Abstract
This review summarizes knowledge on the effects of stress on two catecholamine biosynthetic enzymes, tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT). Information is presented on differential responses of the enzymes to a variety of stressors as well as differential responses of the enzymes localized to the central nervous system vs. peripheral nervous system and tissues. Changes in mRNA and protein or activity are described, including species- and stressor-specific effects. While temporal changes in these parameters may differ for the particular stressor or enzyme, in general, maximal changes in mRNA and protein content occur at 6–8 and 24 h after stressor exposure, respectively. Elevation of TH and PNMT transcriptional activators prior to mRNA induction and nuclear run-on assays show that stress activates the genes encoding these enzymes. Yet, extents of induction of mRNA, protein and enzyme activity are often discordant depending on the stress, its duration and repetition of exposure. The extremes are concordant changes in mRNA and protein/activity vs. highly elevated mRNA with no change in protein/activity. Post-transcriptional and/or post-translational regulatory influences that may contribute to the complex effects of stress on TH, PNMT and the stress hormone epinephrine are explored.
Introduction
Hans Seyle (Citation1975), a Slovakian by birth, described stress in his “General Adaptive Syndrome” as the series of events initiated by a stimulus to the senses that constitutes the “fight or flight” response posited by Walter Cannon in the early 1900s (Cannon and De La Paz Citation1911). The incoming stimulus is forwarded to the brain, activating physical changes that, in turn, permit the individual to cope with a potentially dangerous situation. The activated physiological processes prime the person to take action, whether that action is “fight” or “flight”. The catecholamine epinephrine and the glucocorticoid cortisol are the two major stress hormones, which released into the bloodstream initiate these coping responses. Stress effects on epinephrine and two other catecholamines, dopamine and norepinephrine, have important consequences for both behavioral and physical well being due to their roles in a wide variety of important biological processes.
The catecholamines
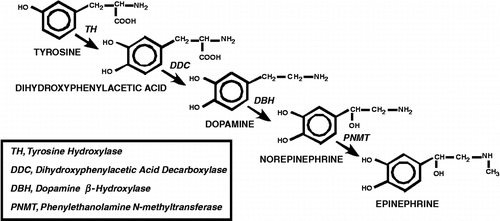
The catecholamines, dopamine, norepinephrine and epinephrine are synthesized via the enzymatic pathway depicted in . Clusters of dopaminergic, noradrenergic and adrenergic cells have been identified in the central and peripheral nervous systems. Release of catecholamine neurotransmitters is elicited by a variety of hormonal and neural stimuli as well as by stress. The intracellular stores of these transmitters are utilized for release, and these stores are then replenished through de novo synthesis and re-uptake by active transport systems, with neurotransmitter synthesis, release, re-uptake and degradation contributing in aggregate to sustaining cellular steady-state levels of the catecholamines.
Figure 1 Catecholamine Biosynthesis. Depiction of steps involved in catecholamine biosynthesis. The amino acid tyrosine is converted to dihydroxyphenylacetic acid by TH. Dihydroxyphenylacetic acid is decarboxylated by dihydroxyphenylacetic acid decarboxylase (DDC) to the neurotransmitter dopamine. Addition of a hydroxyl group to the β carbon of the aliphatic chain by dopamine β‐hydroxylase (DBH) generates norepinephrine, and finally, norepinephrine is converted to epinephrine by N-methylation of the terminal amino group by PNMT.
Epinephrine and cortisol are the two major human stress hormones. The initial response to physical, psychological or environmental stress is the rapid and transient release of epinephrine and norepinephrine into the circulation. Subsequently, cortisol is released from the adrenal cortex into the blood stream as well. These events comprise activation of the tripartite stress axis (hypothalamus, pituitary gland and adrenal gland), and lead to initiation of a series of physiological responses, involving but not limited to the activation of catecholaminergic neurotransmission in the central nervous system, the sympathetic nervous system and heart. Much effort has been directed at delineating molecular mechanisms underlying stress-induced catecholamine responses, and in particular, responses via control of their biosynthetic enzymes. Here, we review present knowledge of stress-induced changes in two enzymes in the pathway, tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine production, and phenylethanolamine N-methyltransferase (PNMT), the final enzyme in the pathway generating epinephrine.
TH and PNMT localization and basal expression
TH. TH is localized in all cells that produce catecholamines; these comprise the adrenal medulla and sympathetic neurons in the periphery, dopamine-producing neurons in the substantia nigra, ventral tegmentum, hypothalamus and olfactory bulb, norepinephrine-producing neurons in the locus coeruleus (LC, A6) and other discrete nuclei of the brainstem and the epinephrine-producing neurons of the brainstem (see below). The expression of this enzyme is tightly controlled in response to stress or other stimuli and is regulated by both short-term and long-term mechanisms (Kumer and Vrana Citation1996; Sabban et al. Citation1998). Its basal expression is dependent on a number of transcriptional regulators, as shown in transgenic mice where TH gene promoter sequences including either ∼4.5 or ∼9 kb of the 5′ flanking region direct accurate tissue-specific expression of reporter genes, with only minor ectopic expression (Banerjee et al. Citation1992; Min et al. Citation1994). The cAMP/calcium response element (CRE) and activator protein-1 (AP1; and adjoining sequences) sites within the most proximal TH gene promoter are required for basal expression in the adrenal medulla and sympathetic ganglia (Kim et al. Citation1993; Lazaroff et al. Citation1995; Trocme et al. Citation1998). In contrast, more upstream elements participate in basal TH expression in the brain (Banerjee et al. Citation1992; Min et al. Citation1994). In midbrain dopaminergic neurons, a number of transcription factors play a role in the development and maintenance of TH expression and consequent dopaminergic phenotype, including Nurr1 (Kim et al. Citation2003), Ptx3 (Cazorla et al. Citation2000). GATA-3 (Hong et al. Citation2006) and AP2 (Kim et al. Citation2001). Factors that maintain TH gene expression in other catecholaminergic cells have yet to be elucidated.
PNMT. In contrast to TH, which identifies cells producing dopamine, norepinephrine and epinephrine, PNMT solely identifies adrenergic cells producing epinephrine. In the CNS, adrenergic neurons are limited to the C1, C2 and C3 nuclei in the ventrolateral regions of the medulla oblongata in the brainstem (Hokfelt et al. Citation1984), clustered dorsolaterally to corresponding noradrenergic A1, A2 and A3 nuclei. Adrenergic cells expressing PNMT and epinephrine are also found in the heart (Ebert et al. Citation1996; Krizanova et al. Citation2001; Ziegler et al. Citation2002), sympathetic ganglia (Kubovcakova et al. Citation2006), spleen (Jelokova et al. Citation2002; Warthan et al. Citation2002; Ziegler et al. Citation2002), thymus (Warthan et al. Citation2002; Ziegler et al. Citation2002), retina (Cohen and Hadjiconstantinou Citation1984; Ziegler et al. Citation2002) and other tissues (Kennedy et al. Citation1990; Kubovcakova et al. Citation2006). However, the most abundant source of PNMT and epinephrine are the chromaffin cells in the adrenal medulla. These cells are the major contributors to circulating epinephrine, particularly in response to stressful stimuli. Not all stressors elicit epinephrine release from the chromaffin cells. Rather, release appears dependent on the type of stress, its intensity and duration (Kvetnansky et al. Citation1984; Kvetnansky Citation2004). Consistent with the variable responses of epinephrine to novel as well as repeated or combined stressors are changes in the expression of its biosynthetic enzyme PNMT (Dronjak et al. Citation2004; Kvetnansky Citation2004).
Stress and up-regulation of TH and PNMT enzyme levels
TH. Since the initial findings that stress up-regulates TH (Kvetnansky et al. Citation1970; Thoenen Citation1970), many different types of stressors have been shown to induce TH, including cold exposure (Fluharty et al. Citation1983; Osterhout et al. Citation1997), immobilization (Nankova et al. Citation1994; Kvetnansky et al. Citation1996; Osterhout et al. Citation1997), isolation (Toru Citation1982), hypoglycemia induced by insulin or 2-deoxyglucose (Fluharty et al. Citation1983; Gagner et al. Citation1985), foot shock (Melia and Duman Citation1991) and exercise (Tumer et al. Citation1992) in a variety of tissues including adrenal medulla, sympathetic ganglia and LC. Increases in TH activity generally have a long onset (12–24 h), require sustained exposure to stress for many hours and are associated with an equivalent rise in TH protein expression. In the adrenal medulla TH induction is mediated transsynaptically, since denervation of the innervating splanchnic nerve abolishes the response. In contrast, hypophysectomy has no effect on induction of adrenal TH by most stressors. Finally, some stressors also increase TH activity rapidly (min) due to activation of pre-existing enzyme molecules by phosphorylation mechanisms (Kumer and Vrana Citation1996).
PNMT. The first indication that stress-mediated increases in epinephrine production might be genetically controlled through its biosynthetic enzyme PNMT arose from studies examining changes in PNMT protein or activity in response to stress. Stress elicits a rise in adrenal PNMT consistent with increased epinephrine production. In particular, 3 h of cold stress (4°C) rapidly induces PNMT enzymatic activity in mice, with inhibition of elevation by the protein synthesis inhibitor, cycloheximide (Ciaranello et al. Citation1972). These effects are not species-specific as a similar increase in adrenal PNMT occurs in rats exposed to cold (Weisberg et al. Citation1989). Social stress also elevates PNMT activity (Thoa et al. Citation1976). If C57BR/cd mice are subjected to 10 min of daily attack by male Swiss Webster mice (aggressive behavior evoked by prolonged isolation) for 14 days, adrenal PNMT and serum epinephrine levels increase. Repeated immobilization similarly stimulates adrenal PNMT activity in Lewis and F344 inbred rats (Cooper and Stolk Citation1979). Curiously, splanchnic denervation of the adrenal gland does not alter responses of PNMT to immobilization in the Lewis rats while ablation of the pituitary gland (hypophysectomy) does. The converse is the case in the F344 rats.
Stress elicits changes in PNMT in the central nervous system as well. Horizontal shaker stress (30 min) elevates PNMT activity in the C1 and C2 nuclei of the medulla oblongata and pons of Sprague Dawley rats (Turner et al. Citation1978). Adrenal PNMT activity does not change despite a marked rise in plasma corticosterone. However, adrenal PNMT protein reaches a peak at 24 h post-stimulus and has a long half-life. The rapid increase in PNMT activity in the brain suggests differences between central and peripheral PNMT regulatory mechanisms, due perhaps to post-transcriptional and/or post-translational controls (Wong et al. Citation1992).
Stress and transcriptional regulation of TH and PNMT
TH. TH mRNA expression increases in both the adrenals and LC in response to most stressors, including cold exposure (Tank et al. Citation1985; Baruchin et al. Citation1990), immobilization (Nankova et al. Citation1994; Kvetnansky et al. Citation1996; Rusnak et al. Citation1996), isolation (Angulo et al. Citation1991), hypoglycemia (Vietor et al. Citation1996; Rusnak et al. Citation2001), foot shock (Chang et al. Citation2000) and exercise (Tumer et al. Citation2001). In most cases, but not all (see section on post-transcriptional regulation), increases in TH mRNA and TH protein are similar. As with TH protein, changes in TH mRNA expression have been primarily reported in the adrenal medulla and LC. However, TH mRNA also increases in response to immobilization stress in sympathetic ganglia (Nankova et al. Citation1996; Micutkova et al. Citation2003), and some stressors elicit small increases in TH mRNA expression in dopaminergic midbrain neurons. This latter response is highly dependent on the strain of the animal (Angulo et al. Citation1991; Ortiz et al. Citation1996; Serova et al. Citation1998a; Serova et al. Citation1999) and it remains unclear whether increased midbrain TH mRNA expression results in induction of TH protein synthesis.
Increases in mRNA levels may be caused by increased gene transcription, altered RNA precursor processing and/or decreased mRNA degradation. Most studies on TH gene regulation by stress have focused on changes in the rate of TH gene transcription, as findings suggest that stress leads to rapid and in some cases, sustained increases in TH gene transcription. Three approaches have been used to measure these transcriptional responses to stress: (1) Nuclear run-on assays provide direct evidence that immobilization stress stimulates TH gene transcription rate in rat adrenal medulla and LC (Nankova et al. Citation1999; Serova et al. Citation1999); (2) Transgenic mouse studies demonstrate that TH gene promoter activity increases in response to stress in the adrenal medulla (Osterhout et al. Citation1997; Osterhout et al. Citation2005); and (3) Measurements of nuclear TH gene primary transcripts show that transcription is induced rapidly after footshock in the LC (Chang et al. Citation2000), and after immobilization stress in both the adrenal medulla and LC (Sun et al. Citation2003; Sun et al. Citation2004). More detailed analyses further indicate that duration of the transcriptional response differs depending upon duration of stress and the cell type. In the adrenal medulla, short-term stress transiently stimulates the TH gene for a few hours, causing a short-term induction of TH mRNA and usually minor effects, if any, on TH protein expression. However, long-term or repeated stress causes sustained stimulation of adrenal TH gene transcription rate (1–2 days), leading to long-term induction of both TH mRNA and TH protein (Osterhout et al. Citation1997; Sun et al. Citation2003; Sun et al. Citation2004; Osterhout et al. Citation2005). In contrast, in the LC, both short-term and long-term stressors activate TH gene transcription rate for short periods of time, but apparently post-transcriptional mechanisms must also be activated to elicit sustained increases in TH mRNA and TH protein (see below) (Sun et al. Citation2004; Osterhout et al. Citation2005).
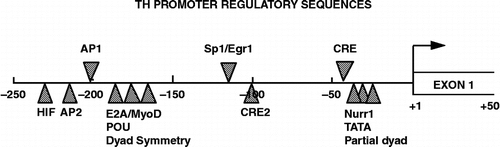
These transcriptional responses are mediated by transcription factors that interact with cognate cis-acting DNA sequences within the promoter region of the TH gene. The TH gene proximal promoter possesses many well-known response elements () (Kumer and Vrana Citation1996; Sabban and Kvetnansky Citation2001; Lewis-Tuffin et al. Citation2004); however, three of these elements appear critical for the stress response, the CRE, AP1 and possibly the Sp1/Egr1 sites. Supportive evidence derives from studies in which signaling pathways known to participate in the stress response are activated in cell culture; there is also correlative in vivo evidence that transcription factors binding to these sites are rapidly activated and/or induced in response to stress (Serova et al. Citation1998b; Papanikolaou and Sabban Citation1999; Sabban and Kvetnansky Citation2001). Several reports have also tentatively identified long-lived transcription factors binding to these sites during long-term stress (Nankova et al. Citation2000; Sun et al. Citation2003; Hebert et al. Citation2005); these factors may be involved in the sustained transcriptional response observed in the adrenal medulla. Finally, we still possess only a rudimentary understanding of how these factors stimulate the TH gene in response to stress, with evidence mostly derived from the adrenal medulla; yet unknown is whether these same factors and promoter elements operate similarly in the brain to regulate TH gene transcription.
Figure 2 Tyrosine hydroxylase (TH) promoter. Schematic of the rat TH promoter depicting known transcription factor regulatory elements identified to date. The regulatory elements that are likely involved in the stress response are shown above the line: AP1 (activator protein-1 site that recognizes Jun and Fos proteins; Kumer and Vrana Citation1996); Sp1/Egr1 site (Papanikolaou and Sabban Citation1999) and the CRE (cAMP/CRE that recognizes members of the CREB, CREM and ATF families; Lewis-Tuffin et al. Citation2004). The sites designated below the line are involved in tissue-specific or developmental expression of the gene or responses to more specific stimuli. These include sites for hypoxia-inducible factor (HIF), AP2, POU domain factors, cAMP and TPA response factors (CRE2) and Nurr1.
PNMT. Cloning of the bovine (Baetge et al. Citation1986), rat (Ross et al. Citation1990), mouse (Morita et al. Citation1992) and human (Baetge et al. Citation1988; Kaneda et al. Citation1988) PNMT genes has provided tools for more detailed investigation of molecular mechanisms underlying stress-mediated changes in PNMT since changes in PNMT protein or activity can be complemented by changes in PNMT mRNA expression. While species-specific variations exist in the gene, a generic structure has emerged. The gene is ∼2.0–2.8 kb in length, consists of three exons and two introns, and excision of intronic sequences from the gene-derived primary transcript yields an mRNA encoding a 31–33 kDa protein.
The proto-typical stress model for studying adrenergic responses is immobilization because it evokes strong and robust changes in the production of catecholamines and their synthetic enzymes. Early rat studies showed that single and repeated immobilization markedly increases PNMT mRNA in the adrenal medulla (Viskupic et al. Citation1994; Sabban et al. Citation1995; Wong et al. Citation1996). Induction is rapid with the response largely due to stress-induced glucocorticoid release (Sabban et al. Citation1995). The RNA synthesis inhibitor, actinomycin D, prevents induction, consistent with transcriptional activation. Changes in PNMT mRNA expression have further been shown to require an intact pituitary–adrenocortical axis as concomitant corticosteroid administration exacerbates while hypophysectomy abolishes the increase in mRNA expression (Kvetnansky et al. Citation1995). Studies with corticotrophin releasing factor (CRF) knock out mice (CRF KO) provide further evidence of these findings. Compared to wild-type mice, immobilization negligibly alters adrenal PNMT protein and mRNA expression in CRF KO mice, consistent with lower levels of plasma epinephrine and corticosterone. Immobilization-induced increases in adrenal medullary PNMT mRNA expression are transient as well. In rats, stimulation is equivalent for single or repeated immobilization for 30 or 120 min, indicating no apparent desensitization to these conditions of stress for up to seven daily exposures (Tai et al. Citation2007). Curiously, the Flinders Sensitive rats, a model of genetic depression, show elevated basal adrenal PNMT, TH and dopamine β-hydroxylase (DBH) mRNA levels, and immobilization evokes a blunted increase in expression of these mRNAs. Finally, in mice with knock out (KO) of monoamine oxidase B (MAOB), which catabolizes dopamine, immobilization for 30 min markedly increases serum corticosterone without any change in adrenal PNMT (Tai et al. Citation2002). The neurochemical trademark of these mice is highly elevated phenylethylamine, a monoamine purportedly affecting behavior (Grimbsy et al. Citation1997) and a PNMT inhibitor. In the Porsolt forced swim test, the mice exhibit greater mobility, indicative of increased vigilance to inescapable stress. The MAOB KO mice may model a hypo-responsive stress axis where stress does not incapacitate the animal, or strength and duration of stress may need to be greater to elicit a response (Wong et al. Citation2002). MAOB preferentially catabolizes dopamine while MAOA preferentially catabolizes epinephrine and norepinephrine. The absence of the former and presence of the latter may effectively lower concentrations of epinephrine and norepinephrine. Inhibition of PNMT by phenylethylamine may further tilt the scales toward epinephrine reduction making it harder for the animal to mount a stress response, particularly when levels of PNMT cannot be elevated either.
Immobilization stimulates PNMT mRNA levels in other tissues as well. Two hours of immobilization elevates heart (atria and ventricles) and spleen (white pulp) PNMT mRNA expression (Krizanova et al. Citation2001; Jelokova et al. Citation2002). Induction depends on the integrity of the pituitary–adrenocortical axis for the cardiac atria and spleen but not for the cardiac ventricles, since adrenalectomy and hypophysectomy markedly blunt atrial and splenic stimulation. As described earlier, PNMT is present in stellate ganglia and in CRF KO mice, ganglionic elevation of PNMT mRNA depends on glucocorticoids too (Kubovcakova et al. Citation2006).
Other stressors known to increase PNMT protein or enzyme activity do so via changes in mRNA expression. Cold stress (4°C for ≤ 3 h) induces a 1.5-fold increase in adrenal polysomal PNMT mRNA (Weisberg et al. Citation1989), mediated via both non-cholinergic neural and non-neural pathways. Oxygen deprivation or hypoxia also elevates PNMT mRNA. Following adrenal innervation in fetal sheep, acute hypoxia increases PNMT mRNA expression in inverse proportion to oxygen reduction (Adams and McMillen Citation2000). In contrast to cold stress, hypoxic stress depends on nicotinic innervation.
Since stressors may selectively activate either neural or hormonal components of the sympathoadrenal axis rather than both, effects of long-term homotypic stress combined with novel heterotypic stress have been examined (Kvetnansky Citation2004). Rats exposed to long-term cold stress show an exacerbated increase in adrenal medullary PNMT mRNA in response to a heterotypic stressor, e.g. immobilization, insulin or 2-deoxyglucose. In contrast, long-term immobilization stress does not alter PNMT mRNA induction by a heterotypic stressor. Thus, selective molecular mechanisms associated with long-term stress and novel heterotypic stress may provide important catecholaminergic adaptive coping responses.
Co-localization of stress-induced increases in PNMT mRNA and enzyme certainly supports the hypothesis that stress activates PNMT gene transcription, particularly when bolstered by studies with the RNA synthesis inhibitor actinomycin D. However, mRNA levels reflect steady-state levels of message derived from the net of mRNA synthesis and degradation.
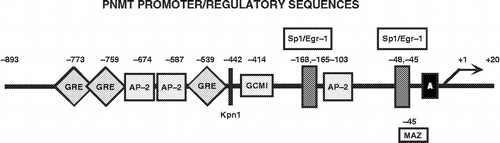
Cloning of the PNMT gene has also provided sequence information by which to dissect the PNMT promoter to identify candidate transcription factor binding sites that can be tested for functionality. Known consensus sites for transcription factors regulating the PNMT gene include Egr-1 (Ebert et al. Citation1994), Sp1 (Ebert and Wong Citation1995), the glucocorticoid receptor (GR) (Ross et al. Citation1990; Tai et al. Citation2002), AP-2 (Ebert et al. Citation1998), MAZ (Her et al. Citation1999) and GCM (Tai and Wong Citation2003) (). In the adrenal gland of rats, at least two of these factors, Egr-1 and Sp1, are activated by immobilization stress (Tai et al. Citation2007), with rapid induction of their proteins preceded by an increase in expression of their mRNAs. Furthermore, the stress-elicited release of corticosteroids would provide abundant amounts of glucocorticoid for GR activation. While these changes are consistent with PNMT transcriptional induction, siRNA strategies blocking transcription factor expression should provide definitive evidence.
Figure 3 Phenylethanolamine N-methyltransferase (PNMT) Promoter. Schematic of the rat PNMT promoter depicting known transcription factor regulatory elements identified to date. Transcriptional activators include Egr-1 (Ebert et al. Citation1994; Ebert et al. Citation1998) and Sp1 (Ebert and Wong Citation1995) with two overlapping consensus sites at − 165/ − 168 and − 45/ − 48 bp upstream of the site of transcription initiation (+1), the GR (Ross et al. Citation1990; Tai et al. Citation2002) with consensus sites at − 539 and two overlapping sites at − 759/ − 773 bp, AP-2 (Ebert et al. Citation1998) with three consensus sites at − 103, − 587 and − 674 bp, MAZ (Her et al. Citation1999; Her et al. Citation2003) with consensus site at − 45 bp and GCM (Tai and Wong Citation2003) with consensus site at − 414 bp.
Stress and post-transcriptional regulation of TH and PNMT
While molecular biological research has placed much emphasis on gene transcription, investigators have recognized the importance of completing the circle and linking mRNA back to protein. Measurements of stress-induced changes in transcription rates, mRNA and protein levels have made it clear that discrepancies in these indices may be the norm rather than the exception. mRNA and protein levels are finely regulated through the balance of synthesis and degradation. RNA binding proteins may control mRNA degradation and translation, adding other dimensions for “regulating genes”. Evidence exists for post-transcriptional regulation of both TH and PNMT expression.
TH. The first defining study showing discrepancies between these parameters in response to stress was one utilizing immobilization stress (Nankova et al. Citation1994). Whereas, repeated daily immobilizations induce both TH mRNA and TH protein in rat adrenal medulla, a single immobilization markedly increases TH mRNA levels (2–8-fold) for up to 24 h, but TH protein levels only increase ∼1.5-fold and TH activity not at all. Short-term exposure to other stressors (2-deoxyglucose-induced hypoglycemia and cold exposure) produces a similar response; 2–3-fold increases in adrenal TH mRNA levels that persist for 12–24 h without concomitant increases in TH protein (unpublished observations). These discrepancies may be explained by kinetic arguments, due to the relatively long half-life of TH protein. However, it is also feasible that short-term stress induces TH mRNA, but other mechanisms must regulate translation of induced mRNA into protein, or TH mRNA may be translated into protein that rapidly degrades when stress is limited. In contrast, long-term, repeated stress, which induces both TH mRNA and TH protein, may activate TH mRNA translation and/or stabilize TH protein. Mechanisms mediating these distinct post-transcriptional responses remain obscure. However, in support of this hypothesis, treatment of rats with the muscarinic (m) AChR agonist bethanechol leads to sustained induction of TH mRNA, and as with short-term stress, TH protein is not induced (Piech-Dumas et al. Citation1999; Yoshimura et al. Citation2004). It is tempting to speculate that short-term stress may act via mAChRs in the adrenal medulla but signals activated by mAChR agonists are not sufficient to induce TH protein. However, more work is required to test this hypothesis and to elucidate these mechanisms.
Mechanisms that control TH mRNA stability have also been described. The best-studied example is hypoxia. Hypoxia stabilizes TH mRNA in PC12 cells by increasing the binding of trans-factors to a pyrimidine-rich tract in the 3′UTR of TH mRNA, with candidate trans-factors including poly(C)-binding proteins (PCBP) (Czyzyk-Krzeska et al. Citation1994; Paulding and Czyzyk-Krzeska Citation1999). Reserpine treatment also leads to a sustained induction of TH mRNA in rat adrenal medulla, persisting at least 24 h; however, transcriptional activation of the TH gene lasts for only a few hr after drug injection (Alterio et al. Citation2001). Similar kinetics have been reported in the LC after foot shock (Chang et al. Citation2000). Morphine treatment up-regulates TH mRNA levels in both the LC and ventral tegmental area (VTA), but increases are associated with transcriptional activation only in the LC and not in the VTA (Boundy et al. Citation1998). In rat LC repeated nicotine treatment for 14 days causes long-term induction of TH mRNA (persisting 3 days after cessation of drug treatment); however, this induction is not due to long-term stimulation of TH gene transcription rate (Sun et al. Citation2004). Most recently, it has been shown that TH promoter activity is not stimulated in the LC of transgenic mice but TH mRNA is induced in response to a number of different stressors (Osterhout et al. Citation2005). The simplest explanation for these apparent dissociations between TH gene activation and TH mRNA induction is that all of these stimuli stabilize TH mRNA. However, molecular mechanisms mediating this stabilization remain obscure. In PC12 cells, there is evidence that TH mRNA stability increases with PKC activation or PACAP treatment (Vyas et al. Citation1990; Corbitt et al. Citation2002). Based on the hypoxia model, these effects may involve PCBP or other binding proteins identified in rat adrenal medulla and bovine adrenal chromaffin cells that can bind to sequences in the TH mRNA 3′UTR and/or possibly coding sequences (Alterio et al. Citation2001; Roe et al. Citation2004). However, the identities of these factors, the RNA sequences to which they bind and the signals that regulate them remain to be determined.
Finally, an intriguing study suggests that TH protein stability may be regulated by nicotine in bovine adrenal chromaffin cells (Fernandez and Craviso Citation1999). Pulse-chase experiments show that constitutively-expressed TH protein is degraded by mechanisms dependent on short-lived factors that destabilize the protein, and that these mechanisms are modulated by agonist occupation of nicotinic AChRs. More work is needed to clarify this observation and to determine whether TH protein stability is regulated in response to stress.
PNMT. Early studies examining hormonal and neural effects on PNMT provided the first evidence of its post-transcriptional control. As expected, type II glucocorticoid agonists (RU28362 and dexamethasone) markedly stimulate adrenal PNMT mRNA but a decline in enzymatic activity changes ensues (Wong et al. Citation1992).
Similar discontinuity in adrenal PNMT mRNA and PNMT activity/protein occurs with stress. Contrary to the robust mRNA induction by single or repeated immobilization, PNMT activity only shows a trend towards an increase (Wong et al. Citation2002). Immobilization does increase PNMT protein (western blotting) in a pattern corresponding to that of mRNA (Tai et al. Citation2007). However, the increase in protein is far less than that of the mRNA, and with prolonged, repeated stress, protein level reverts to basal while mRNA reaches an acme. Similarly, in the heart, where single and repeated immobilization increase PNMT mRNA expression in the atria and ventricles, PNMT activity changes insignificantly except in the left ventricle. In rat spleen, a single immobilization does not stimulate PNMT activity. Multiple immobilizations show a trend towards increased protein, reaching significance after seven immobilizations but the striking increase in mRNA is again highly discordant with PNMT protein/activity.
Discrepant increases of these indices do not appear to be stressor-specific. While cold stress elicits a rapid and prolonged increase in adrenal PNMT mRNA with concomitant elevation of immunoreactive PNMT protein, these matches are qualitative, rather than quantitative in nature (Weisberg et al. Citation1989). The same is the case for PNMT mRNA and protein changes associated with treatment with insulin and 2-deoxyglucose.
An important caveat for many studies, however, is the mismatch between sampling time and the lag time for stimulus-induced changes in PNMT protein. Generally, 24 h from the initiation of the stimulus is required for protein changes. However, recent studies assessing immobilization stress effects on two PNMT transcriptional activators, Egr-1 and Sp1 in the rat adrenal medulla, indicate that sampling time does not solely account for the discrepancies and that important post-transcriptional regulatory mechanisms may underlie the delay. Egr-1 and Sp1 are both modified by phosphorylation. For Egr-1, modification by phosphorylation enhances its binding to consensus sequences and thereby increases its ability to stimulate gene transcription. For Sp1, unphosphorylated protein does not bind to its DNA recognition site and therefore will not activate transcription. The pattern of change for Egr-1 and Sp1 mRNA, protein and protein-DNA binding complex formation are, for the most part, coincident with two important Sp1 exceptions. Six daily 120 min immobilizations markedly increase levels of Sp1 mRNA and protein-DNA complex, while protein is unchanged from controls. In addition, seven daily immobilizations, irrespective of duration or time to sacrifice, do not alter Sp1 mRNA, protein or protein-DNA complex. Thus, for this transcription factor, post-translational control of its expression may contribute to stress-induced changes in PNMT.
Finally, post-translational control of PNMT itself was one of the first regulatory modes described for hormone action. Glucocorticoids sustain levels of S-adenosylmethionine (AdoMet), the methyl donor permitting conversion of norepinephrine to epinephrine. To effect the methyl transfer, AdoMet must bind to PNMT, and in so doing proteolytically vulnerable sites are protected so that PNMT enzyme degradation does not occur.
Conclusions
Through the years of stress research, understanding of mechanisms controlling stress-induced changes in catecholaminergic function and the catecholamine enzymes, TH and PNMT, has expanded exponentially. Yet, there are “miles to go before we sleep”. Present knowledge shows differences in short- and long-term mechanisms that involve transcriptional as well as post-transcriptional regulatory controls and suggest studies that will keep us all busy for decades.
Acknowledgements
This work was supported by NIH Grant DK51025, The Spunk Fund, Inc., the Sobel and Keller Research Support Fund and McLean Hospital (dlw) and NIH grants DA05014 and NS22675 (awt).
Notes
†This review article celebrates the lifetime scientific contributions to our understanding of stress by Dr Richard Kvetnansky, from Slovakia. Over several decades, Dr Kvetnansky has helped define catecholaminergic responses to a variety of stressors and contributed to our understanding of underlying molecular mechanisms. As importantly, he has encouraged and mentored subsequent generations of stress researchers through his animated and provoking questions and discussions, his broad web of collaborative stress research and for those of us so fortunate, the gathering of stress researchers to engage in “eating, living and sleeping” a wide array of stress-related topics every four years in the exquisite and intimate environment of Smolenice Castle, the meeting place designee for events under the auspices of the Slovak Academy of Sciences.Richard, we thank you for your contributions to the field of catecholamines and stress, for your encouragement, support and enthusiasm for our own research efforts in the same and for providing us with a recurring and challenging forum to further test the waters. May your 70th birthday be a hallmark of many more years of both personal and professional “joie de vivre”!
References
- Adams MB, McMillen IC. Actions of hypoxia on catecholamine synthetic enzyme mRNA expression before and after development of adrenal innervation in the sheep fetus. J Physiol (Lond) 2000; 529: 519–531
- Alterio J, Mallet J, Biguet NF. Multiple complexes involved in tyrosine hydroxylase mRNA stability in rat adrenal medulla, after reserpine stimulation. Mol Cell Neurosci 2001; 17: 179–189
- Angulo JA, Printz D, Ledoux M, McEwen BS. Isolation stress increases tyrosine hydroxylase mRNA in the locus coeruleus and midbrain and decreases proenkephalin mRNA in the striatum and nucleus accumbens. Brain Res Mol Brain Res 1991; 11: 301–308
- Baetge EE, Suh YH, Joh TH. Complete nucleotide and deduced amino acid sequence of bovine phenylethanolamine N-methyltransferase: Partial amino acid homology with rat tyrosine hydroxylase. Proc Natl Acad Sci USA 1986; 83: 5454–5458
- Baetge EE, Behringer RR, Messing A, Brinster RL, Palmiter RD. Transgenic mice express the human phenylethanolamine N-methyltransferase gene in adrenal medulla and retina. Proc Natl Acad Sci USA 1988; 85: 3648–3652
- Banerjee SA, Hoppe P, Brilliant M, Chikaraishi DM. 5′ flanking sequences of the rat tyrosine hydroxylase gene target accurate tissue-specific, developmental, and transsynaptic expression in transgenic mice. J Neurosci 1992; 12: 4460–4467
- Baruchin A, Weisberg EP, Miner LL, Ennis D, Nisenbaum LK, Naylor E, Stricker EM, Zigmond MJ, Kaplan BB. Effects of cold exposure on rat adrenal tyrosine hydroxylase: An analysis of RNA, protein, enzyme activity, and cofactor levels. J Neurochem 1990; 54: 1769–1775
- Boundy VA, Gold SJ, Messer CJ, Chen J, Son JH, Joh TH, Nestler EJ. Regulation of tyrosine hydroxylase promoter activity by chronic morphine in TH9.0-LacZ transgenic mice. J Neurosci 1998; 18: 9989–9995
- Cannon WG, De La Paz D. Emotional stimulation of adrenal secretion. Am J Physiol 1911; 28: 64–70
- Cazorla P, Smidt MP, O'Malley KL, Burbach JP. A response element for the homeodomain transcription factor Ptx3 in the tyrosine hydroxylase gene promoter. J Neurochem 2000; 74: 1829–1837
- Chang MS, Sved AF, Zigmond MJ, Austin MC. Increased transcription of the tyrosine hydroxylase gene in individual locus coeruleus neurons following footshock stress. Neuroscience 2000; 101: 131–139
- Ciaranello RD, Dornbusch JN, Barchas JD. Rapid increase of phenylethanolamine N-methyltransferase by environmental stress in an inbred mouse strain. Science 1972; 175: 789–790
- Cohen J, Hadjiconstantinou M. Identification of epinephrine and phenylethanolamine N-methyltransferase activity in rat retina. Fed Proc 1984; 43: 2725–2728
- Cooper DO, Stolk JM. Differences between inbred rat strains in the alteration of adrenal catecholamine synthesizing enzyme activities after immobilization stress. Neuroscience 1979; 4: 1163–1172
- Corbitt J, Hagerty T, Fernandez E, Morgan WW, Strong R. Transcriptional and post-transcriptional regulation of tyrosine hydroxylase messenger RNA in PC12 cells during persistent stimulation by VIP and PACAP38: Differential regulation by protein kinase A and protein kinase C-dependent pathways. Neuropeptides 2002; 36: 34–45
- Czyzyk-Krzeska MF, Dominski Z, Kole R, Millhorn DE. Hypoxia stimulates binding of a cytoplasmic protein to a pyrimidine-rich sequence in the 3′-untranslated region of rat tyrosine hydroxylase mRNA. J Biol Chem 1994; 269: 9940–9945
- Dronjak S, Jezova DA, Kvetnansky R. Different effects of novel stressors on sympathoadrenal system activation in rats exposed to long-term immobilization. Ann N Y Acad Sci 2004; 1018: 113–123
- Ebert SN, Wong DL. Differential activation of the rat phenylethanolamine N-methyltransferase gene by Sp1 and Egr-1. J Biol Chem 1995; 270: 17299–17305
- Ebert SN, Balt SL, Hunter JPB, Gashler A, Sukhatme V, Wong DL. Egr-1 activation of rat adrenal phenylethanolamine N-methyltransferase gene. J Biol Chem 1994; 269: 20885–20898
- Ebert SN, Baden J, Mathers LH, Siddall BJ, Wong DL. Expression of phenylethanolamine N-methyltransferase in the embryonic rat heart. J Mol Cell Cardiol 1996; 28: 1653–1658
- Ebert SN, Ficklin MB, Her S, Siddall BJ, Bell RA, Morita K, Ganguly K, Wong DL. Glucocorticoid-dependent action of neural crest factor AP-2: Stimulation of phenylethanolamine N-methyltransferase gene expression. J Neurochem 1998; 70: 2286–2295
- Fernandez E, Craviso GL. Protein synthesis blockade differentially affects the degradation of constitutive and nicotinic receptor-induced tyrosine hydroxylase protein level in isolated bovine chromaffin cells. J Neurochem 1999; 73: 169–178
- Fluharty SJ, Snyder GL, Stricker EM, Zigmond MJ. Short-and long-term changes in adrenal tyrosine hydroxylase activity during insulin-induced hypoglycemia and cold stress. Brain Res 1983; 267: 384–387
- Gagner JP, Gauthier S, Sourkes TL. Differential effects of transection of the spinal cord and splanchnic nerve on adrenal tyrosine hydroxylase and catecholamines. Neuroscience 1985; 14: 907–920
- Grimbsy J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, Karoum F, Gal J, Shih JC. Increased stress response and b-phenylethylamine in MAOB-deficient mice. Nat Genet 1997; 17: 206–210
- Hebert MA, Serova LI, Sabban EL. Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem 2005; 95: 484–498
- Her S, Bell RA, Bloom AK, Siddall BJ, Wong DL. Phenylethanolamine N-methyltransferase gene expression: Sp1 and MAZ potential for tissue specific expression. J Biol Chem 1999; 274: 8698–8707
- Her S, Claycomb R, Tai TC, Wong DL. Regulation of the rat phenylethanolamine N-methyltransferase gene by transcription factors Sp1 and MAZ. Mol Pharmacol 2003; 64: 1180–1188
- Hokfelt T, Johansson O, Goldstein M. Central catecholamine neurons as revealed by immunochemistry with special reference to the adrenaline neurons. Handbook of neuroanatomy, A Bjorklund, T Hokfelt. Elsevier, Amsterdam 1984; Vol. 2: 157–276
- Hong SJ, Huh Y, Chae H, Hong S, Lardaro T, Kim KS. GATA-3 regulates the transcriptional activity of tyrosine hydroxylase by interacting with CREB. J Neurochem 2006; 98: 773–781
- Jelokova J, Rusnak M, Kubovcakova L, Buckendahl P, Krizanova O, Sabban EL, Kvetnansky R. Stress increases gene expression of phenylethanolamine N-methyltransferase in spleen of rats via pituitary–adrenocortical mechanism. Psychoneuroendocrinology 2002; 27: 619–633
- Kaneda N, Ichinose H, Kobayashi K, Oka K, Kishi F, Nakazawa A, Kurosawa Y, Fujita K, Nagatsu T. Molecular cloning of cDNA and chromosomal assignment of the gene for human phenylethanolamine N-methyltransferase, the enzyme for epinephrine biosynthesis. J Biol Chem 1988; 263: 7672–7677
- Kennedy B, Elayan H, Ziegler M. Lung epinephrine synthesis. Am J Physiol 1990; 258: L227–L231
- Kim KS, Lee MK, Carroll J, Joh TH. Both the basal and inducible transcription of the tyrosine hydroxylase gene are dependent upon a cAMP response element. J Biol Chem 1993; 268: 15689–15695
- Kim HS, Hong SJ, LeDoux MS, Kim KS. Regulation of the tyrosine hydroxylase and dopamine beta-hydroxylase genes by the transcription factor AP-2. J Neurochem 2001; 76: 280–294
- Kim KS, Kim CH, Hwang DY, Seo H, Chung S, Hong SJ, Lim JK, Anderson T, Isacson O. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem 2003; 85: 622–634
- Krizanova O, Micutkova L, Jelokova J, Filipenko M, Sabban E, Kvetnansky R. Existence of cardiac PNMT mRNA in adult rats: Elevation by stress in a glucocorticoid-dependent manner. Am J Physiol Heart Circ Physiol 2001; 281: H1372–H1379
- Kubovcakova L, Micutkova L, Barttosova Z, Sabban EL, Krizanova O, Kvetnansky R. Identification of phenylethanolamine N-methyltransferase gene expression in stellate ganglia and its modulation by stress. J Neurochem 2006; 97: 1419–1430
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem 1996; 67: 443–462
- Kvetnansky R. Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann N Y Acad Sci 2004; 1032: 117–129
- Kvetnansky R, Weise VK, Kopin IJ. Elevation of adrenal tyrosine hydroxylase and phenylethanolamine-N-methyltransferase by repeated immobilization of rats. Endocrinology 1970; 87: 744–749
- Kvetnansky R, Nemeths S, Vigas M, Oprsalova Z, Jur-.Covicova J. Plasma catecholamines in rats during adaptation to intermittent exposure to different stressors. Gordon and Breach Sci., New York, NY 1984; 1: 537–562
- Kvetnansky R, Pacak K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ. Sympathoadrenal system in stress. Interaction with the hypothalamic–pituitary–adrenocortical system. Ann N Y Acad Sci 1995; 771: 131–158
- Kvetnansky R, Nankova B, Hiremagalur B, Viskupic E, Vietor I, Rusnak M, McMahon A, Kopin IJ, Sabban EL. Induction of adrenal tyrosine hydroxylase mRNA by single immobilization stress occurs even after splanchnic transection and in the presence of cholinergic antagonists. J Neurochem 1996; 66: 138–146
- Lazaroff M, Patankar S, Yoon SO, Chikaraishi DM. The cyclic AMP response element directs tyrosine hydroxylase expression in catecholaminergic central and peripheral nervous system cell lines from transgenic mice. J Biol Chem 1995; 270: 21579–22159
- Lewis-Tuffin LJ, Quinn PG, Chikaraishi DM. Tyrosine hydroxylase transcription depends primarily on cAMP response element activity, regardless of the type of inducing stimulus. Mol Cell Neurosci 2004; 25: 536–547
- Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci USA 1991; 88: 8382–8386
- Micutkova L, Rychkova N, Sabban EL, Krizanova O, Kvetnansky R. Quantitation of changes in gene expression of norepinephrine biosynthetic enzymes in rat stellate ganglia induced by stress. Neurochem Int 2003; 43: 235–242
- Min N, Joh TH, Kim KS, Peng C, Son JH. 5′ upstream DNA sequence of the rat tyrosine hydroxylase gene directs high-level and tissue-specific expression to catecholaminergic neurons in the central nervous system of transgenic mice. Brain Res Mol Brain Res 1994; 27: 281–289
- Morita S, Kobayashi K, Hidaka H, Nagatsu T. Organization and complete nucleotide sequence of the gene encoding mouse phenylethanolamine N-methyltransferase. Mol Brain Res 1992; 13: 313–319
- Nankova B, Kvetnansky R, McMahon A, Viskupic E, Hiremagalur B, Frankle G, Fukuhara K, Kopin IJ, Sabban EL. Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc Natl Acad Sci USA 1994; 91: 5937–5941
- Nankova B, Kvetnansky R, Hiremagalur B, Sabban B, Rusnak M, Sabban EL. Immobilization stress elevates gene expression for catecholamine biosynthetic enzymes and some neuropeptides in rat sympathetic ganglia: Effects of adrenocorticotropin and glucocorticoids. Endocrinology 1996; 137: 5597–5604
- Nankova BB, Tank AW, Sabban EL. Transient or sustained transcriptional activation of the genes encoding rat adrenomedullary catecholamine biosynthetic enzymes by different durations of immobilization stress. Neuroscience 1999; 94: 803–808
- Nankova BB, Rivkin M, Kelz M, Nestler EJ, Sabban EL. Fos-related antigen 2: Potential mediator of the transcriptional activation in rat adrenal medulla evoked by repeated immobilization stress. J Neurosci 2000; 20: 5647–5653
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology 1996; 14: 443–452
- Osterhout CA, Chikaraishi DM, Tank AW. Induction of tyrosine hydroxylase protein and a transgene containing tyrosine hydroxylase 5′ flanking sequences by stress in mouse adrenal gland. J Neurochem 1997; 68: 1071–1077
- Osterhout C, Sterling CR, Chikaraishi DM, Tank AW. Induction of tyrosine hydroxylase in the locus coeruleus of transgenic mice in response to stress or nicotine treatment: Lack of activation of tyrosine hydroxylase promoter activity. J Neurochem 2005; 94: 731–741
- Papanikolaou NA, Sabban EL. Sp1/Egr1 motif: A new candidate in the regulation of rat tyrosine hydroxylase gene transcription by immobilization stress. J Neurochem 1999; 73: 433–436
- Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase mRNA stability by protein-binding, pyrimidine-rich sequence in the 3′-untranslated region. J Biol Chem 1999; 274: 2532–2538
- Piech-Dumas KM, Sterling CR, Tank AW. Regulation of tyrosine hydroxylase gene expression by muscarinic agonists in rat adrenal medulla. J Neurochem 1999; 73: 153–161
- Roe DF, Craviso GL, Waymire JC. Nicotinic stimulation modulates tyrosine hydroxylase mRNA half-life and protein binding to the 3′UTR in a manner that requires transcription. Brain Res Mol Brain Res 2004; 120: 91–102
- Ross ME, Evinger MJ, Hyman SE, Carroll JM, Mucke L, Comb M, Reis DJ, Joh TH, Goodman HM. Identification of a functional glucocorticoid response element in the phenylethanolamine N-methyltransferase promoter using fusion genes introduced into chromaffin cells in primary culture. J Neurosci 1990; 10: 520–530
- Rusnak M, Zorad S, Smith MA, Hiremagalur B, Nankova B, Sabban EL, Kvetnansky R. Stress: Molecular genetic and neurobiological advances, R McCarty, G Aguilera, E Sabban, R Kvetnansky. Gordon and Breach Science Publishers, New York, NY 1996; Vol. 2: 711–722
- Rusnak M, Kvetnansky R, Jelokova J, Palkovits M. Effect of novel stressors on gene expression of tyrosine hydroxylase and monoamine transporters in brainstem noradrenergic neurons of long-term repeatedly immobilized rats. Brain Res 2001; 899: 20–35
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: Dynamics of transcriptional events. Trends Neurosci 2001; 24: 91–98
- Sabban EL, Hiremagalur B, Nankova B, Kvetnansky R. Molecular biology of stress-elicited induction of catecholamine biosynthetic enzymes. Ann N Y Acad Sci 1995; 771: 327–338
- Sabban EL, Nankova BB, Serova LI, Hiremagalur B, Rusnak M, Saez E, Spiegelman B, Kvetnansky R. Regulation of gene expression of catecholamine biosynthetic enzymes by stress. Adv Pharmacol 1998; 42: 564–567
- Serova L, Sabban EL, Zangen A, Overstreet DH, Yadid G. Altered gene expression for catecholamine biosynthetic enzymes and stress response in rat genetic model of depression. Brain Res Mol Brain Res 1998a; 63: 133–138
- Serova LI, Saez E, Spiegelman BM, Sabban EL. c-Fos deficiency inhibits induction of mRNA for some, but not all, neurotransmitter biosynthetic enzymes by immobilization stress. J Neurochem 1998b; 70: 1935–1940
- Serova LI, Nankova BB, Feng Z, Hong JS, Hutt M, Sabban EL. Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry 1999; 45: 853–862
- Seyle H. The stress of life. McGraw Hill, New York, NY 1975
- Sun B, Sterling CR, Tank AW. Chronic nicotine treatment leads to sustained stimulation of tyrosine hydroxylase gene transcription rate in rat adrenal medulla. J Pharmacol Exp Ther 2003; 304: 575–588
- Sun B, Chen X, Xu L, Sterling CR, Tank AW. Chronic nicotine treatment leads to induction of tyrosine hydroxylase in locus coeruleus neurons: The role of transcriptional activation. Mol Pharmacol 2004; 66: 1011–1021
- Tai TC, Wong DL. Protein kinase A and protein kinase C signaling pathway interaction in phenylethanolamine N-methytransferase gene regulation. J Neurochem 2003; 85: 816–829
- Tai TC, Claycomb R, Her S, Bloom AK, Wong DL. Glucocorticoid responsiveness of the rat phenylethanolamine N-methyltransferase gene. Mol Pharmacol 2002; 61: 1385–1392
- Tai TC, Claycomb R, Siddall BJ, Bell RA, Kvetnansky R, Wong DL. Stress-induced changes in epinephrine expression in the adrenal medulla in vivo. J Neurochem 2007; 101: 1108–1118
- Tank AW, Lewis EJ, Chikaraishi DM, Weiner N. Elevation of RNA coding for tyrosine hydroxylase in rat adrenal gland by reserpine treatment and exposure to cold. J Neurochem 1985; 45: 1030–1033
- Thoa NB, Tizabi Y, Kopin IJ, Maengwyn-Davies GD. Alterations of mouse adrenal medullary catecholamines and enzymes in response to attack: Effect of pre- and post-treatment with phenobarbital. Pscyhopharmacology 1976; 51: 53–57
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurons by cold exposure. Nature 1970; 228: 861–862
- Toru M. Increased tyrosine hydroxylase activity in frontal cortex of rats after long-term isolation stress. Encephale 1982; 8: 315–317
- Trocme C, Sarkis C, Hermel JM, Duchateau R, Harrison S, Simonneau M, Alshawi R, Mallet J. CRE and TRE sequences of the rat tyrosine hydroxylase promoter are required for TH basal expression in adult mice but not in the embryo. Eur J Neurosci 1998; 10: 508–521
- Tumer N, Hale C, Lawler J, Strong R. Modulation of tyrosine hydroxylase gene expression in the rat adrenal gland by exercise: Effects of age. Brain Res Mol Brain Res 1992; 14: 51–56
- Tumer N, Demirel HA, Serova L, Sabban EL, Broxson CS, Powers SK. Gene expression of catecholamine biosynthetic enzymes following exercise: Modulation by age. Neuroscience 2001; 103: 703–711
- Turner BB, Katz RJ, Roth KA, Carroll BJ. Central elevation of phenylethanolamine N-methyltransferase activity following stress. Brain Res 1978; 153: 419–422
- Vietor I, Rusnak M, Viskupic E, Blazicek P, Sabban EL, Kvetnansky R. Glucoprivation by insulin leads to trans-synaptic increase in rat adrenal tyrosine hydroxylase mRNA levels. Eur J Pharmacol 1996; 313: 119–127
- Viskupic E, Kvetnansky R, Sabban EL, Fukuhara K, Weise VK, Kopin IJ, Schwartz JP. Increase in rat adrenal phenylethanolamine N-methyltransferase mRNA level caused by immobilization stress depends on intact pituitary–adrenocortical axis. J Neurochem 1994; 63: 808–814
- Vyas S, Faucon Biguet N, Mallet J. Transcriptional and post-transcriptional regulation of tyrosine hydroxylase gene by protein kinase C. EMBO J 1990; 9: 3707–3712
- Warthan MD, Freeman JG, Loesser KE, Lewis CW, Min H, Conway CM, Stewart JK. Phenylethanolamine N-methyltransferase expression in mouse thymus and spleen. Brain Behav and Immun 2002; 16: 493–499
- Weisberg EP, Baruchin A, Stachowiak MK, Stricker EM, Zigmond MJ, Kaplan BB. Isolation of rat adrenal cDNA clone encoding phenylethanolamine N-methyltransferase and cold-induced alterations in adrenal PNMT mRNA and protein. Mol Brain Res 1989; 6: 159–166
- Wong DL, Lesage A, Siddall B, Funder JW. Glucocorticoid regulation of phenylethanolamine N-methyltransferase in vivo. FASEB J 1992; 6: 3310–3315
- Wong DL, Ebert SN, Morita K. Glucocorticoid control of phenylethanolamine N-methyltransferase gene expression: Implications for stress and disorders of the stress axis. Stress: Molecular genetic and neurobiological advances, R McCarty, G Aguilera, E Sabban, R Kvetnansky. Gordon and Breach Science Publishers, New York, NY 1996; Vol. 2: 677–693
- Wong DL, Her S, Tai TC, Bell RA, Rusnak M, Farkas R, Kvetnansky R, Shih J. Stress: Neural, endocrine and molecular studies, R McCarty, G Aguilera, EL Sabban, R Kvetnansky. Taylor and Francis, London 2002; 129–135
- Yoshimura R, Xu L, Sun B, Tank AW. Nicotinic and muscarinic acetylcholine receptors are essential for the long-term response of tyrosine hydroxylase gene expression to chronic nicotine treatment in rat adrenal medulla. Mol Brain Res 2004; 126: 188–197
- Ziegler MG, Bao X, Kennedy BP, Joyner A, Enns R. Location, development, control and function of extraadrenal phenylethanolamine N-methyltransferase expression. The chromaffin cell: Transmitter biosynthesis, storage, release, actions, and informatics, DT O'Connor, LE Eiden. Ann. NY Acad. Sci, New York, NY 2002; Vol. 971: 76–82