Abstract
Acute stress and corticosterone enhance 5-HT1A receptor-mediated responses in rat hippocampal CA1 cells within 1–2 h, through a process involving transcriptional regulation of unknown genes. Earlier studies showed that regulation of the 5-HT1A receptor gene cannot explain the functional effects. We here tested the hypothesis that corticosterone targets genes encoding RGS4 or SGK1, which can both affect the 5-HT1A receptor associated Kir channel, thus affecting 5-HT1A receptor function. To this end, the effect of a single corticosterone injection on hippocampal expression of RGS4 and SGK1 mRNAs, measured by in situ hybridization, was studied. Expression of RGS4 or SGK1 mRNA was not affected by the corticosterone injection, neither in the CA1 area nor in other hippocampal subregions. Strikingly, SGK1 mRNA expression was strongly up-regulated in the corpus callosum. We reject, however, the hypothesis that the effect of corticosterone on 5-HT1A responsiveness is mediated via altered RGS4 or SGK1 mRNA expression.
Introduction
In response to stress, the hypothalamus–pituitary–adrenal (HPA) axis is activated, leading to the release of corticosterone from the adrenal glands. Corticosteroids have previously been found to affect serotonin (5-hydroxytryptamine; 5-HT) 1A receptor-mediated responses in CA1 cells of the rat hippocampus. Specifically, after acute stress, a single corticosterone injection, or incubation of hippocampal slices with high levels of corticosterone, increased 5-HT1A receptor-mediated responses were found (Joels and De Kloet Citation1992; Hesen and Joels Citation1996; Karst et al. Citation2000). The effect of corticosterone or stress could be reversed by treatment with the glucocorticoid receptor (GR) antagonist RU38486 (Joels and De Kloet Citation1992; Hesen and Joels Citation1996). Also, it was found to depend on homodimerization and binding of the GR to the DNA (Karst et al. Citation2000), indicating that transcriptional changes are necessary for alterations in 5-HT1A receptor-mediated responses to occur. Since increased 5-HT1A receptor-mediated responses were observed already 1–2 h after corticosterone treatment (Joels and De Kloet Citation1992), transcriptional modulation should take place before this time-point and be visible already 1 h after steroid treatment. In view of this rapid time-frame, corticosterone most likely acts by targeting a gene, the product of which is involved in the altered biological response to 5-HT via the 5-HT1A receptor.
The most likely transcriptional target would be the 5-HT1A receptor gene itself, assuming that corticosterone does not affect 5-HT1A receptor mRNA stability or its translation. Yet, while some studies reported a decrease in 5-HT1A receptor mRNA in the hippocampal CA1 area after acute administration of a high corticosterone dose (Czyrak et al. Citation2002) and others reported no effect on 5-HT1A receptor mRNA or binding capacity (Joels et al. Citation1991; Meijer and De Kloet Citation1995), an increased expression (which could explain the increased functional response) was never seen. Therefore, we here tested if corticosterone targets within 1 h the transcription of other genes that might be involved in altered 5-HT1A-mediated responses. To find the rapidly induced GR-responsive genes, either large scale expression analysis or a candidate gene approach may be used. Here, we have opted to test with in situ hybridization for regulation of two candidate genes that were earlier (1) shown to be sensitive to stress or corticosterone within hours, and (2) found to affect 5-HT1A receptor function post-translationally. High sensitivity combined with high spatial resolution are the main advantages of this approach, as opposed to serial analysis of gene expression (Evans et al. Citation2002).
The first candidate is regulator of G protein signaling 4 (RGS4). Like other members of the RGS family, this molecule decreases G protein-mediated signaling by increasing GTPase activity (Watson et al. Citation1996). RGS4, like RGS10 and RGSZ1, was found to suppress 5-HT1A receptor-mediated inwardly rectifying K+ (Kir) currents (Doupnik et al. Citation1997; Willars Citation2006). Interestingly, RGS4 mRNA expression is affected by acute and chronic corticosterone elevations in locus coeruleus and in the paraventricular nucleus (PVN) of the hypothalamus (Ni et al. Citation1999). If corticosterone effects on 5-HT1A responses involve transcriptional regulation of RGS4, we expect to see decreased expression shortly after corticosterone treatment. The second candidate is serum- and glucocorticoid-regulated kinase 1 (SGK1), which regulates cell surface expression of various ion channels, including the Kir channel which is activated via the 5-HT1A receptor (Yoo et al. Citation2003). SGK1 mRNA expression was found to be increased by stress stimuli and glucocorticoid treatment in various cell lines and in the brain (Webster et al. Citation1993; Chen et al. Citation1999; Koya et al. Citation2005; Murata et al. Citation2005).
In the present study, we tested if a high dose of corticosterone changes the transcription of these potential target genes in the CA1 area such that this could explain the earlier observed increased 5-HT1A receptor-mediated responses. The corticosterone dose (Hesen and Joels Citation1996) and time-frame (Joels and De Kloet Citation1992) were selected so that they are optimal for examining transcriptional changes contributing to the functional effects.
Methods
The experiments were performed according to the European Community Council Directive 86/609/EEC and with approval of the local Animal Experiment Committee (protocol number DED108). All efforts were made to prevent suffering of animals and to limit the number of animals used. Male Wistar rats (n = 16; Harlan, Horst, The Netherlands) weighing 197 ± 9 g were housed individually on a 12 h light/dark cycle (lights on at 8.00 a.m.) with food and drinking water ad libitum. On the day of the experiment, at 9.30 a.m., animals received a subcutaneous injection with arachide oil (vehicle group; n = 8) or corticosterone (Sigma, The Netherlands; 10 mg/animal) (cort group; n = 8) in a total volume of 800 μl oil. One hour after the injection, rats were quickly decapitated without anesthetic. Trunk blood was collected in tubes containing the anticoagulant EDTA and plasma was stored at − 20°C until use in a radioimmunoassay (RIA; ICN Biomedicals Inc., Costa Mesa, CA, USA) to determine corticosterone concentrations. The brain was rapidly dissected out of the skull, frozen on dry ice, and stored at − 80°C.
For in situ hybridization, 12 μm thick coronal sections containing the hippocampus were cut on a cryostat and mounted with four sections per slide on SuperFrost Plus slides (Menzel-Glaser, Braunschweig, Germany). In situ hybridizations were performed essentially as described previously (van Riel et al. Citation2004). To study RGS4 mRNA expression, ∼240 bp sense and antisense riboprobes labeled with [35S]-UTP were generated from a linearized cDNA clone (courtesy of Koelle and Horvitz Citation1996) using T7 and T3 polymerase, respectively. After fixation, 100 μl hybridization mix containing 3 × 106 cpm of the RGS4 probe was applied to each slide. Slides were then incubated overnight at 55°C. The next day, slides were washed in saline sodium citrate (SSC) to a final stringency of 0.1 × SSC at 55°C for 30 min. Sections were then dehydrated in an alcohol series, air-dried, and exposed to a Kodak Biomax MR film for 3 weeks.
For SGK1 mRNA expression, α-[33P]-dATP end-labeled desoxyoligonucleotide probes were used. Sequences were 5′ tctggaaagagaagtgaaggcccaccaggaaagggtgcttcacat (match; reverse complement of nucleotides 456–501 of rat SGK1 coding sequence) and 5′ gctggacagagacgtgaatgcccaacaggacagggttcttcaaat (mismatch). After fixation, 100 μl hybridization mix containing 1 × 106 cpm of the SGK1 probe was applied per slide and slides were incubated overnight in a moist chamber at 42°C. The next day, sections were washed to a final stringency of 1 × SSC at 50°C for 30 min. Subsequently, the slides were dehydrated in an alcohol series, and exposed to Kodak Biomax MR film for 2 weeks.
Control experiments for mRNA expression of the mineralocorticoid receptor on hippocampal sections from the same animals were performed as described elsewhere (van Riel et al. Citation2004).
Eight hippocampal sections per rat were scanned from film and loaded into Image J (Image J 1.31 v). The cell layers of the CA1 region as well as the CA3 and dentate gyrus regions were analyzed for grey value. Expression was further tested by analyzing grey values of the medial part of the corpus callosum (CC) directly above the hippocampus, and the average of all layers of the cerebral cortex (CX) adjacent to the hippocampus. The value of the stratum lacunosum/moleculare of the CA3 area, which shows no detectable signal in all experimental conditions, was used as tissue background. The grey values for each region were averaged per rat. After this, the values of all animals of the same group were averaged. Data are presented as group mean ± standard error of mean (SEM). Since the effects of corticosterone on mRNA expression in different brain areas are independent of each other, and our question particularly addressed expression changes in the CA1 area, statistical testing was performed by means of multiple unpaired Student's t-tests between the groups. A P-value < 0.05 was considered statistically significant.
Results
As expected, plasma corticosterone levels were significantly increased by the injection with a high dose of corticosterone (vehicle 1.3 ± 0.1 μg/dl; cort 60.0 ± 11.3 μg/dl; P < 0.001). Although we did not asses functional 5-HT1A receptor function in these animals, the corticosterone values are comparable to the elevated plasma corticosterone concentration that was earlier shown to result in large 5-HT1A receptor-mediated responses (Hesen and Joels Citation1996; Karten et al. 1999).
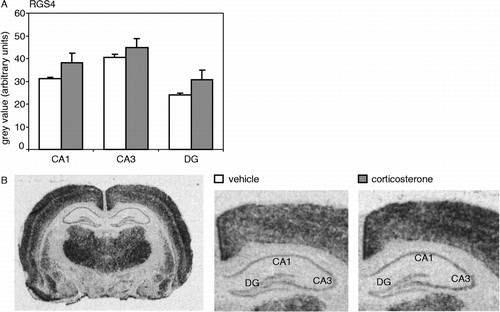
RGS4 mRNA expression showed a clear distribution in the hippocampus, with moderate expression in the CA1 and CA3 regions when compared to thalamus and CX, and lower expression in the dentate gyrus (). This is in line with previous findings (Gold et al. Citation1997). A single injection with a high dose of corticosterone did not significantly affect RGS4 mRNA expression measured 1 h later in the CA1 area nor in any of the other hippocampal subfields tested. In the CC and CX, also no effect of corticosterone on RGS4 mRNA expression was found (data not shown).
Figure 1 (A) RGS4 mRNA expression in the hippocampal subfields (CA1, CA3 and DG) was not significantly affected by an injection with a high dose of corticosterone (CA1: P = 0.17; CA3: P = 0.39; DG: P = 0.18). (B) Autoradiogram of a complete coronal brain section from a vehicle-injected animal (left) shows the overall expression pattern of RGS4 mRNA in the rat brain. On the right, hybridization signals of the hippocampus are shown separately for a vehicle- and a corticosterone-injected animal. Hybridization with the sense probe did not yield any specific signal (data not shown). N = 8 per group.
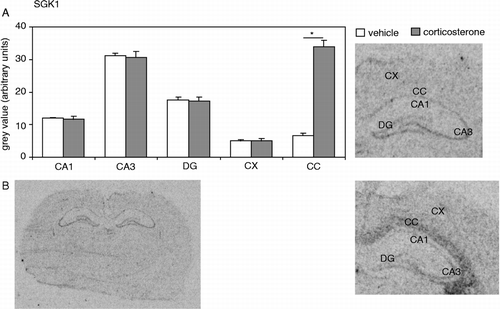
The hippocampal expression of SGK1 also showed a clear distribution, with low levels in CA1 and dentate gyrus, and relatively high levels in the CA3 area (). This was previously described by most (e.g. Lee et al. Citation2001) but not all groups (Tsai et al. Citation2002). A single corticosterone injection did not affect SGK1 mRNA expression in any of the hippocampal subfields tested or in the CX. However, corticosterone did induce a more than 5-fold increase in SGK1 mRNA expression in the CC (P < 0.001; ). The latter strongly suggests that the lack of effect of corticosterone treatment on RGS4 and SGK1 expression in the CA1 area was not due to ineffective corticosterone administration or quality of the tissue. This was confirmed by a control experiment on sections from the same animals, showing that expression of the mineralocorticoid receptor was significantly reduced in the dentate gyrus (optical density in arbitrary units for vehicle: 108 ± 4; cort: 97 ± 1, P = 0.03) though not in the CA1 area (vehicle: 98 ± 5; cort: 90 ± 2, P = 0.30).
Figure 2 (A) SGK1 mRNA expression in several brain areas after a vehicle or corticosterone injection. Expression in the hippocampal subfields (CA1, CA3 and DG), or in the CX, was not affected by corticosterone (CA1: P = 0.75; CA3: P = 0.84; DG: P = 0.78; CX: P = 0.99). In the CC, SGK1 mRNA expression was significantly (P < 0.001, indicated by an asterisk) increased 1 h following a single injection with a high dose of corticosterone. (B) Autoradiogram of a complete coronal brain section from a vehicle-injected animal (left) shows the overall expression pattern of SGK1 mRNA in the rat brain. On the right, hybridization signals of the hippocampus and adjacent CC are shown separately for a vehicle- and a corticosterone-injected animal. Hybridization with the mismatch probe did not yield any specific signal (data not shown). N = 8 per group.
Discussion
This study was initiated to examine two potential transcriptional targets of corticosterone, RGS4 or SGK1, that could contribute to the earlier observed increase of 5-HT1A receptor-mediated responses 1–2 h after corticosterone administration in hippocampal CA1 cells (Joels et al. Citation1991; Joels and De Kloet Citation1992; Hesen and Joels Citation1996; Karst et al. Citation2000). We hypothesized that in view of the rapid onset of functional corticosterone effects, the activated hormone receptor most likely targets a gene, the product of which directly affects 5-HT1A receptor function. Earlier studies had shown that, with respect to the relatively rapid functional effects, the 5-HT1A receptor gene itself is probably not the primary target of GRs (Joels et al. Citation1991; Meijer and De Kloet Citation1995; Czyrak et al. Citation2002). Our present findings indicate that transcriptional regulation through corticosteroid receptors of the RGS4 or SGK1 genes also most likely do not contribute to the observed corticosterone-induced changes in 5-HT1A receptor-mediated responses in the CA1 area.
Thus, the first candidate we tested was RGS4. A single injection with corticosterone was previously found to differentially affect RGS4 mRNA expression in locus coeruleus and PVN 6 h later (Ni et al. Citation1999). In other brain regions no dramatic changes in RGS4 mRNA expression were found, indicating that the effect of corticosterone on RGS4 expression in the brain is region specific. Also in our study, 1 h after a single corticosterone injection no differences in RGS4 mRNA expression were found in any of the hippocampal subfields tested. Since we did not include the PVN or locus coeruleus in our study, we can not be certain whether the lack of effect in the hippocampus is due to the difference in timing between the studies or to regional differences of RGS4 mRNA reactivity to corticosterone. It should be noted that there was a (non-significant) trend towards enhanced RGS4 mRNA expression in each of the hippocampal subregions. Yet, in view of the decreased responsiveness to 5-HT caused by RGS4, enhanced levels of this protein could certainly not explain the increased 5-HT1A receptor-mediated responses seen after stress and corticosterone treatment.
SGK1 is a direct glucocorticoid target gene, which was previously found to be up-regulated by various glucocorticoid treatments and stress stimuli in cell cultures (Webster et al. Citation1993), in an immediate early gene-like time frame (Chen et al. Citation1999). Moreover, SGK1 expression in the brain was up-regulated after various treatments (Koya et al. Citation2005; Murata et al. Citation2005). After exposure to an elevated plus maze, SGK1 mRNA expression was increased in the ventral tegmental area (VTA) but not the nucleus accumbens or prefrontal CX, indicating regional specificity in the transcriptional regulation of SGK1 (Koya et al. Citation2005). It should be realized, though, that exposure to an elevated plus maze most likely alters the release of many hormones, of which corticosterone is only one; it is therefore not clear at this moment whether or not a single injection with corticosterone would also increase SGK1 mRNA expression in the VTA. The present study, however, unequivocally shows that a single injection with corticosterone does not result in elevated SGK1 mRNA levels in any of the hippocampal subfields tested. Increased SGK1 expression in the hippocampus was previously found after psychophysiological stress (Murata et al. Citation2005); again, it cannot be excluded that stress-related factors other than corticosterone, like corticotrophin releasing hormone or noradrenaline, induced this effect.
In the CC, however, a specific and marked increase of SGK1 mRNA expression was found 1 h after injection with corticosterone. This marked effect on SGK1 mRNA expression in the CC indicates that corticosterone treatment and quality of the tissue were adequate in our study. This was confirmed in a control experiment, examining expression of the mineralocorticoid receptor in the dentate gyrus. The upregulation of SGK1 gene expression in the CC was not a general, non-specific effect, since RGS4 and MR mRNA expression in the CC were not affected (data not shown).
Previously, increased SGK1 mRNA expression in the CC has been found 2 h after transient global cerebral ischemia (Nishida et al. Citation2004). Also, from days 3 to 14 after brain injury increased SGK1 expression was found in this area (Imaizumi et al. Citation1994). In the latter case, oligodendrocytes were likely to be responsible for the SGK1 mRNA upregulation; this might also be the case in our present findings. Peripheral and central glial cell types can show a strong transcriptional response to glucocorticoids (Grenier et al. Citation2005). The cell specific mechanisms of GR signaling found in different types of glial cells (Grenier et al. Citation2006) may form part of the explanation for the regional specificity of the presently observed effect. In addition, corticosterone might indirectly affect SGK1 expression in glia by causing cell shrinkage, since SGK1 mRNA expression has previously been found to be highly responsive to cell volume, where dehydration and shrinkage of cells led to increased SGK1 transcript levels (Wärntges et al. Citation2002). Notably, corticosteroids have been shown to cause a strong shrinkage of cultured microglial cells (Tanaka et al. Citation1997). Although less likely, it is also possible that the increased SGK1 mRNA expression found in the CC was caused by increased expression in fiber tracts, instead of glial cells. Double-labeling with neuronal and glial markers could provide more information on this issue. Whether oligodendrocytes, other glial cells, or fiber tracts are responsible for the increased SGK1 mRNA expression in CC found after corticosterone injection thus remains to be studied. This also holds for the functional consequences of the SGK1 mRNA up-regulation. The finding does raise the possibility that the transmission of information by the axons in the CC may be subject to regulation by corticosterone.
In summary, the objective of the present study was to identify primary targets of activated corticosteroid receptors that could contribute to the corticosterone-induced changes of 5-HT1A receptor-mediated responses in CA1 hippocampal cells, which already occur after 1–2 h (Joels and De Kloet Citation1992). Any changes in transcriptional regulation that might account for these functional changes should take place earlier in time, i.e. within 1 h after corticosterone injection. This time frame is indeed sufficient for changes in mRNA levels to occur, as demonstrated by a recent study where corticosterone was found to affect transcriptional regulation of 81 genes in the hippocampus already after 1 h (Morsink et al. Citation2006). With respect to RGS4 we are not aware of such rapid transcriptional regulation, as the effect of corticosterone on mRNA levels was assessed previously after longer periods of time (Ni et al. Citation1999). The SGK1 gene, however, has the capacity to react to corticosteroids within 30 min, even at the protein level (Chen et al. Citation1999)—as is also evident from the effect observed in CC in the present study. Although we can not exclude the possibility that corticosterone affects translational or post-translational modifications of RGS4 or SGK1, the focus of our study was to search for the transcriptional changes induced by corticosterone which are essential for changes in 5-HT1A receptor-mediated responses to occur (Karst et al. Citation2000). We conclude that the increased 5-HT1A responsiveness after exposure to a high level of corticosterone is not preceded by changes in the mRNA expression of 5-HT1A, RGS4 or SGK1 in the CA1 hippocampal region. This indicates that less obvious candidate genes are probably transcriptionally regulated by corticosteroid receptors thus changing 5-HT1A receptor function.
Acknowledgements
Maaike van der Mark is acknowledged for the performance of the radioimmunoassay. Siem van der Laan and Peter Steenbergen are acknowledged for their help with the in situ hybridizations.
References
- Chen S-Y, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 1999; 96: 2514–2519
- Czyrak A, Mackowiak M, Chocyk A, Fijal K, Tokarski K, Bijak M, Wedzony K. Prolonged corticosterone treatment alters the responsiveness of 5-HT1A receptors to 8-OH-DPAT in rat CA1 hippocampal neurons. Naunyn Schmiedebergs Arch Pharmacol 2002; 366: 357–367
- Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gbeta gamma-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA 1997; 94: 10461–10466
- Evans SJ, Datson NA, Kabbaj M, Thompson RC, Vreugdenhil E, De Kloet ER, Watson SJ, Akil H. Evaluation of Affymetrix Gene Chip sensitivity in rat hippocampal tissue using SAGE analysis. Eur J Neurosci 2002; 16: 409–413
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: Region-specific expression of nine subtypes in rat brain. J Neurosci 1997; 17: 8024–8037
- Grenier J, Tomkiewicz C, Trousson A, Rajkowski KM, Schumacher M, Massaad C. Identification by microarray analysis of aspartate aminotransferase and glutamine synthetase as glucocorticoid target genes in a mouse Schwann cell line. J Steroid Biochem Mol Biol 2005; 97: 342–352
- Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. Mol Endocrinol 2006; 20: 254–267
- Hesen W, Joels M. Modulation of 5HT1A responsiveness in CA1 pyramidal neurons by in vivo activation of corticosteroid receptors. J Neuroendocrinol 1996; 8: 433–438
- Imaizumi K, Tsuda M, Wanaka A, Tohyama M, Tagaki T. Differential expression of sgk mRNA, a member of the Ser/Thr protein kinase family, in rat brain after CNS injury. Brain Res Mol Brain Res 1994; 26: 189–196
- Joels M, De Kloet ER. Coordinative mineralocorticoid and glucocorticoid receptor-mediated control of responses to serotonin in rat hippocampus. Neuroendocrinology 1992; 55: 344–350
- Joels M, Hesen W, de Kloet ER. Mineralocorticoid hormones suppress serotonin-induced hyperpolarization of rat hippocampal CA1 neurons. J Neurosci 1991; 11: 2288–2294
- Karst H, Karten YJG, Reichardt HM, de Kloet ER, Schutz G, Joels M. Corticosteroid actions in hippocampus require DNA binding of glucocorticoid receptor homodimers. Nat Neurosci 2000; 3: 977–978
- Koelle MR, Horvitz HR. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell 1996; 84: 115–125
- Koya E, Spijker S, Homberg JR, Voorn P, Schoffelmeer ANM, De Vries TJ, Smit AB. Molecular reactivity of mesocorticolimbic brain areas of high and low grooming rats after elevated plus maze exposure. Mol Brain Res 2005; 137: 184–192
- Lee E, Lein ES, Firestone GL. Tissue-specific expression of the transcriptionally regulated serum and glucocorticoid-inducible protein kinase (Sgk) during mouse embryogenesis. Mech Dev 2001; 103: 177–181
- Meijer OC, De Kloet ER. A role for the mineralocorticoid receptor in a rapid and transient suppression of hippocampal 5-HT1A receptor mRNA by corticosterone. J Neuroendocrinol 1995; 7: 653–657
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol 2006; 18: 239–252
- Murata S, Yoshiara T, Lim CR, Sugino M, Kogure M, Ohnuki T, Komurasaki T, Matsubara K. Psychophysiological stress-regulated gene expression in mice. FEBS Lett 2005; 579: 2137–2142
- Nishida Y, Nagata T, Takahashi Y, Sugahara-Kobayashi M, Murata A, Asai S. Alteration of serum/glucocorticoid regulated kinase-1 (sgk-1) gene expression in rat hippocampus after transient global ischemia. Mol Brain Res 2004; 123: 121–125
- Ni YG, Gold SJ, Iredale PA, Terwilliger RZ, Duman RS, Nestler EJ. Region-specific regulation of RGS4 (regulator of G-protein-signaling protein type 4) in brain by stress and glucocorticoids: In vivo and in vitro studies. J Neurosci 1999; 19: 3674–3680
- Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids. Glia 1997; 20: 23–37
- Tsai KJ, Chen SK, Ma YL, Hsu WL, Lee EH. Sgk, a primary glucocorticoid-induced gene, facilitates memory consolidation of spatial learning in rats. Proc Natl Acad Sci USA 2002; 99: 3990–3995
- Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature 1996; 383: 172–175
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 1993; 13: 2031–2040
- Willars GB. Mammalian RGS proteins: Multifunctional regulators of cellular signalling. Semin Cell Dev Biol 2006, in press
- Wärntges S, Friedrich B, Henke G, Duranton C, Lang PA, Waldegger S, Meyermann R, Kuhl D, Speckmann EJ, Obermüller N, Witzgall R, Mack AF, Wagner HJ, Wagner CA, Bröer S, Lang F. Cerebral localization and regulation of the cell volume-sensitive serum- and glucocorticoid-dependent kinase SGK1. Pflugers Arch Eur J Physiol 2002; 443: 617–624
- Yoo D, Kim Y, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1 and protein kinase A. J Biol Chem 2003; 278: 23066–23075
- van Riel E, van Gemert NG, Meijer OC, Joels M. Effect of early life stress on serotonin responses in the hippocampus of young adult rats. Synapse 2004; 53: 11–19