Abstract
AMP-activated protein kinase (AMPK) plays a central role in maintaining the energy balance of organisms under physiological and environmental stresses. Here two AMPK alpha subunit gene transcripts (named Afr-AMPKalpha1 and Afr-AMPKalpha2) from Artemia franciscana were isolated and gene expression was characterized by semiquantitive reverse transcription-polymerase chain reaction (RT-PCR). Afr-AMPKalpha1 was differentially expressed during Artemia developmental stages as well as in response to stresses, such as heat-shock, starvation and a hyperosmotic environment. Afr-AMPKalpha1 mRNA expression in adult Artemia decreased under heat shock, but not in a time- and temperature-dependent manner. By contrast, the transcript sharply decreased in heat-shocked cysts in a time-dependent manner. Under hyperosmotic stress, however, the mRNA level in adults first declined and then increased with prolonged exposure. In the case of starvation, the gene expression in adults decreased and was undetectable after day 9. In addition, Afr-AMPKalpha2 mRNA expression was too low to be detected without nested PCR. Southern blot analysis, moreover, indicated AMPK alpha subunit was present in multiple copies in the Artemia genome. Furthermore, our results demonstrate that the Afr-AMPKalpha1 mRNA level sharply decreases in Artemia carrying diapause-destined embryos and this indicates the possibility that Afr-AMPKalpha1 is involved in determining the reproductive mode in Artemia.
Introduction
Adenosine monophosphate-activated protein kinase (AMPK), a member of the metabolite-sensing kinase family, plays key roles in regulating cellular energy balance under stressed conditions (Hardie Citation2004). It is known that factors elevating the ratio of AMP: ATP, including physiological stresses, like starvation and prolonged exercise, and environmental stresses, such as heat shock, and oxidative stresses, lead to AMPK activation. (Hardie et al. Citation1994; Wilson et al. Citation1996; Rasmussen and Winder Citation1997; Choi et al. Citation2001; Fisher et al. Citation2002). Once activated, AMPK switches off a number of ATP-consuming processes, including fatty acid, cholesterol and protein synthesis, while on the other hand it switches on catabolic pathways such as fatty acid oxidation and glycolysis, which generate ATP (Hardie Citation2004). Recent studies have shown that AMPK is an important signaling molecule that integrates nutritional and hormonal signals for feeding behavior and energy metabolism (Kim and Lee Citation2005). Thus, AMPK, as an important energy sensor, is crucial for organism survival under adverse conditions.
AMPK families are functionally conserved throughout the eukaryotic kingdom including plants, yeast, nematodes, insects and mammals. In mammals, AMPK influences various metabolic pathways, including glucose uptake, glycolysis, protein synthesis and fatty acid oxidation (Hardie and Pan Citation2002; Ferre et al. Citation2003; Hardie Citation2004). The yeast sucrose non-fermenting 1 (SNF1) kinase, which has strong structural and functional similarities with mammalian AMPK, plays important roles in transcriptional, metabolic and developmental responses to glucose limitation (Celenza and Carlson Citation1986; Ferre et al. Citation2003; Kuchin et al. Citation2003). Similar functions of AMPK and SNF1 have also been found in other members of AMPK families such as the plant protein kinase SNF1-related protein kinase-1 (SnRK1) (Halford et al. Citation2003).
AMPKs are heterotrimeric complexes consisting of a catalytic alpha subunit, and two regulatory subunits, beta and gamma. Genes encoding orthologues of the alpha, beta and gamma subunits are found in all eukaryotic species whose genome has been sequenced (Hardie Citation2004). In Drosophila, single genes encoding alpha, beta and gamma subunits have been identified (Pan and Hardie Citation2002). In mammals, each of the three subunits is encoded by two or three distinct genes (alpha 1, alpha 2; beta 1, beta 2; gamma 1, gamma 2, gamma 3), suggesting the existence of 12 AMPK complexes (Hardie Citation2004). The varied AMPK complexes may be partly responsible for the diversity of AMPK functions concerning with the responses to environmental and physiological stresses. Moreover, alternative splicing of some of these subunit encoding genes have been reported and some of these genes have been found to be expressed in different tissues or developmentally regulated (Carling et al. Citation1994; Verhoeven et al. Citation1995; Hardie Citation2004). Such variations have made the AMPK system more complex.
The brine shrimp, Artemia, possesses strong adaptability to extreme environment, being found alive in supersaturated brine at salinities as high as 34.0 g/l (Moens et al. Citation1991). The adaptability to such environment is also reflected by their reproductive modes. Artemia have two modes of reproduction: one ovoviviparity in which females produce free-swimming nauplii and the other one oviparity in which females produce diapaused encysted embryos (cysts) (Criel and Macias Citation2002). The encysted embryos can survived for years with a very low metabolic rate, and resume their development with the approach of favorable conditions (Clegg Citation1997; MacRae Citation2003). Determination of the reproductive mode occurs at some unknown point in the reproductive cycle and the physiological mechanisms controlling the reproductive mode are not known. However, intrinsic factors such as brood number and density affect the reproductive mode (Drinkwater and Clegg Citation1991). Such a trade-off between the two reproductive modes of Artemia is also affected by numerous environmental factors as photoperiod, temperature, oxygen concentration and salinity (Drinkwater and Clegg Citation1991; Clegg and Trotman Citation2002; Nambu et al. Citation2004). The ability to sense the changes of environmental factors and trade-off between reproductive modes indicates the dynamic nature of the Artemia AMPK system which may play important roles in stress signaling.
However, information about structures of AMPK subunit genes and their expression in crustaceans remains unknown and the role of AMPK in crustaceans has not been studied previously. Here we isolated two cDNAs of Artemia franciscana AMPK alpha subunit encoding genes, named Afr-AMPKalpha1 and Afr-AMPKalpha2, as the first step to study the function of Artemia AMPK. We also studied the gene expression patterns in different stages of Artemia life cycles and also under stressed conditions in adults and cysts, in addition to Southern blot analysis to determine whether there are multiple genes encoding AMPK alpha subunit in the A. franciscana genome.
Materials and methods
Culture of brine shrimps and embryos for sampling
Encysted embryos of A. franciscana, gifted by Freshwater Fisheries Institute of Zhejiang (China), were hydrated in ice-cold artificial sea water (2%) for 5 h (0 h cysts). Cysts were divided into two groups. Group-I was given heat shock of 55°C for sample collection. Group-II was cultured for normal development under continuous light with adequate O2 at 27°C until completion of hatching; sampling was done at 0, 1, 2, 4, 8, 12 and 14 h, and at the free-swimming nauplius stage.
Artemia were maintained in room temperature (25–27°C) and fed once a day with Chlorella powder. Samples were collected as larvae at different sizes (2, 3 and 5 mm), males and adult females without ovary formation (10 mm), females with white shell gland (FWSG) and brown shell gland (FBSG) but both with oocyte-filled lateral pouches, females both with diapause-destined embryos (FDE) and nondiapause embryos (FNE), the head and thoracic region of both females with diapause-destined embryos (HFDE) and nondiapause embryos (HFNE), and adult males. All samples were snap-frozen in liquid nitrogen and stored at − 80°C until isolation of RNA.
Stress treatments
A heat shock of 55°C was given to one group of hydrated cysts for 15, 30, 60 and 90 min. Another group of adult Artemia (0.8–1.2 cm) was given heat shock of 37°C for 5 and 30 min and 42°C for 30 min. In starvation treatments, Artemia were transferred to a dietless tank (with change of water every day) and after 24 h the next day was designated as day 0. Then samples were collected on days 2, 4, 6 and 9. For osmotic stress, Artemia were transferred to saturated solution of NaCl for 5, 30 and 60 min.
Nucleic acid isolation
All samples were homogenized in Trizol Reagent (Invitrogen), and total RNA was prepared according to the manufacturer's instructions. Genomic DNA was extracted using the standard phenol/chloroform method. Total RNA and genomic DNA were quantified on a Genova UV/visible spectrophotometer at 260 nm.
Reverse transcription-polymerase chain reaction (RT-PCR)
First strand cDNAs were synthesized from a mixture of nauplius and adult Artemia total RNAs using a FirstChoice™ RLM-RACE Kit (Ambion, USA) following the manufacturer's protocol. The first strand cDNAs for 3′ rapid amplification of cDNA ends (3′-RACE) were used as the template, and a cDNA fragment encoding the AMPK alpha subunit was amplified by two rounds of PCR amplification. Degenerate primers for the AMPK alpha subunit were designed on the basis of the highly conserved amino acid sequences of Drosophila melanogaster (GenBank accession number AAV36959), Aedes aegypti (AAX20150), Caenorhabditis elegans (AAP13770), Xenopus laevis (AAH84741), Sus scrofa (AAO17789), and Homo sapiens (NP_006242). In the first round of PCR, amplification was primed by the degenerate primer DF and the 3′-RACE adapter outer primer provided in the kit (, ), and the following program was used: 94°C for 4 min, 35 cycles of 94°C for 30 s, 48°C for 30 s and 72°C for 2 min, and a final step of 72°C for 10 min. In the second round, the first PCR products were used as templates, and amplification was performed with degenerate primers DF and DR (, ); the following program was used: 94°C for 4 min, 35 cycles of 94°C for 30 s, 46°C for 30 s and 72°C for 30 s, and a final step of 72°C for 10 min. The PCR product was subcloned into PCR® 2.1-TOPO plasmid using the TOPO™ TA cloning kit (Invitrogen) and sequenced.
Figure 1 Schematic diagram of Afr-AMPKalpha1 (A) and Afr-AMPKalpha2 (B) cDNAs showing locations of primers for PCR and strategy of the gene cloning. Arrowheads represent primers and lines under them indicate cDNA fragments amplified with the primers by PCR, 5′- and 3′-RACE. The dashed lines indicate the 244-bp deletion in cDNA sequence of Afr-AMPKalpha2 compared with that of Afr-AMPKalpha1.
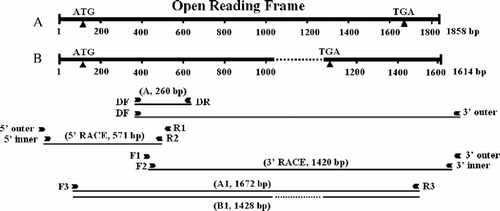
Table I. Nucleotide sequences and positions of primers used in PCR.
5′- and 3′-rapid amplification of cDNA ends (5′- and 3′-RACE)
The rapid amplification of cDNA ends (RACE) method was employed to isolate terminal cDNA fragments of Artemia AMPK alpha subunit gene. First strand cDNAs for 5′-and 3′-RACE were synthesized as described above, and used as templates of PCR. Two pairs of nested gene-specific primers (F1, F2, R1 and R2 in and ) were synthesized on the basis of the nucleotide sequences of Afr-AMPK alpha subunit cDNA fragment amplified by RT-PCR ().
Two rounds of PCR were used to amplify the cDNA fragment encoding the 5′-region of the Artemia AMPK alpha subunit. The first round of PCR was performed using gene-specific primer R1 and the 5′-RACE adapter outer primer included in the 5′-RACE kit (, ). The programs for PCR amplification were as follows: 4 min at 94°C, 35 cycles of 30 s at 94°C, 30 s at 58°C and 40 s at 72°C, and a final step of 10 min at 72°C. In the second PCR, the first PCR products were used as templates, and amplification was performed using nested gene-specific primer R2 (, ) and the 5′-RACE adapter inner primer. The programs for nested PCR amplification were the same as with the first PCR. The nested PCR product, a fragment of 570 bp, was subcloned and sequenced.
cDNA fragment encoding the 3′-region of the Artemia AMPK alpha subunit was amplified by two rounds of PCR, respectively. The first round of PCR was performed using Z-Taq (TaKaRa, Shiga, Japan), and gene-specific primer F1 and the 3′-RACE adapter outer primer (, ). The programs for PCR amplification were as follows: 30 cycles of 5 s at 98°C and 40 s at 66°C. In the nested PCR, the first PCR products were used as templates, and amplification was performed using nested gene-specific primer F2 (, ) and the 3′-RACE adapter inner primer. The programs for nested PCR amplification were the same as with the first PCR. The largest nested PCR product, a fragment of about 1.4 kb, was gel-purified using the QIAquick® Gel Extraction Kit (Qiagen), subcloned and sequenced.
To make sure that the 3′- and 5′-RACE fragments belonged to the same molecule, the first-strand cDNAs described for 3′-RACE were used as templates for PCR amplification. Two gene-specific primers (F3 and R3) were designed based on the nucleotide sequences of the 3′- and 5′-RACE fragments (, ). Amplification was primed by F3 and R3, and the following program was used: 35 cycles of 94°C for 30 s (4 min only for the first cycle), 56°C for 30 s, and 72°C for 2 min (10 min only for the last cycle). The PCR product was subcloned. The insert lengths of 16 independent clones were analyzed by PCR amplification performed with primer pairs, F3 and R3 (, ). Two independent clones, Number 1 and Number 13 (with insert sizes of approximately 1.6 and 1.4 kb), were subjected to sequencing analysis.
Probe preparation
The probe containing 1672 bp (, A1) used for Northern blot analysis and Southern blot analysis was generated by PCR amplification. Purified recombinant plasmid of the Number 1 clone was used as template for PCR amplification. Amplification was achieved using a pair of primers, F3 and R3 ( and ). The PCR product was gel-purified, and labeled with digoxigenin-dUTP using the DIG High Prime Labeling Kit (Roche). Pairs of primers (), tubulinF and tubulinR, were synthesized on the basis of a partial cDNA sequence of the Artemia alpha tubulin derived from GenBank (GenBank accession no. AF427598), and used to amplify a 350 bp tubulin cDNA fragment. The PCR product was purified, and labeled as described above.
Northern blot analysis
Aliquots containing 20 μg of total RNA corresponding to each sample were separated through a 1.0% agarose gel, transferred to a nylon membrane (Millipore immobilon-NY+), UV cross-linked, and then hybridized with the 1672 probe (, A1). Hybridization was performed at 50°C overnight and hybridized probes were detected using the DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche). The membranes were reprobed with a tubulin probe (350 bp) as a control after washed twice with 10 mM Tris–Cl/1 mM EDTA/0.1% SDS (pH 7.5) at 95°C for 5 min.
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR)
Semiquantitative RT-PCR experiments were performed to measure levels of Afr-AMPKalpha1 and Afr-AMPKalpha2 mRNAs in Artemia and cysts during developmental stages and in response to stresses. First-strand cDNA was synthesized from 2 μg of total RNA from each sample using oligo (dT) and M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) in a 20 μl reaction system. For each sample, an aliquot of 0.5 μl first-strand cDNA was used as template in PCR amplification. Primer pair, F2 and R3 (, ), was used to amplify a 1307-bp Afr-AMPKalpha1 cDNA fragment or a 1063-bp Afr-AMPKalpha2 cDNA fragment. Primer pair, tubulinF and tubulinR (), was used to amplify a 350-bp fragment of the constitutively expressed alpha tubulin gene which served as an internal control. PCR was carried out in a 25-μl reaction containing 10 pmol F2 and R3, and 3 pmol tubulinF and tubulinR (). The following program was used in these reactions: 94°C for 5 min, followed by 32 cycles of 94°C for 30 s, 56°C for 30 s, 72°C for 1.5 min, and a final extension step at 72°C for 10 min. Aliquots (7 μl) of the PCR products were electrophoresed in 1.5% agarose gels stained with ethidium bromide and the bands were analyzed using Dolphin 1D Version 1.1.0 (Wealtec Corp., USA). Relative abundances were expressed as Afr-AMPKalpha1 cDNA levels to those of alpha tubulin. In each case, peak values were set to 100 and the rest of the values normalized. Representative gels for Afr-AMPKalpha1 and tubulin are shown. All data are given as means ± SEM of independent experiments from separate RNA pools (n = 3). Statistical analyses were performed using one-way ANOVA ( and ). The differences were considered significance for P < 0.05.
Southern blot analysis
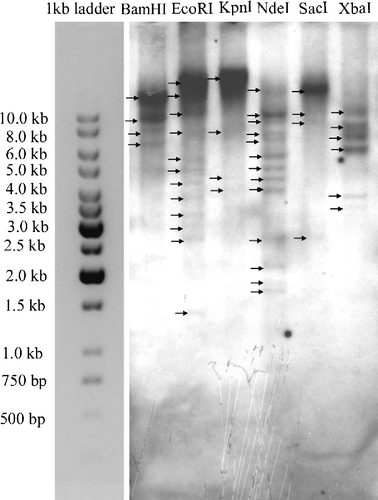
Six aliquots of genomic DNA (10 μg each) were digested overnight at 37°C with BamHI, EcoRI, KpnI, NdeI, SacI and XbaI (TaKaRa), precipitated with isopropanol and then dissolved in 15 μl of sterilized water. The restriction fragments and 5 μl of 1 kb DNA Ladder (Fermentas, MBI) were separated on a 0.8% (w/v) agarose gel and transferred onto a millipore nylon membrane. The probe described above (, A1) was also used to detect the digested genomic DNA.
Nucleotide sequence accession numbers
The nucleotide sequences of the cDNAs encoding Afr-AMPKalpha1 and Afr-AMPKalpha2 were submitted to GenBank under accession numbers DQ825653 and DQ825654, respectively.
Results
Identification Of Afr-ampkalpha1 And Afr-ampkalpha2 Genes
A 260-bp fragment () was amplified by pairs of degenerate primers, DF and DR ( and ) and sequenced. The sequence was conceptually translated into an 82-amino acid residue sequence that exhibited 76–95% identity with known AMPK alpha subunits of other animals. On the basis of the 260-bp cDNA fragment two pairs of nested gene-specific primers (F1, F2, R1 and R2 in and ) were designed and synthesized for 3′- and 5′-RACE. The 5′-RACE and the largest 3′-RACE fragments (571 and 1420 bp, ) were subcloned and sequenced. These fragments overlapped with the cDNA fragment A (). Then primers located near the 5′ and 3′ termini of the 5′- and 3′-RACE fragments (F3 and R3, and ) were designed and used for PCR amplification to make sure that the 3′- and 5′-RACE fragments belong to the same molecule. The PCR product was subcloned. The insert lengths of 16 independent clones were analyzed using PCR amplification performed with pairs of primers, F3 and R3 ( and ). Among them, the inserts of six clones were amplified by the primer pair, F3 and R3. Five of the six clones were found to have the same insert sizes of about 1.6 kb, one (Number 13) was found to have an insert size of about 1.4 kb. Number 1 and Number 13 clone (with insert sizes of approximately 1.6 and 1.4 kb) were subjected to sequencing analysis. Thus, two cDNA fragments of 1672 and 1428 bp (, A1 and B1) were obtained. Two full-length cDNAs (termed Afr-AMPKalpha1 and Afr-AMPKalpha2) were determined to be 1858 and 1614 bp, based on PCR, 5′-RACE and 3′-RACE ().
The Afr-AMPKalpha1 clone isolated was 1858 bp long with a 1548 bp open reading frame (ORF), a 136-nucleotide 5′ untranslated region (5′ UTR) and a 174-nucleotide 3′ UTR that contained two overlapped polyadenylation signal (AATTAA or ATTAAA) 14 or 13 bp upstream from the poly (A) tail in the cDNA (). The ORF of Afr-AMPKalpha1 was conceptually translated into a 515-amino acid protein (termed Afr-AMPKALPHA1) with a calculated molecular mass of approximately 58 kDa. The basic serine/threonine protein kinase domain was identified (). Afr-AMPKALPHA1 amino acid sequence exhibited highest identities (78.3 and 78.1%) with AMPK alpha subunits from the yellow fever mosquito, A. aegypti and the fruit fly, D. melanogaster, and showed high identity (33–77%) with other known AMPK alpha subunits.
Figure 2 Nucleotide sequences and deduced amino acid sequences of Afr-AMPKalpha1 and Afr-AMPKalpha2 cDNAs. The basic serine/threonine protein kinase domain is boxed. The amino acid sequence found in both Afr-AMPKalpha1 and Afr-AMPKalpha2 is shown in bold and italics. The amino acid sequence only in Afr-AMPKalpha2 is shaded. The 244-bp deletion of Afr-AMPKalpha2 compared with Afr-AMPKalpha1 is shown in lower case as a dashed line. The start and stop codons are underlined. The stop codon for Afr-AMPKalpha2 is italicized while that of Afr-AMPKalpha1 is shaded. Two putative polyadenylation signals AATTAA and ATTAAA are underlined and boxed, respectively.
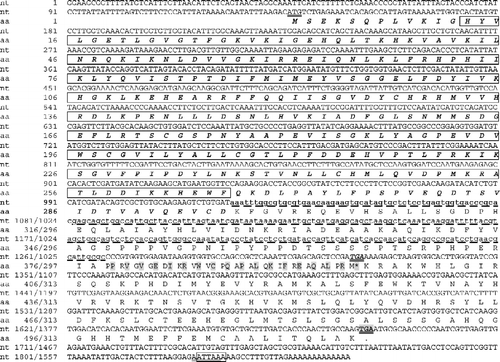
The Afr-AMPKalpha2 clone was 1614 bp long with a 942-bp ORF coding for a 313-amino acid protein containing the basic serine/threonine protein kinase domain (). The 5′ UTR of the Afr-AMPKalpha2 was 136 bp long, while the 3′ UTR was 536 bp. The nucleotide sequence of Afr-AMPKalpha2 was found to be identical to that of Afr-AMPKalpha1 except for a 244-bp deletion in the ORF region ( and ). The ORF of Afr-AMPKalpha2 was conceptually translated into a 313-amino acid protein (termed Afr-AMPKALPHA2) with a calculated molecular mass of approximately 36 kDa. Compared with Afr-AMPKalpha1, this 244-bp deletion caused a frameshift, introduced a new translation stop codon and shortened the C-terminal of the Afr-AMPKALPHA2 protein ().
Expression of AMPK alpha subunit genes
Expression of AMPK alpha subunit gene during Artemia life cycle
The transcripts from Artemia AMPK alpha subunit gene expression during the Artemia life cycle were determined by Northern blot analysis (). One AMPK alpha subunit transcript of approximately 5 kb was detected by Northern blot, as shown in . This gene showed different expression during the developmental stages of Artemia. During pre-emergence development of the cysts, the 5 kb mRNA level increased as development proceeded (, lanes 1–4). The mRNA level declined when the cysts developed into free-swimming nauplii (, lane 5), and then accumulated as the nauplii became adults (, lanes 5–8).
Figure 3 Expression of AMPK alpha subunit gene in Artemia and cysts at various developmental stages. (A) Northern blot analysis of Artemia AMPK alpha subunit gene expression in developing cysts incubated for 0, 4, 8 and 12 h, and in developing Artemia (nauplii, 2, 5 and 10 mm). (B) Semi-quantitative RT-PCR analysis of Afr-AMPKalpha1 expression in IC, dry activated cysts (DAC), hydrated activated cysts that were incubated for 0–14 h, and in nauplii. (C) Semi-quantitative RT-PCR analysis of Afr-AMPKalpha1 expression in developing Artemia (nauplii, 2–10 mm), in adult males and females (FWSG, females with two oocyte-filled lateral pouches and white shell gland; FBSG, females with two oocyte-filled lateral pouches and brown shell gland; FDE, females with diapause-destined embryos; FNE, females with non-diapause embryos). (D) Semi-quantitative RT-PCR analysis of Afr-AMPKalpha1 expression in the head and thoracic region of Artemia with diapause-destined embryos (HFDE), and with non-diapause embryos (HFNE). Asterisks (**) indicate significant differences (P < 0.01, by one-way ANOVA).
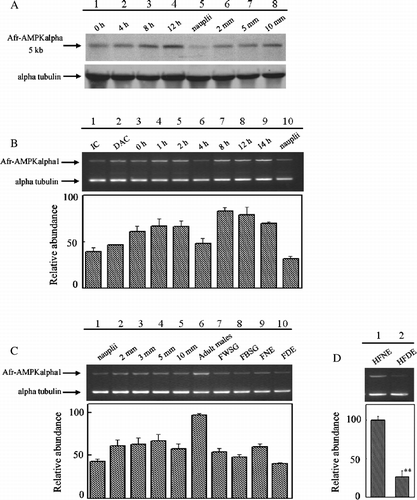
Northern blot analysis using 20 μg total RNA did not detect Afr-AMPKalpha1 or Afr-AMPKalpha2 expression. Therefore, semi-quantitative RT-PCR was used to characterize the expression of the Afr-AMPKalpha1 and Afr-AMPKalpha2 gene during Artemia development. The expression of the Afr-AMPKalpha1 gene was found to be developmentally regulated during pre-emergence development of the cysts (). As shown in , the Afr-AMPKalpha1 mRNA level in inactivated cysts (IC, lane 1) was lower than in hydrated-activated cysts (0 h, lane 3). The transcript of Afr-AMPKalpha1 accumulated during the first 2 h of the cysts hatching (, lanes 3–5). With a small decline after 4 h of incubation (, lane 6), the mRNA level reached the maximal value after 8 h of incubation (, lane 7), then the mRNA level remained relatively high (, lanes 8 and 9) until the free-swimming nauplii emerged (, lane 10). The Afr-AMPKapha1 mRNA level in free-swimming nauplii was the lowest (,C, lanes 10 and 1). No significant expression change was found in other post-emergence developmental stages of Artemia (, lanes 2–5). The comparison of mRNA levels among adult males, females with white shell gland and two pouches of unfertilized oocytes (FWSG), females with brown shell gland and two pouches of unfertilized oocytes (FBSG), females with non-diapause embryos (FNE) and females with diapause-destined embryos (FDE) (, lanes 6–10) showed that the Afr-AMPKalpha1 mRNA level was higher in adult males (, lane 6) than in females of different stages (, lanes 7–10).
To investigate whether Afr-AMPKalpha1 played roles in influencing the reproductive modes (ovoviviparity and oviparity) of females, the total RNAs of the head and thoracic region of females having non-diapause embryos (HFNE) or diapause-destined embryos (HFDE) were isolated, and used as templates for semiquantitative RT-PCR. A statistically significant difference (P < 0.01 by One-way ANOVA; ) was found between the Afr-AMPKalpha1 mRNA levels of these two kinds of females, with 4-fold greater level in females producing non-diapause embryos (ovoviviparity) as compared to those producing diapause-destined embryos (oviparity).
Expression of AMPK alpha subunit genes in Artemia and cysts in response to varied stresses
Whether the expression of Artemia AMPK alpha subunit gene was up- or down-regulated in response to stresses was analyzed by Northern blot. Northern blot analysis using 20 μg total RNA did not detect Afr-AMPKalpha1 or Afr-AMPKalpha2 expression in adults and hydrated cysts in response to varied stresses. The 5 kb transcript described above was also detected in adults and cysts in response to stresses by Northern blot, as shown in . The expression of this 5 kb gene product in Artemia was down-regulated by starvation (, lanes 2 and 3), heat shock (, lanes 4–6) and hyperosmotic stress (, lanes 9–11); while prolonged exposure time to these stresses did not decrease the 5 kb mRNA level further (). The 5 kb mRNA level in hydrated cysts decreased with increasing time of exposure to heat stress (50°C) (, lanes 7 and 8).
Figure 4 Expression of Artemia alpha subunit gene in Artemia and cysts in response to stresses. (A) Northern blot analysis of AMPK alpha subunit gene in response to varied stresses. Lane 1, RNAs from Artemia in normal conditions; Lanes 2 and 3, RNAs from starved adults for 2 or 9 days (ST2 and ST9); Lanes 4–6, RNAs from heat-shocked adults; Lanes 7 and 8, RNAs from heat shocked cysts; Lanes 9–11, RNAs from hyperosmotically shocked adults (HO 15, HO 30 and HO 60). (B) Semi-quantitative RT-PCR analysis of Afr-AMPkalpha1 expression in adults in response to heat-shock stress, hyperosmotic stress and starvation stress. (C) Semi-quantitative RT-PCR analysis of Afr-AMPKalpha1 expression in cysts subjected to heat-shock stress. (D) Semi-quantitative RT-PCR analysis of Afr-AMPKalpha1 expression in starved adults. Asterisks (**) indicate significant differences (P < 0.01, by one-way ANOVA versus control).
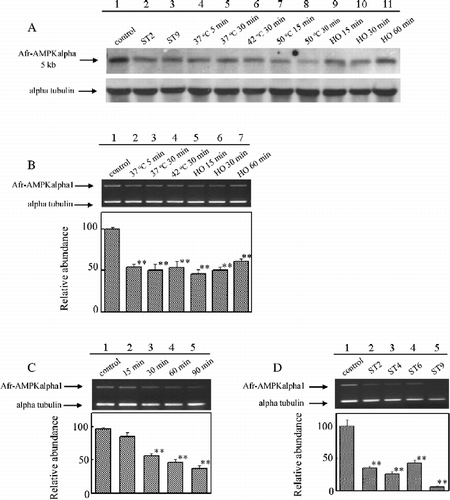
Semiquantitative RT-PCR analysis showed that, upon heat shock stress, the expression of Afr-AMPKalpha1 in Artemia decreased, while prolonged exposure time or elevated temperature did not decrease the Afr-AMPKalpha1 mRNA level further (, lanes 2–4). The Afr-AMPKalpha1 mRNA level decreased after 15 min of incubation in saturated saline solution, and then increased as the exposure time was extended (, lanes 5–7). After 9 days of starvation, expression of Afr-AMPKalpha1 was not detected (, lane 5). A decline of Afr-AMPKalpha1 mRNA level was also detected in hydrated cysts in response to heat shock for 15 min at 50°C (, lane 2 compared with lane 1). As the exposure time to 50°C increased, the mRNA level sharply decreased (, lane 2–5).
However, mRNA level of Afr-AMPKalpha2 was too low to be detected in all conditions unless nested PCR was used (data not shown).
Southern blot analysis
AMPK alpha subunit gene copy number in genomic DNA was examined by Southern blot analysis of Artemia fransicana using a 1672 probe for Afr-AMPKalpha1. Multiple bands were detected in Artemia genomic DNA digested by various enzymes ().
Discussion
AMPK is a metabolic and stress sensor that has been functionally conserved throughout eukaryotic evolution. AMPK families are found in many eukaryotes, including plants, yeast, nematode, insects and mammals (Hardie Citation2004). However, members of AMPK families have not been reported previously in crustaceans. Here, we described the molecular cloning and characterization of two cDNAs encoding two AMPK alpha subunits from the brine shrimp, A. franciscana, as a first step towards a more detailed understanding of Artemia AMPK.
Two Artemia cDNAs coding for different isoforms of the AMPK alpha subunit (Afr-AMPKalpha1 and Afr-AMPKalpha2) have been characterized. The nucleotide sequence of Afr-AMPKalpha2 was the same as Afr-AMPKalpha1, except for a 244-bp deletion in the 3′ region ( and ). The lack of GT–AG and AT–AC signals in the deletion region () suggests that the Afr-AMPKalpha2 is not a direct spliced product of Afr-AMPKalpha1. However, the deletion might be achieved by alternative splicing. It is possible that the two cDNAs originate by alternative splicing of a single gene. For example, the 244 nt fragment, as an exon for Afr-AMPKalpha1, may be located between two adjacent introns. While for Afr-AMPKalpha2, this 244 nt fragment, as part of an intron, may be spliced together with the two adjacent introns.
The AMPK alpha subunits contain a conventional kinase domain at the N-terminal regions (Hardie Citation2004), while the C-terminal regions of the alpha subunits are required to form a complex with beta and gamma subunits (Crute et al. Citation1998; Hardie Citation2004). The putative amino acid sequences of both Afr-AMPKALPHA1 (515 amino acids in length) and Afr-AMPKALPHA2 (313 amino acids in length) contain the basic serine/threonine protein kinase domain. However, the C-terminal region of Afr-AMPKALPHA2 is truncated, indicating the inability of Afr-AMPKALPHA2 to form functional complexes with other subunits. The amino acid sequence of Afr-AMPKALPHA2 and Afr-AMPKALPHA1 showed great identities (94%) with each other. The amino acid sequence of Afr-AMPKALPHA1 shows a relatively high identity (33–78%) with other members of the AMPK/SNF1 family, while that of Afr-AMPKALPH2 shows an even higher identity (45–88%), which may be due to a truncated C-terminal region of Afr-AMPKALPHA2 (). The highest sequence identity is found in the basic serine/threonine protein kinase domain that confers biological functions. This domain, spanning amino acids 13–266 of Afr-AMPKALPHA1 and Afr-AMPKALPHA2 showed >64% identity among species. The fact that all AMPK alpha subunits show high identities in their functional domains may partially explain why various species share the highly-conserved AMPK systems.
Multiple transcripts of AMPK alpha subunit genes, as well as the existence of large AMPK alpha subunit mRNAs, have been found in many organisms, for example, rat and human (Aguan et al. Citation1994; Beri et al. Citation1994; Carling et al. Citation1994; Gao et al. Citation1995). In rat, 10–15% of AMPK alpha subunit mRNA is alternatively spliced in most tissues (Aguan et al. Citation1994). As shown by Northern blot, a single 5 kb transcript was differently regulated during development and in response to stress ( and ). Whether Afr-AMPkalpha1 or Afr-AMPKalpha2 is an alternatively spliced product of this transcript remains to be studied. Southern blot analysis indicates the possibility that the Artemia AMPK alpha subunit gene is present in multiple copies. However, because intron compositions are not known, the number of Artemia AMPK alpha subunit genes could only be estimated. To further calculate the gene number, additional analyses of genomic cloning and Southern blots are required.
Artemia is a model animal to study embryonic development and diapause because it tends to produce gastrula stage diapaused embryos under adverse environmental conditions. The diapaused embryos can resume their development with the approach of favorable conditions, so that the activation of the cyst and its posterior embryonic development can be studied in the laboratory (Clegg Citation1997; MacRae Citation2003). The expression study of several genes showed an accumulation of their mRNAs during 4–16 h of Artemia embryonic development (Ortega et al. Citation1992; Escalante et al. Citation1994). Analysis of Artemia alpha subunit gene expression using Northern blot and semiquantitative RT-PCR showed similar results (,B). However, no detection of Afr-AMPKalpha1 mRNA in all samples analyzed by Northern blot analysis indicates that the Afr-AMPKalpha1 mRNA levels were very low. The adult male Artemia contained more Afr-AMPKalpha1 mRNAs than adult females (, lane 6 compared with lanes 7–10), suggesting a differential role of the gene between males and females.
Artemia produce diapaused encysted embryos in adverse conditions (MacRae Citation2003). Multiple environmental factors may influence biological signal factors for Artemia to reproduce oviparously. However, the questions about the physiological nature of the signal transduction pathway, by which Artemia senses the environment stimuli and changes their reproductive mode, are still unknown. Recent studies suggest that AMPK functions not only as a peripheral sensor of energy balance, but also in the central nervous system as a multifunctional metabolic sensor (Ramamurthy and Ronnett (Citation2006)). Thus, we analyzed the expression of Afr-AMPKalpha1 in the head and thoracic region of Artemia carrying diapause-destined or nondiapause embryos to see whether AMPK is involved in affecting the reproductive mode of Artemia. Results of semiquantitative RT-PCR revealed a significant difference (P < 0.01) in the Afr-AMPKalpha1 mRNA level between these two kinds of females (). This result suggests a relation between the expression of the Afr-AMPKalpha1 gene and the reproductive modes of A. franciscana: decreases in the mRNA level of Afr-AMPKalpha1 in the head and thoracic region may be one of the reasons why Artemia change reproductive mode to produce diapause-destined embryos.
We also investigated Afr-AMPKalpha1 gene expression in response to hyperosmotic, heat shock and starvation stresses (). The mRNA level of Afr-AMPKalpha1 in cysts was significantly decreased in response to heat shock in a time-dependent manner (). In contrast, in Artemia, the Afr-AMPKalpha1 mRNA level decreased in response to heat shock, but was not further decreased with prolonged stress or with increased stress temperature (, lanes 2–4). The mechanism controlling its differential expression with heat stress at these different stages needs further studies. Upon osmotic stress, the level of Afr-AMPKalpha1 transcript in Artemia decreased and then slightly increased with exposure time (), suggesting a role of AMPK alpha subunit in Artemia in response to hyperosmotic stress. Similarly, the expression of SIK, another member of the AMPK/SNF1 family, in rat adrenal glands is also induced by high sodium concentration (Wang et al. Citation1999). Oviparity in Artemia is generally considered to be induced by environmental stresses (Tackaert and Sorgeloos Citation1991). Decreasing expression of the transcript of Afr-AMPKalpha1 under stresses (), combined with the finding that Afr-AMPKalpha1 mRNA level declined in the head and thoracic region of Artemia carrying diapause-destined embryos, indicates the possibility that Afr-AMPKalpha1 is involved in signaling stress to determine the reproductive mode.
Previous studies have shown that AMPK plays key roles in regulating cellular energy balance under stressed conditions in which it is allosterically-activated by AMP or by upstream kinases at a threonine residue within the kinase domain of the alpha subunit (Hardie Citation2004). Although AMPK is activated in response to stresses, its activated level may decline during the course of stress. For example, time course studies revealed that H2O2 induced a rapid but transient activation of AMPK in NIH-3T3 cells, which peaked within 5 min, then declined but remained slightly elevated until 20 min of exposure, and thereafter gradually returned to the basal level in 1 h (Choi et al. Citation2001). In contrast in the present study the level of Afr-AMPKalpha1 mRNA in cysts and in adults under stress conditions was lower than found under normal conditions (). It is possible that Afr-AMPKalpha1 mRNA expression is increased at other times after stress. It should be emphasized that we measured Afr-AMPKalpha1 mRNA expression, and further studies are needed to measure the content of Afr-AMPKALPHA1 protein, for example with western blot analysis. Changes in mRNA expression need not reliably reflected in changes in expression, or activity, of the corresponding protein. Thus, in our further studies, we will focus on expression of the AMPK alpha subunit proteins and AMPK kinase activity during the Artemia life cycle as well as under stress.
AMPK is a complex system that plays significant roles in many physiological activities. We have demonstrated expression of AMPK subunit gene expression in a crustacean, though this system has been found in many other eukaryotes. Our finding reinforces the universality of AMPK. In addition, cloning and characterization of AMPK alpha subunit genes provide information useful in the further investigation of the functions of this kinase in Artemia, especially under stress.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (30225034 and 30371097).
References
- Aguan K, Scott J, See CG, Sarkar NH. Characterization and chromosomal localization of the human homologue of a rat AMP-activated protein kinase-encoding gene: A major regulator of lipid metabolism in mammals. Gene 1994; 149(2)345–350
- Beri RK, Marley AE, See CG, Sopwith WF, Aguan K, Carling D, Scott J, Carey F. Molecular cloning, expression and chromosomal localisation of human AMP-activated protein kinase. FEBS Lett 1994; 356(1)117–121
- Carling D, Aguan K, Woods A, Verhoeven AJ, Beri RK, Brennan CH, Sidebottom C, Davison MD, Scott J. Mammalian AMP-activated protein kinase is homologous to yeast and plant protein kinases involved in the regulation of carbon metabolism. J Biol Chem 1994; 269(15)11442–11448, < http://www.jbc.org/cgi/reprint/269/15/11442>
- Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science 1986; 233(4769)1175–1180
- Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. The regulation of AMP-activated protein kinase by H(2)O(2). Biochem Biophys Res Commun 2001; 287(1)92–97
- Clegg J. Embryos of Artemia franciscana survive four years of continuous anoxia: The case for complete metabolic rate depression. J Exp Biol 1997; 200(Pt 3)467–475, < http://www.jeb.biologists.org/cgi/reprint/200/3/467>
- Clegg JS, Trotman CNA. Physiological and biochemical aspects of Artemia ecology. ARTEMIA: Basic and applied biology. Biology of aquatic organisms, vol. 1, THJ Abatzopoulos, JA Beardmore, JS Clegg, P Sorgeloos. Kluwer Academic Publishers, Dordrecht 2002; 129–170
- Criel GRJ, Macias MT. Reproductive biology of Artemia. ARTEMIA: Basic and applied biology. Biology of aquatic organisms, vol. 1, THJ Abatzopoulos, JA Beardmore, JS Clegg, P Sorgeloos. Kluwer Academic Publishers, Dordrecht 2002; 39
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem 1998; 273(52)35347–35354, < http://www.jbc.org/cgi/content/full/273/52/35347>
- Drinkwater LE, Clegg JS. Experimental biology of cyst diapause. Artemia biology, RA Browne, P Sorgeloos, CNA Trotman. CRC Press, Boca Raton/Ann Arbor/Boston 1991; 93–117
- Escalante R, Garcia-Saez A, Ortega MA, Sastre L. Gene expression after resumption of development of Artemia franciscana cryptobiotic embryos. Biochem Cell Biol 1994; 72(3–4)78–83
- Ferre P, Azzout-Marniche D, Foufelle F. AMP-activated protein kinase and hepatic genes involved in glucose metabolism. Biochem Soc Trans 2003; 31(Pt 1)220–223, < http://www.biochemsoctrans.org/bst/031/0220/bst0310220.htm>
- Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 2002; 282(1)E18–E23, < http://www.ajpendo.physiology.org/cgi/content/full/282/1/E18>
- Gao G, Widmer J, Stapleton D, Teh T, Cox T, Kemp BE, Witters LA. Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochim Biophys Acta 1995; 1266(1)73–82
- Halford NG, Hey S, Jhurreea D, Laurie S, McKibbin RS, Paul M, Zhang Y. Metabolic signalling and carbon partitioning: Role of Snf1-related (SnRK1) protein kinase. J Exp Bot 2003; 54(382)467–475, < http://www.jxb.oxfordjournals.org/cgi/content/full/54/382/467>
- Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci 2004; 117(Pt 23)5479–5487, < http://www.jcs.biologists.org/cgi/content/full/117/23/5479>
- Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 2002; 30(Pt 6)1064–1070
- Hardie DG, Carling D, Halford N. Roles of the Snf1/Rkin1/AMP-activated protein kinase family in the response to environmental and nutritional stress. Semin Cell Biol 1994; 5(6)409–416
- Kim MS, Lee KU. Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J Mol Med 2005; 83(7)514–520
- Kuchin S, Vyas VK, Carlson M. Role of the yeast Snf1 protein kinase in invasive growth. Biochem Soc Trans 2003; 31(Pt 1)175–177, < http://www.biochemsoctrans.org/bst/031/0175/bst0310175.htm>
- MacRae TH. Molecular chaperones, stress resistance and development in Artemia franciscana. Semin Cell Dev Biol 2003; 14(5)251–258
- Moens L, Wolf G, Van Hauwaert ML, De Baere I, Van Beeumen J, Wodak S, Trotman CNA. The extracellular hemoglobins of Artemia: Structure of the oxygen carrier and respiration physiology. Artemia biology, RA Browne, P Sorgeloos, CNA Trotman. CRC Press, Boca Raton/Ann Arbor/Boston 1991; 187–219
- Nambu Z, Tanaka S, Nambu F. Influence of photoperiod and temperature on reproductive mode in the brine shrimp, Artemia franciscana. J Exp Zoolog A Comp Exp Biol 2004; 301(6)542–546, < http://www3.interscience.wiley.com/cgi-bin/fulltext/109061165/PDFSTART>
- Ortega MA, Macias MT, Martinez JL, Palmero I, Sastre L. Expression of actin isoforms in Artemia. Symp Soc Exp Biol 1992; 46: 131–137
- Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem J 2002; 367(Pt 1)179–186, < http://www.biochemj.org/bj/367/0179/bj3670179.htm>
- Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol 2006; 574(Pt 1)85–93, < http://www.jp.physoc.org/cgi/content/full/574/1/85>
- Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol 1997; 83(4)1104–1109, < http://www.jap.physiology.org/cgi/content/full/83/4/1104>
- Tackaert W, Sorgeloos P. Semi-intensive culturing in fertilized ponds. Artemia biology, RA Browne, P Sorgeloos, CNA Trotman. CRC Press, Boca/Raton/Ann Arbor/Boston 1991; 287–315
- Verhoeven AJ, Woods A, Brennan CH, Hawley SA, Hardie DG, Scott J, Beri RK, Carling D. The AMP-activated protein kinase gene is highly expressed in rat skeletal muscle. Alternative splicing and tissue distribution of the mRNA. Eur J Biochem 1995; 228(2)236–243
- Wang Z, Takemori H, Halder SK, Nonaka Y, Okamoto M. Cloning of a novel kinase (SIK) of the SNF1/AMPK family from high salt diet-treated rat adrenal. FEBS Lett 1999; 453(1–2)135–139
- Wilson WA, Hawley SA, Hardie DG. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP: ATP ratio. Curr Biol 1996; 6(11)1426–1434