbidity assessment index for newborns. Journal of Epidemiology and Community Health, 59(5), 420–426. https://doi.org/10.1136/jech.2003.019109
- Volqvartz, T., Andersen, H. H. B., Pedersen, L. H., & Larsen, A. (2023). Obesity in pregnancy—Long-term effects on offspring hypothalamic-pituitary-adrenal axis and associations with placental cortisol metabolism: A systematic review. European Journal of Neuroscience, 58(11), 4393–4422. https://doi.org/10.1111/ejn.16184
- Weiss, S. J., Keeton, V., Richoux, S., Cooper, B., & Niemann, S. (2023). Exposure to antenatal corticosteroids and infant cortisol regulation. Psychoneuroendocrinology, 147, 105960. https://doi.org/10.1016/j.psyneuen.2022.105960
- Wesarg, C., Van Den Akker, A. L., Oei, N. Y. L., Hoeve, M., & Wiers, R. W. (2020). Identifying pathways from early adversity to psychopathology: A review on dysregulated HPA axis functioning and impaired self-regulation in early childhood. European Journal of Developmental Psychology, 17(6), 808–827. https://doi.org/10.1080/17405629.2020.1748594
- Wu, Q., & Feng, X. (2020). Infant emotion regulation and cortisol response during the first 2 years of life: Association with maternal parenting profiles. Developmental Psychobiology, 62(8), 1076–1091. https://doi.org/10.1002/dev.21965
- Zhong, Q., Gelaye, B., Fann, J. R., Sanchez, S. E., & Williams, M. A. (2014). Cross-cultural validity of the Spanish version of PHQ-9 among pregnant Peruvian women: A Rasch item response theory analysis. Journal of Affective Disorders, 158, 148–153. https://doi.org/10.1016/j.jad.2014.02.012

