Abstract
A decreased reactivity to stressors during lactation might heighten the expression of maternal care (including defense of offspring) by minimizing the extent to which stress can impact maternal care. Although stressors applied during pregnancy have variable effects on maternal aggression (or defense of offspring), to date no study has examined the effects of stress applied during the postpartum period on maternal aggression. In this study, we examined the effects of both daily and acute restraint stress (30 min) applied postpartum on maternal aggression and other maternal behaviors. Daily restraint (ending 2 h before testing) did not alter any measure of maternal behavior, including nursing, licking and grooming of pups and pup retrieval, or any measure of maternal aggression. In contrast, acute stress significantly impaired total time aggressive and number of attacks, but pup retrieval was normal. c-Fos levels were significantly elevated in a number of brain regions in association with acute stress, including lateral septum (LS), caudal periaqueductal gray and medial amygdala (MeA), suggesting possible sites where stress reactivity could alter aggression. Together, the results indicate that acute restraint stress impairs maternal aggression and provide a starting point for future studies examining how stress reactivity pathways may intersect with maternal aggression pathways.
Introduction
The expression of maternal behaviors, such as nursing, pup retrieval, nest building and defense of offspring (maternal aggression) (Numan and Insel Citation2003), is associated with an alteration in how dams respond to stressors. For example, in association with lactation in rodents: (1) the CNS shows decreased response to stressors (da Costa et al. Citation1996); (2) the CNS is less responsive to centrally injected corticotrophin releasing factor (CRF) (da Costa et al. Citation1997), an anxiogenic peptide that helps mediate the behavioral responses to stress (for reviews, see Koob and Heinrichs Citation1999; Smagin et al. Citation2001); (3) CRF-enhanced startle response is decreased (Walker et al. Citation2003); and (4) females exhibit decreased indices of fear and anxiety using a wide range of testing paradigms (Lonstein and Gammie Citation2002; Lonstein Citation2005). In support of the idea that a decreased response to stressors (or central CRF) during lactation supports maternal aggression, recent work has shown that central injections of CRF and related peptides dose-dependently impair maternal aggression in mice (Gammie et al. Citation2004; D'Anna et al. Citation2005). Also, an earlier study found centrally injected CRF decreased maternal care in maternally sensitized virgin female rats (Pedersen et al. Citation1991). A number of signaling molecules (besides CRF) are involved in the behavioral response to stress and whether CRF plays a role in reactivity to stress during lactation, still needs to be tested. The adaptive value of a suppressed response to stressors for full expression of maternal care is supported by a range of studies (Carter et al. Citation2001; Russell et al. Citation2001; Groer et al. Citation2002; Walker et al. Citation2004).
Four studies in mice have applied stressors prepartum and examined maternal aggression postpartum. Interestingly, in two studies, one using restraint or novel environment stress (Maestripieri et al. Citation1991) and the other using a combination of mild stressors (Pardon et al. Citation2000), prepartum stress (applied during pregnancy) decreased maternal aggression. In the former case, greater maternal care was found in the stressed group, but in the latter study no effect of stressor on maternal behavior was found. In contrast, in two other studies, one using heat or restraint stress (Kinsley and Svare Citation1988) and the other using heat, light, or noise stress (Meek et al. Citation2001), maternal aggression was elevated in the stressed mice. The latter study also found enhanced maternal care in stressed mice. In rats, two studies found prepartum stressors increase maternal care (Muir et al. Citation1985; Neumann et al. Citation2005), one found no effect (Melniczek and Ward Citation1994) and a third found an inhibition (Moore and Power Citation1986). Maternal aggression was elevated in high, but not low anxiety rats following application of a prepartum stressor (Neumann et al. Citation2005). Why opposing effects of prepartum stress on maternal aggression was found in these studies is unclear. Given that CRF and related peptides applied postpartum decrease maternal aggression (Gammie et al. Citation2004; D'Anna et al. Citation2005), the finding of prepartum stressors impairing aggression are consistent with the idea of elevated CRF neurotransmission in the stressed animals. It is also possible, though, that stressors applied prepartum could result in adaptations of the CNS that then alter behavior during lactation.
To our knowledge, no study to date has examined whether or how stress affects maternal aggression when applied during the lactation period. In one study examining effects of stress during lactation on maternal care, application of a noise stress during lactation increased nest building and pup cleaning activities (Windle et al. Citation1997). In another study, prolonged exposure to a male resulted in decreased nursing (Lau and Simpson Citation2004). The main aim of this study was to examine whether or how either daily or acute restraint stress applied during lactation affects maternal aggression, but other maternal behaviors were also examined. We chose to apply restraint stress both acutely and daily to assess whether the method of application affected response in the females. Given that centrally injected CRF impairs maternal aggression in mice (Gammie et al. Citation2004) and some behaviors in maternally sensitized virgin rats (Pedersen et al. Citation1991), we hypothesized that both chronic and acute restraint stress would impair maternal aggression and other maternal behaviors. This finding would be consistent with the idea that a suppressed stress response during lactation helps to ensure the full expression of maternal care and minimizes the deleterious effects of stress on parental behavior. To gain insights into which brain regions showed altered activity with stress, we examined c-Fos activity in the CNS of lactating mice either exposed or not exposed to restraint stress.
Materials and methods
Animals
Outbred hsd:ICR (CD-1) mice were purchased from vendor (Harlan, Madison, WI). Females (∼50 days old) were singly housed with a breeder male (hsd:ICR strain) and following impregnation (∼2 weeks), each female mouse was housed individually for the remainder of the study. Just prior to parturition, female mice were given precut nesting material. Polypropylene cages were changed once weekly, but after parturition, cages were not changed for the duration of the experiments. Pups were culled to 11 on postpartum Day 1. All female mice were given ad lib access to Harlan Tekland Mouse Breeder Diet 7004 (Harlan) and tap water. Intruder male mice (hsd:ICR strain; Harlan, Madison, WI) were sexually naïve and group-housed (4 mice/cage). Intruder males (∼2 months old) were given ad libitum access to regular chow. Intruder males were never used more than once per day and used for ∼3 tests each. Intruder males from the same cage were used to test mice from all three groups, so any effect of previous aggression on the intruder mice was distributed equally among the test groups. All animals were housed on a 14:10 h light/dark cycle with lights on at 0600 CST. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Animals (NIH Publications No. 80 23, revised 1978) and approved by the Animal Care and Use Committee of the University of Wisconsin.
Restraint stress
Restraint stress was applied by placing individual mice into a ventilated clear Plexiglas tube with a flat bottom and a curved (semi-circle shaped) side (width = 5.5 cm; base to top = 2.5 cm; length = 6.5, 7.5 or 8.5 cm, depending on size of mouse). In all cases, mice were removed from their home cage and placed in the restraint tubing in a separate testing room under low light conditions for 30 min. Restraint was applied in one of two groups: daily (n = 10) and acute (n = 9).
Daily stress group
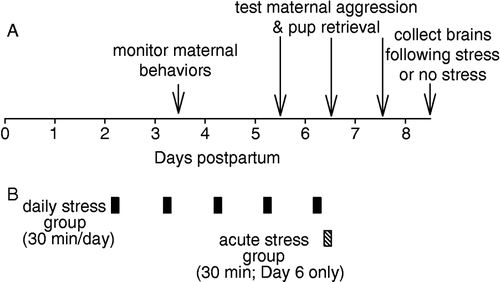
Each day beginning on postpartum Day 2, mice were exposed to restraint stress each morning for five consecutive days (). Restraint in this group always occurred between 0845 and 0915 CST. Maternal behaviors were observed for both daily stress and control (unstressed) mice on Day 3 () from 1115 to 1215 h. Thus, the delay from cessation of stress to maternal behavior observations was 2 h. Comparisons of aggression with the control group were made on postpartum Days 5, 6 and 7 () between 1200 and 1300 h. Thus, the delay from cessation of stress to aggression testing was 165 min. On Day 7, with no stress, maternal aggression was examined to determine whether aggression was altered with the cessation of stress (). Stress ended at least 2 h (and not immediately) prior to behavioral examination so that acute effects of stress, if any, would have minimal effects on behavior. For all maternal aggression testing, pups were removed from both groups 30 min prior to testing, so that control mice could be used for comparisons against both stress groups (for rationale, see below). Control mice were left in their homecage and were not handled. Because, we wanted to apply daily stress early during lactation (Day 2) and aggression does not peak until Day 4 (see below), we were unable to pretest daily stress group animals for maternal aggression.
Figure 1 Overview of the timeline for behavioral observations (A) and restraint stress application for the daily and acute stress groups (B). See Methods for additional details. Daily stress ended 2 h prior to maternal behavior observations and 165 min prior to aggression testing. Acute stress ended just prior to aggression testing.
Acute stress group
Mice were exposed to restraint stress for 30 min just prior to testing for maternal aggression on postpartum Day 6. Control (same mice as used for daily stress comparisons) and test mice in this group were all first tested on postpartum Day 5 to provide a baseline level of aggression for the two groups. Separation from pups for longer periods (>5 h) prior to maternal aggression testing decreases aggression, but separation for shorter time periods does not in rats and mice (Svare and Gandelman Citation1973; Stern and Kolunie Citation1993). Still, to control for any subtle effects that 30 min separation from pups might have on aggression, control mice were also separated from their pups for the identical 30 min period prior to testing. All mice in all groups were age matched, housed together in the same room, and tested within a 10 day window.
Maternal behavior examination
On postpartum Day 3, each dam was observed between 1115 and 1215 h. Dams were observed within their home cages and within the home housing room. Every minute for 1 h, observers blind to treatment noted the maternal behaviors of the dam. The behaviors examined included: off nest, on nest, low arched-back nursing, high arched-back nursing, licking and grooming pups, self grooming, eating and drinking, nest building, or no activity (Champagne et al. Citation2003; D'Anna and Gammie Citation2006). The proportion of time spent in the varying activities was determined for the one day of observation and these data were used for analysis. provides an overview of the timeline for all observations and testing performed.
Maternal aggression and pup retrieval testing
On postpartum Days 5, 6 and 7 (), each female was exposed to an intruder male for 5 min in her home cage between 1200 and 1300 h. The pups were removed from the cage prior to the behavioral test as described above. The days of testing occurred within the window of peak maternal aggression that occurs from postpartum Day 4 though 10 in mice (Svare Citation1990). An intruder male mouse was placed in the dam's home cage and each test session was recorded on videotape and subsequently analyzed off-line to quantify maternal aggression. Maternal aggression scoring was conducted by individuals blind to experimental conditions and treatments. For all behavioral scoring, each individual scored an equal number of mice from the different groups to maintain consistency. Further, scoring is regularly examined in the laboratory and recalibrated to maintain consistency within and across studies. For quantification of maternal aggression, the following features were measured: latency to first attack, number of attacks and total duration of attacks, as previously performed (Gammie and Nelson Citation2001; Gammie et al. Citation2004). At the completion of each test, the pups were randomly scattered in the home cage and the time to retrieve the first pup to the nest site within a 10 min test period was recorded (Gammie and Nelson Citation1999). Dams and pups were weighed on Day 1 (prior to first application of stress) and on Day 7. On Day 7, pups were weighed just prior to being returned to the home cage for a pup retrieval test.
Data analysis
Maternal behavior and aggression variables were analyzed using a one-way ANOVA. In cases where the differences between the two groups were not normally distributed, then a nonparametric ANOVA on ranks (Dunn's method or Kruskal-Wallis) was used. For all analysis Sigma Stat software was used (SPSS Inc., Chicago IL). In the case of time to first attack, if an animal was not aggressive (no aggression shown during the test period), a time of 300 s was assigned (the maximum possible for the test). In the case that the first pup was not retrieved within the test window, a time of 600 s was assigned (the maximum possible for the test).
Immunohistochemistry for c-Fos
On postpartum Day 8, half of the control mice (never previously exposed to restraint stress) were exposed to restraint stress for 30 min and the other half of the mice were not. Also, on postpartum Day 8, half of the daily stress mice were exposed to restraint stress and the other half were not. All non-stressed mice on Day 8 also had their pups removed for 30 min as for test mice. 100 min following the onset of the restraint (or no restraint), mice were briefly exposed to isoflurane anesthesia. Mice were then decapitated and the brains removed. Brains were post-fixed overnight in 5% acrolein (Sigma) in phosphate buffered saline (PBS) and cryoprotected in 30% sucrose in PBS for two days. Brains were frozen on a platform and cut into 40 μm thick coronal sections using a sliding microtome (Leica, Microsystems, Heidelberg, Germany) and stored in a cryoprotectant solution at − 20 C until processing for immunohistochemistry. Immunohistochemistry was run for all mice in all groups in one batch. Sections were washed in PBS in the presence of 0.2% Triton-X-100 (PBS-X), blocked in 5% normal goat serum for 1 h and incubated for two days at 4 C with rabbit anti-Fos antibodies (1:15,000; Calbiochem, San Diego, CA, catalog # PC38). After washes in PBS-X, the sections were incubated for 90 min at room temperature in biotinylated goat anti-rabbit secondary antibodies (1:500, Vector Laboratories, Burglingame, CA), washed in PBS-X, exposed to an avidin-biotin complex (Vector) for 1 h, washed again in PBS-X and stained using diaminobenzidine (Sigma) as a chromagen, enhanced with 0.008% nickel chloride. The sections were then mounted, dehydrated in a series of ethyl alcohols and xylene and coverslipped.
Analysis of c-Fos immunoreactivity (c-Fos-IR)
Bright field microscopy was used for counting c-Fos-positive cells. The images of brain sections were projected from an Axioskop Zeiss light microscope using a 10 X objective (Zeiss, Gottingen, Germany) through an Axiocam Zeiss high resolution digital camera attached to the microscope and interfaced with a computer. Counting from specified brain regions was based on a previously used paradigm (Rhodes et al. Citation2003; Gammie et al. Citation2004; D'Anna et al. Citation2005). Brain regions were chosen for examination based on whether they had previously been implicated in maternal aggression and/or reactivity to stress. For locations of regions examined, see (Gammie et al. Citation2004; D'Anna et al. Citation2005; Hasen and Gammie Citation2005). One section per brain region was used to quantify c-Fos-IR in each animal and a box of a preset size was used for a given region. The box was placed in the same site within a given region using overt landmarks. To ensure c-Fos-IR was measured consistently between samples; (1) all sections were exposed to diaminobenzidine for 10 min during the above immunocytochemical processing, (2) the backgrounds were normalized by adjusting light levels, (3) a threshold of staining levels was used to automatically distinguish c-Fos-positive cells, (4) all slides were coded and the counting for each specific brain region was performed by one individual, blind to the experimental conditions, (5) only c-Fos-positive nuclei within a specified size range were counted (30–1000 pixels squared), and (6) all group sections were run in one batch.
Evaluation of c-Fos-IR among groups was conducted using a two-way ANOVA to examine the effect of acute restraint stress and of prior stress exposure as the two variables using Sigma Stat software. In the cases where the data were not normally distributed, then nonparametric tests were used as described above. Additionally, a one-way ANOVA was used within the two starting groups to test the effects of stress on c-Fos.
In order to correct for the increased type 1 error possible with multiple comparisons, the false discovery rate method of Storey (Storey Citation2002) was applied using the open source software QVALUE, http://faculty.washington.edu/ ∼ jstorey/qvalue/. This method can be used to estimate the p-value cutoff to use for each test that will yield a global, experiment-wide, false discovery rate of 5%. It has been used previously to correct for multiple comparisons (Gammie et al. Citation2004; D'Anna et al. Citation2005; Rhodes et al. Citation2005). For the two-way ANOVA tests examining effects of acute stress on c-Fos expression, QVALUE arrived at a p-value of 0.039 as the cutoff for significance.
Results
Daily stress effects on maternal behaviors
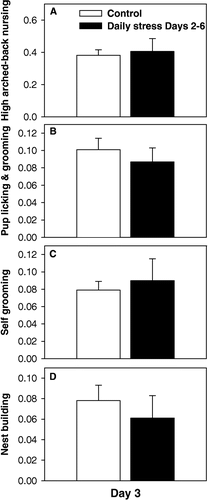
After exposure to 30 min restraint stress episodes on postpartum Days 2 and 3, mice were examined for maternal behaviors on Day 3 2 h following the last restraint. As shown in , mice stressed daily did not exhibit any differences in maternal care relative to control mice in terms of either proportion of time high arched-back nursing (F(1,28) = 0.09; p = 0.757; one-way ANOVA) (), licking and grooming of pups (F(1,28) = 0.41; p = 0.523; one-way ANOVA) (), self-grooming (F(1,28) = 0.24; p = 0.625; one-way ANOVA) (), or nest building (F(1,28) = 0.44; p = 0.509; one-way ANOVA) (). Additionally, for other measures such as percentage of time on nest not nursing (F(1,28) = 0.04; p = 0.825; one-way ANOVA), time low arched-back nursing (H(1,28) = 0.62; p = 0.428; one-way ANOVA on ranks), and time eating and drinking (F(1,28) = 0.19; p = 0.660; one-way ANOVA), no differences between groups were detected.
Figure 2 Analysis of maternal behaviors in controls and mice exposed to daily restraint stress. On Day 3, no differences between groups were found in terms of proportion of time spent either (A) high arched-back nursing; (B) licking and grooming of pups; (C) self grooming; or (D) nest building. Bars represent means ± SE. White bars indicate control mice (n = 19) and black bars indicate mice stressed daily (n = 10).
Mean pup weight was similar between the two groups on postpartum Day 1 (1.8 ± 0.07 g for control mice; 1.8 ± 0.08 g for mice stressed daily) (F(1,19) = 0.15; p = 0.698; one-way ANOVA) and Day 7 (4.6 ± 0.1 g for control mice; 4.5 ± 0.2 g for mice stressed daily) (F(1,19) = 0.09; p = 0.768; one-way ANOVA), suggesting that daily stress had no adverse effects on maternal care that would affect pup weight. Consistent with the above results, mean dam weight was similar between groups when examined on postpartum Day 1 (39.9 ± 1.6 g for control mice; 40.7 ± 1.2 g for daily stress mice) (F(1,19) = 0.148; p = 0.705; one-way ANOVA) and on postpartum Day 7 (50.7 ± 2.0 g for control mice; 49.3 ± 2.2 g for daily stress mice) (F(1,19) = 0.210; p = 0.652; one-way ANOVA).
Effects of daily stress on maternal aggression and pup retrieval
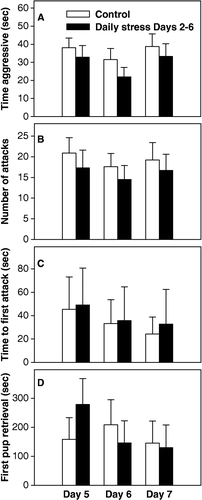
Maternal aggression did not differ between mice stressed daily (stress ended 165 min prior to testing) and control mice on postpartum Days 5 or 6 in terms of time aggressive (p = 0.542, Day 5; p = 0.263, Day 6) (), number of attacks (p = 0.541, Day 5; p = 0.522, Day 6) (), or time to first attack (p = 0.519, Day 5; p = 0.494, Day 6) (). Further, pup retrieval did not differ between groups on either of these two days (p = 0.399, Day 5; p = 0.732, Day 6) (). On postpartum Day 7, both groups were tested for aggression and retrieval, but the stress group was not exposed to restraint stress that day. As seen in –D, maternal aggression, in terms of time aggressive (p = 0.598), number of attacks (p = 0.671), and time to first attack (p = 0.879) and pup retrieval (p = 0.239) were still similar between groups when daily restraint stress had been removed.
Figure 3 Analysis of maternal aggression and pup retrieval in controls and mice exposed to daily restraint stress. On neither Days 5, 6, or 7, were any differences between groups found for either (A) time aggressive; (B) number of attacks; (C) time to first attack; or (D) time to retrieve first pup. Bars represent means ± SE. White bars indicate control mice (n = 10) and black bars indicate daily stress mice (n = 10).
Effects of acute stress on maternal aggression and pup retrieval
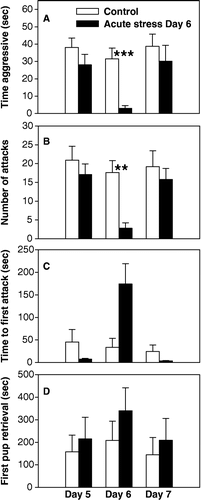
Prior to stress application, maternal aggression and pup retrieval were examined on postpartum Day 5 to establish baseline levels for the control and acute stress groups. As seen in –C, no initial differences between groups existed in terms of either time aggressive (p = 0.234), number of attacks (p = 0.442), or time to first attack (p = 0.306). In contrast, on postpartum Day 6 when acute stress was applied just before testing, a significant decrease in maternal aggression occurred in terms of time aggressive (H(1,18) = 11.06; p < 0.001; one-way ANOVA on ranks) () and number of attacks (H(1,18) = 11.01; p = 0.002; one-way ANOVA on Ranks) (). The apparently longer latency to attack for restraint stress mice was not significant (H(1,18) = 2.84; p = 0.092; one-way ANOVA on ranks) (). Pup retrieval did not differ between the two groups on either postpartum Day 5 (p = 0.869) or Day 6 (p = 0.420) (). On postpartum Day 7, both groups were tested for aggression and retrieval, but the acute stress group was not exposed to restraint stress that day. As seen in –C, maternal aggression levels were again similar between the two groups in terms of time aggressive (p = 0.470), number of attacks (p = 0.623) and time to first attack (p = 0.454), suggesting that the acute stress the previous day did not have any long term effects on aggression. Pup retrieval remained similar between the groups on Day 7 (p = 0.934).
Figure 4 Analysis of maternal aggression in control and acutely stressed (stress only on Day 6) mice. On Day 5 (prior to stress application), no differences existed between groups in any aggression measure. On Day 6, significant decreases in aggression were found in acutely stressed mice in terms of (A) time aggressive, (B) number of attacks, but not in time to first attack (p = 0.09) (C). On Day 7 (one day after acute stress on Day 6), no differences in aggression existed between groups. Time to retrieve first pup remained similar between groups on all test days. Bars represent means ± SE. White bars indicate control mice (n = 10) and black bars indicate acute stress on Day 6 mice (n = 9). **, p < 0.01; ***, p < 0.001; one-way ANOVA.
c-Fos immunoreactivity in association with restraint stress
As shown in , for 14 of 33 regions examined, significant increases in c-Fos labeling were found in association with restraint stress using the p-value cutoff of 0.039 as determined by QVALUE (see Methods). Restraint stress effect on c-Fos was examined with a two-way ANOVA using two groups of mice: control mice (which had never previously experienced stress) and daily restraint stress mice. shows separate means for both starting groups both with and without stress. For only three brain regions were differences based on prior experience (or group) found and in each case daily restraint stress mice showed lower c-Fos levels than control mice. This occurred for central amygdala (CeA) (p = 0.024); paratenial thalamic nucleus (PT) (p = 0.047) and lateral habenular nucleus (LhB) (p = 0.010). In no cases was a significant interaction found between prior stress exposure and exposure to stress (data not shown). Overall, then, acute stress itself was the dominant factor in alterations of c-Fos activity. Using separate one-way ANOVAs, the effect of restraint stress was also examined independently within each starting group. The c-Fos counts from regions with differences in expression (p < 0.05 from the one-way ANOVAs) are highlighted in bold in . Brain regions examined that did not exhibit significant differences in c-Fos expression with stress using either approach included: anterior hypothalamic area (AH), anterior part; AH, central part; bed nucleus of the stria terminalis (BNST), lateral division, posterior part; BNST, medial division, posterolateral part; lateroanterior hypothalamic nucleus; LhB; lateral preoptic area; lateral septal nucleus, ventral part; mediodorsal thalamic nucleus; medial preoptic area, paraventricular hypothalamic nucleus, anterior parvicellular part; PT; paraventricular thalamic nucleus, anterior part; supraoptic nucleus; nucleus of the vertical limb of the diagonal band.
Table I. Mean±SE c-Fos expression for two groups (control and daily stress) 100 min following either no restraint (control) or acute restraint (acute) on Day 8. P-values reflect overall effect of restraint stress (using a two-way ANOVA; see Methods for details) on c-Fos and only brain regions with p-values less than 0.07 are shown. Level of significance as determined by QVALUE was 0.039 (see Methods for details). Bold indicates a significant effect of restraint stress on c-Fos (p<0.05) within a given group using a one-way ANOVA.
Discussion
In this study, we examined for the first time the effects of stress applied postpartum on maternal aggression. We found a significant inhibitory effect of acute restraint stress on maternal defense, but pup retrieval was not affected. This finding is consistent with recent work showing that central injections of CRF and related peptides (that are thought to mediate some of the behavioral responses to stress) impair maternal aggression (Gammie et al. Citation2004; D'Anna et al. Citation2005). Interestingly, we found no effect of daily restraint stress on either maternal behaviors or maternal aggression. Because daily restraint stress ended 165 min prior testing, but did not affect aggression, we can conclude that the inhibitory effects of restraint (seen with acute treatment) have diminished prior to this time. Our examination of changes in brain activity with acute restraint stress highlight possible areas where response to stress could influence defense of offspring.
In this study, we examined the effects of postpartum stress on maternal aggression. One explanation for the inhibitory effect of acute restraint stress is that the central release of the neuropeptides that are released in association with stress, such as CRF (Koob and Heinrichs Citation1999; Smagin et al. Citation2001), impair aggression. This is consistent with recent work in our laboratory showing that CRF and related peptides impair aggression. Interestingly, for urocortin (Ucn) 1 and 3 (peptides related to CRF), while maternal aggression was impaired, pup retrieval was unaltered by treatment with these peptides (D'Anna et al. Citation2005). The similar decrease in aggression in the absence of effect on retrieval with stress supports the possible release of CRF and related peptides mediating this response. A number of other signaling molecules contribute to the behavioral responses to stress, though and whether or how CRF is released during stress in lactation and has an effect on aggression needs to be directly tested. Because mice stressed daily exhibited no alterations in aggression (even though restraint ended 165 min prior to testing), we speculate that whatever modulators were released in the CNS in association with stress have lost their impact within this time period.
Although it is possible that peripheral increases in corticosterone level could also mediate decreased maternal defense, this is less likely because peripheral administration of this steroid was unable to alter maternal aggression in mice (Al-Maliki Citation1980). Further, because intruder males trigger an aggressive response from females and a significant elevation in corticosterone in the dam (especially when pups are present) (Deschamps et al. Citation2003), it is not likely that the steroid is an acute aggression suppressor. Thus, the central release of neurotransmitters or neuropeptides most likely mediates the behavioral effects of restraint stress on maternal aggression and not changes in peripheral glucocorticoids.
The finding of no effect of daily restraint stress (ended 2 h before testing) on maternal care is consistent with previous work in mice showing a lack of effect of restraint stress on maternal behavior 30 min after stressor exposure has ended (Yamada et al. Citation2002). It is possible that the mice stressed daily habituated to the stressor and the use of chronic variable stressors would be valuable in future work to rule out effects of habituation. Further, it is possible that restraint stress would have been found to affect maternal care if maternal behavior had been examined at differing time points following stress application. Interestingly, pup retrieval was found to be impaired immediately after restraint stress in inbred C57 mice (Yamada et al. Citation2002), but in this study we found this measure to be unaffected. One possible reason for this discrepancy is that inbred C57 mice show faster retrieval times relative to hsd:ICR outbred mice (Gammie and Nelson Citation1999; D'Anna et al. Citation2005), so perturbations may be easier to observe when the behavior is more reliably produced.
In this study, we also examined c-Fos changes in the brain in response to restraint stress in lactating mice. Previous work in rats found that a suppressed response to stress occurs with lactation. For example, stress-induced increases in c-Fos expression in LS and CeA in virgin rats are reduced in lactating rats (da Costa et al. Citation1996). Lactating rats also show a blunted c-Fos response to CRF relative to virgin rats (da Costa et al. Citation1997), suggesting that lactating females can minimize the deleterious effects stress can have on maternal behaviors. Using one-way ANOVAs to independently examine the effects of stress on c-Fos within the two starting groups in the present study revealed similar trends in both groups, but regions in which significance was reached were not always the same (); indeed only the locus coeruleus showed significant increases in c-Fos expression with acute stress in both naïve mice and mice previously exposed to daily stress. This outcome highlights that starting background factors (i.e. prior repeated stress exposure or not) may affect reactivity to stress. LS and MeA are regions previously implicated in the regulation of maternal aggression (Consiglio and Lucion Citation1996; Giovenardi et al. Citation1998; Lonstein et al. Citation1998; Gammie and Nelson Citation2001; Lonstein and Gammie Citation2002; Hasen and Gammie Citation2005; Hasen and Gammie Citation2006) and using a two-way ANOVA we identified increases in c-Fos expression within these regions with restraint. However, the LS showed increased c-Fos expression only in the mice exposed to daily stress before acute stress (), which may correspond with the finding that acute, but not daily, stress inhibited aggressive behavior. Similarly, the finding that the MeA showed increased c-Fos expression only in the mice not exposed to daily stress before acute stress (), suggests that MeA activation may be involved in inhibition of maternal aggression by acute stress. The c-Fos findings here are consistent with other studies in rats and mice (da Costa et al. Citation1996; Nomura et al. Citation2003; Patel et al. Citation2005) and suggest these (and others) as sites with altered activity with stress. One possibility is that altered neurotransmitter release occurs in these regions with stress that then alters performance of neurons in the same regions that regulate normal production of maternal aggression. However, the results from this study only highlight candidate brain regions that respond to stress and are not an endpoint. Whether or how stress reactivity pathways and those governing maternal aggression intersect needs to be addressed in subsequent studies. Further, because the signaling molecules released with stress that affect aggression are unknown, double-labeling immunohistochemistry studies will be useful in helping to determine which neurotransmitters are involved.
LS is an example of an interesting candidate region in the negative regulation of maternal aggression for a few reasons. (1) For CRF, Ucn 1 and Ucn 3, all three alter c-Fos activity in LS at the dose that also impairs maternal aggression (Gammie et al. Citation2004; D'Anna et al. Citation2005). (2) LS shows decreased responsiveness to stress (da Costa et al. Citation1996) and CRF (da Costa et al. Citation1997) with lactation, suggesting blunted stress reactivity in this region. (3) Chlordiazepoxide (a benzodiazepine) decreases c-Fos expression in LS at the same dose that elevates maternal aggression (G. Lee and S.C. Gammie, unpublished observations). (4) CRF injected into LS is sufficient to impair maternal aggression (K.L. D'Anna and S.C. Gammie, unpublished observations). It is possible that CRF or related peptides released during stress into LS or possibly into some of these other regions may be the basis for the reduction of maternal aggression, but this needs to be tested. The finding of lower c-Fos counts in CeA, PT, and LHb in the mice exposed to daily stress versus control mice in the unrestrained condition was unexpected. One possibility for this lowered c-Fos activity in daily stress mice was an adaptation to daily restraint stress that had long-lasting effects (persisted 2 days after stress had been removed). The basis of this altered activity is unclear, but could involve elevated GABAergic neurotransmission which itself can reduce c-Fos labeling (Imaki et al. Citation1995; Hitzemann and Hitzemann Citation1999; van Luijtelaar et al. Citation2001). However, the basis of lowered c-Fos in daily stress mice would need to be examined in subsequent studies.
Our finding of impaired aggression with acute stress, but not 165 min after stress (as seen in the daily stress group) suggests the deleterious effect of stress on aggression is short-lived. Given previous work indicating that lactating females have blunted or diminished reactivity to stress and/or CRF relative to non-lactating females, we suggest this altered reactivity helps to mitigate the inhibitory effects of stress on protective behavior. We speculate that linking maternal aggression to environmental stressors makes ecological sense. If stressful environmental conditions (e.g. too hot, cold, or wet) are inappropriate for reproduction, then losing a potentially dangerous behavior (protection of offspring) and decreasing investment in a given litter would be beneficial, especially if the animal is geared towards producing multiple litters. In humans that invest far greater resources per offspring, it would be expected that stressors have less effect on maternal protection. However, dysregulation of CRF neurotransmission is linked to some forms of depression (Nemeroff Citation2002) and depression, itself, has been linked to decreases in maternal care (Newport et al. Citation2002). It is possible, then, that examining whether or how specific modulators of stress reactivity, such as benzodiazepines (Mechiel Korte and De Boer Citation2003; Breese et al. Citation2005), oxytocin (Neumann et al. Citation2000), or serotonin reuptake inhibitors (Zhang et al. Citation2000), can prevent stress-induced decreases in maternal aggression in mice, may provide a model for understanding interventions for maternal deficiencies during the postpartum period.
Acknowledgements
This work was supported by National Institutes of Health Grant R01MH066086 to S.C.G. The authors wish to thank Emily Bethea, Michael Foley, Allen Irgens and Caleigh Mandel-Brehm for technical assistance and Kate Skogen and Jeff Alexander for animal care.
References
- Al-Maliki S. Influences of stress-related hormones on a variety of attack behaviour in laboratory mice. Adaptive capabilities of the nervous system, P McConnell. Elsevier, Amsterdam 1980; Vol 53: 421–426
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: Inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology 2005; 30: 1662–1669
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res 2001; 133: 241–249
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 2003; 79: 359–371
- Consiglio AR, Lucion AB. Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol Behav 1996; 59: 591–596
- da Costa AP, Wood S, Ingram CD, Lightman SL. Region-specific reduction in stress-induced c-fos mRNA expression during pregnancy and lactation. Brain Res 1996; 742: 177–184
- da Costa AP, Kampa RJ, Windle RJ, Ingram CD, Lightman SL. Region-specific immediate-early gene expression following the administration of corticotropin-releasing hormone in virgin and lactating rats. Brain Res 1997; 770: 151–162
- D'Anna KD, Gammie SC. Hypocretin-1 dose-dependently modulates maternal behaviour in mice. J Neuroendocrinol 2006; 18: 553–566
- D'Anna KD, Stevenson SA, Gammie SC. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci 2005; 161–171
- Deschamps S, Woodside B, Walker CD. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J Neuroendocrinol 2003; 15: 486–497
- Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci 1999; 19: 8027–8035
- Gammie SC, Nelson RJ. cFOS and pCREB activation and maternal aggression in mice. Brain Res 2001; 898: 232–241
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci 2004; 118: 805–814
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: Effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav 1998; 63: 351–359
- Groer MW, Davis MW, Hemphill J. Postpartum stress: Current concepts and the possible protective role of breastfeeding. J Obstet Gynecol Neonatal Nurs 2002; 31: 411–417
- Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav 2005; 84: 684–695
- Hasen NS, Gammie SC. Maternal aggression: New insights from Egr-1. Brain Res 2006; 1108: 147–156
- Hitzemann B, Hitzemann R. Chlordiazepoxide-induced expression of c-Fos in the central extended amygdala and other brain regions of the C57BL/6J and DBA/2J inbred mouse strains: Relationships to mechanisms of ethanol action. Alcohol Clin Exp Res 1999; 23: 1158–1172
- Imaki T, Wang XQ, Shibasaki T, et al. Chlordiazepoxide attenuates stress-induced activation of neurons, corticotropin-releasing factor (CRF) gene transcription and CRF biosynthesis in the paraventricular nucleus (PVN). Brain Res Mol Brain Res 1995; 32: 261–270
- Kinsley C, Svare B. Prenatal stress alters maternal aggression in mice. Physiol Behav 1988; 42: 7–13
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 1999; 848: 141–152
- Lau C, Simpson C. Animal models for the study of the effect of prolonged stress on lactation in rats. Physiol Behav 2004; 82: 193–197
- Lonstein JS. Resolving apparent contradictions concerning the relationships among fear or anxiety and aggression during lactation: Theoretical comment on D'Anna, Stevenson, and Gammie (2005). Behav Neurosci 2005; 119: 1165–1168
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev 2002; 26: 869–888
- Lonstein JS, Simmons DA, Stern JM. Functions of the caudal periaqueductal gray in lactating rats: Kyphosis, lordosis, maternal aggression, and fearfulness. Behav Neurosci 1998; 112: 1502–1518
- Maestripieri D, Badiani A, Puglisi-Allegra S. Prepartal chronic stress increases anxiety and decreases aggression in lactating female mice. Behav Neurosci 1991; 105: 663–668
- Mechiel Korte S, De Boer SF. A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol 2003; 463: 163–175
- Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR. Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav 2001; 72: 473–479
- Melniczek JR, Ward IL. Patterns of ano-genital licking mother rats exhibit toward prenatally stressed neonates. Physiol Behav 1994; 56: 457–461
- Moore CL, Power KL. Prenatal stress affects mother infant interaction in Norway rats. Devel Psychobiol 1986; 19: 235–245
- Muir JL, Pfister HP, Ivinskis A. Effects of prepartum stress and postpartum enrichment on mother infant interaction and offspring problem-solving ability in Rattus-Norvegicus. J Comp Psychol 1985; 99: 468–478
- Nemeroff CB. New directions in the development of antidepressants: The interface of neurobiology and psychiatry. Human Psychopharm 2002; 17(1)S13–S16
- Neumann ID, Torner L, Wigger A. Brain oxytocin: Differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience 2000; 95: 567–575
- Neumann ID, Kromer SA, Bosch OJ. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology 2005; 30: 791–806
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: Animal models of an adverse life event. Am J Psychiatry 2002; 159: 1265–1283
- Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. J Neuroendocrinol 2003; 15: 1054–1061
- Numan M, Insel TR. The neurobiology of parental behavior. Springer, New York 2003
- Pardon M, Gerardin P, Joubert C, Perez-Diaz F, Cohen-Salmon C. Influence of prepartum chronic ultramild stress on maternal pup care behavior in mice. Biol Psychiatry 2000; 47: 858–863
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 2005; 21: 1057–1069
- Pedersen CA, Caldwell JD, McGuire M, Evans DL. Corticotropin-releasing hormone inhibits maternal behavior and induces pup-killing. Life Sci 1991; 48: 1537–1546
- Rhodes JS, Garland T, Jr., Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci 2003; 117: 1243–1256
- Rhodes JS, Ryabinin AE, Crabbe JC. Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci 2005; 119: 759–771
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity—adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res 2001; 133: 1–38
- Smagin GN, Heinrichs SC, Dunn AJ. The role of CRH in behavioral responses to stress. Peptides 2001; 22: 713–724
- Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiol Behav 1993; 54: 861–868
- Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B 2002; 64: 479–498
- Svare B. Maternal aggression: Hormonal, genetic, and developmental determinants. Mammalian parenting: Biochemical, neurobiological, and behavioral determinants, NA Krasnegor, RS Bridges. Oxford University Press, New York 1990; 118–132
- Svare B, Gandelman R. Postpartum aggression in mice: Experiential and environmental factors. Horm Behav 1973; 4: 323–324
- van Luijtelaar G, Fabene PF, de Bruin N, Jongema C, Ellenbroek BA, Veening JG. Neural correlates of sensory gating in the rat: Decreased Fos induction in the lateral septum. Brain Res Bull 2001; 54: 145–151
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 2003; 463: 199–216
- Walker CD, Deschamps S, Proulx K, et al. Mother to infant or infant to mother?. Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. J Psychiatry Neurosci 2004; 29: 364–382
- Windle RJ, Wood S, Shanks N, et al. Endocrine and behavioural responses to noise stress: Comparison of virgin and lactating female rats during non-disrupted maternal activity. J Neuroendocrinol 1997; 9: 407–414
- Yamada K, Santo-Yamada Y, Wada K. Restraint stress impaired maternal behavior in female mice lacking the neuromedin B receptor (NMB-R) gene. Neurosci Lett 2002; 330: 163–166
- Zhang Y, Raap DK, Garcia F, et al. Long-term fluoxetine produces behavioral anxiolytic effects without inhibiting neuroendocrine responses to conditioned stress in rats. Brain Res 2000; 855: 58–66