Abstract
Brief neonatal handling permanently alters hypothalamic-pituitary-adrenal axis function resulting in increased ability to cope with stress. Since stress is known to affect cognitive abilities, in the present study we investigated the effect of brief (15 min) handling on learning and memory in the Morris water maze, following exposure to an acute restraint stress either before training or recall. Exposure of non-handled rats to the acute stress prior to training resulted in quicker learning of the task, than in the absence of the stressor. When acute stress preceded acquisition, male handled rats showed an overall better learning performance, and both sexes of handled animals were less impaired in the subsequent memory trial, compared to the respective non-handled. In addition, the number of neurons immunoreactive for GR was higher in all areas of Ammon's horn of the handled rats during the recall. In contrast, the number of neurons immunoreactive for MR was higher in the CA1 and CA2 areas of the non-handled males. When the acute restraint stress was applied prior to the memory test, neonatal handling was not effective in preventing mnemonic impairment, as all animal groups showed a similar deficit in recall. In this case, no difference between handled and non-handled rats was observed in the number of GR positive neurons in the CA2 and CA3 hippocampal areas during the memory test. These results indicate that early experience interacts with sex and acute stress exposure in adulthood to affect performance in the water maze. Hippocampal corticosterone receptors may play a role in determining the final outcome.
| Abbreviations | ||
| ANOVA | = | analysis of variance |
| CRH | = | corticotropin releasing hormone |
| CRH-R | = | corticotropin releasing hormone receptor |
| DAB | = | 3,3′-diaminobenzidine |
| GR | = | glucocorticoid receptor type II |
| HPA | = | hypothalamic-pituitary-adrenal |
| MR | = | mineralocorticoid receptor/ glucocorticoid receptor type I |
| MWM | = | Morris water maze |
| NRS | = | normal rabbit serum |
| PBS | = | phosphate buffered saline |
| PND | = | postnatal day |
| RIA | = | radioimmunoassay |
| RT | = | room temperature |
| SEM | = | standard error of the mean |
Introduction
In rats, brief neonatal handling (15 min daily) of pups during the first 3 weeks of life permanently alters hypothalamic–pituitary–adrenal (HPA) axis function and the ability to cope with stress. More specifically, this treatment increases the mRNA and protein levels of type II glucocorticoid receptors (GRs) in the hippocampus of adult animals (Meaney et al. Citation1985a,Citationb, Citation1989, Citation1992, Citation1996; O'Donnell et al. Citation1994). Consequently, these rats exhibit increased negative feedback sensitivity to glucocorticoids (Meaney et al. Citation1989) and as a result, handled rats show a dampened neuroendocrine response to stress, in terms of corticosterone, CRH and ACTH secretion (Plotsky and Meaney Citation1993; Bhatnagar and Meaney Citation1995; Vallee et al. Citation1996, Citation1997; Liu et al. Citation1997). Furthermore, handled animals display reduced emotional reactivity in anxiety- and fear-related behaviours (Ferre et al. Citation1995; Fernandez-Teruel et al. Citation1997).
In adulthood, handled animals show improved performance in several types of hippocampal-dependent learning paradigms, as in the two-way active avoidance (Escorihuela et al. Citation1994; Pryce et al. Citation2003), in the contextual fear conditioning (Beane et al. Citation2002) and in spatial tasks, including the water maze (Wong and Jamieson Citation1968; Huot et al. Citation2002; Fenoglio et al. Citation2005). During aging the beneficial effects of neonatal handling are even more evident, as handled rats lose fewer hippocampal neurons (Meaney et al. Citation1991a), have an attenuated age-related increase in basal corticosterone levels, and perform better in the spatial task of the Morris water maze (MWM), compared to the non-handled rats (Meaney et al. Citation1988).
Corticosterone and its receptors in the brain are critical components of hippocampal-dependent learning and memory (de Kloet et al. Citation1999). Pharmacological (Oitzl and de Kloet Citation1992) and genetic (Oitzl et al. Citation2001) approaches have confirmed the indispensable role of GRs in the consolidation and retrieval of spatial information. On the other hand, corticosterone affects cognitive function in a U-shaped manner (de Kloet et al. Citation1999), as both elimination and hypersecretion of this stress hormone impair learning and memory (Oitzl and de Kloet Citation1992; Conrad et al. Citation1997; Pugh et al. Citation1997).
Stress can have differential effects on hippocampal-dependent learning and memory depending on its nature and duration and on the phase of the process that stress is applied (Joels et al. Citation2006). When an acute stressor is applied around the time of the acquisition of information, is in the context of learning and leads to moderate increases in corticosterone levels, it can facilitate consolidation and retrieval (Sandi et al. Citation1997; de Kloet et al. Citation1999). In contrast, when the stressor is applied shortly prior to retrieval, it often impairs memory recall (de Quervain et al. Citation1998). Notably, stress exposure alters CRH and its receptors (CRH-R1 and CRH-R2) (Bale and Vale Citation2004; Chen et al. Citation2004, Citation2006) as well as corticosteroid receptor mRNA and protein levels in the hippocampus (Sapolsky et al. Citation1984; Herman et al. Citation1995; Tritos et al. Citation1999; Karandrea et al. Citation2000, Citation2002; Kitraki et al. Citation2004). Stress can thus influence the final outcome of both learning under stress and the stress response per se.
The aim of this study was to elucidate whether the improved ability of neonatally handled rats to cope with stress, in conjunction with their increased hippocampal GR content, would enable them to perform better in the spatial task of the MWM under stress. Having in mind that the timing of stress exposure differently affects the cognitive procedure, we have exposed rats to acute stress either prior to learning, or prior to retrieval of the water maze task. We hypothesized that stressed handled rats would perform better than stressed non-handled, based on the previous observations that the former have an earlier reduction of corticosterone levels following stress (Vallee et al. Citation1996, Citation1997) and an overall reduced threshold of HPA axis activation. HPA axis reactivity in our system was assessed through determination of hippocampal GR and plasma corticosterone levels at the end of the memory test.
Materials and methods
Animals
Wistar rats of both sexes reared in our laboratory were kept under standard conditions (24°C; 12:12 h light/dark cycle, lights on at 8:00 am, three animals of the same sex per cage) and received food and water ad libitum. Virgin females were exposed to stud males and pregnancy was determined by the presence of sperm in the vaginal smear (day 0 of pregnancy). Prior to birth litters from each dam were randomly assigned to either the handled or non-handled category (nine litter in each of the two categories). The litter size range (6–12) did not differ between non-handled and handled groups. Litters were not culled, since it has been shown that litter size within this range (5–18) does not affect maternal behaviour (Champagne et al. Citation2003). Neither the litter size nor the sex ratio was different among the litters employed in the different animal groups (average litter size [mean ± SEM]: non-handled litters 9.2 ± 0.7 pups, handled litters 9.1 ± 0.7 pups; average sex ratio [males:females; mean ± SEM]: non-handled litters 1.18 ± 0.16, handled litters 1.11 ± 0.14). The day of birth was defined as postnatal day 0 (PND0).
Two cohorts of adult rats (PND90-PND120) were used in this study: Cohort A was exposed to acute restraint stress 60 min before the learning trials in the MWM–Experiment I (nine male non-handled, nine male handled, nine female non-handled, nine female handled; one animal of each sex from each litter employed); Cohort B was exposed to acute restraint stress 60 min before the memory trial of the MWM–Experiment II (nine male non-handled, nine male handled, nine female non-handled, nine female handled; one animal of each sex from each litter employed). In all rats of both experimental cohorts performance in the learning and memory trials of the MWM was recorded, and levels of circulating corticosterone were measured. Additionally, in both experimental cohorts GR and MR immunohistochemistry was performed on brain sections from six animals per experimental group (in each experimental cohort: six male non-handled, six male handled, six female non-handled, six female handled). All animal experiments were carried out in agreement with ethical recommendation of the European Communities Council Directive of 24 November 1986 (86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
Neonatal handling
The “neonatal handling” protocol employed was as originally described by Levine (Citation1957), which involves removal of the pups from the nest for 15 min daily during the neonatal period and placing them in a separate container, taking care to control for the pups' body temperature. In the present experiments “handling” was performed from the first postnatal day (PND1) until weaning (PND22).
Specifically, every day between 9.00 and 10.00 am mothers of the pups to be subjected to “handling” were removed from their home cages and temporarily placed separately into cages (the same cage for each mother every day for the duration [22 days] of “handling”). Their pups were then removed and placed into plastic containers, lined with paper towels. After 15 min the pups, and then their mothers, were returned to their home cages. “Non-handled” pups were left undisturbed with their mothers in their home cage until weaning.
Acute restraint stress
Experiment I
Rats were placed for 30 min in plastic cylinders 5 cm in diameter and 15 cm in length, laid horizontally. Following the restraint stress, rats were returned to their home cages, where they remained for 60 min before being trained in the MWM. Memory testing was performed 24 h following the last training trial.
Experiment II
Rats were trained in the MWM and memory testing was performed 24 h following the last training trial. Ninety minutes before memory testing rats were exposed to a 30 min acute restraint stress (as described above). Following the restraint stress, rats were returned to their home cages, where they remained for 60 min before being tested in the memory trial in the MWM.
Spatial learning—Morris water maze
A circular pool, 140 cm in diameter, was filled with water (22 ± 1°C), rendered opaque with milk and a transparent movable platform (13 × 13 cm) was submerged 1 cm below the water surface. Rats were trained to find the hidden platform (in a fixed position relative to visible extra-maze cues) according to an intense, one-day long protocol (de Quervain et al. Citation1998), composed of eight consecutive 90 s learning trials, each beginning at a different starting position with the four possible locations alternated in the same sequence. If a rat failed to find the platform, it was gently guided and allowed to climb and stay on it. At the end of each trial the rat was left on the platform for 20 s. Two behavioural parameters were recorded during spatial learning in the MWM: escape latency (time in seconds to reach the platform) and the total distance swum until the platform was reached (in centimetres).
Spatial memory—Morris water maze
After 24 h, following the last training trial in the MWM, rats were tested in a probe trial in the absence of the platform (60 s duration, starting position opposite to the target quadrant, i.e. the quadrant in which the platform was located during training). Two behavioural parameters were recorded during spatial memory testing in the MWM: time spent (in s) and distance swum (in cm) in each pool quadrant.
The behaviour of the rats was analyzed using the EthoVision software (Noldus, The Netherlands). To ensure that behavioural differences in the MWM between experimental groups did not simply reflect differences in activity, swimming speed was also measured both during learning and memory trials.
Circulating corticosterone concentrations
Immediately following the memory trial in the MWM, each rat was deeply anaesthetized with chloral hydrate (injected i.p. at a dose of 500 mg/kg b.w.) and blood was collected by cardiac puncture using heparinized syringes. Blood was centrifuged at 4°C, 12,000 rpm for 15 min. Plasma was collected and stored at − 40°C until further use. Determination of plasma corticosterone levels was performed by radioimmunoassay (RIA) using a commercially available kit (Amersham Int. plc, Little Chalfront Buckingharmshire, England) containing [125I]-labelled corticosterone and a highly specific and sensitive rabbit anti-corticosterone antibody. The sensitivity of the assay was 6 pg corticosterone per sample and the variability within and between assays was 5% and 4.0–5.9%, respectively.
Tissue preparation
In both Experiment I and II, animals were killed immediately after the MWM memory trial. They were deeply anesthetized with chloral hydrate (injected i.p. at a dose of 500 mg/kg b.w.) and perfused transcardially with 400 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were isolated, post-fixed overnight in the perfusion medium at 4°C, further processed and embedded in paraffin. Serial sagittal sections (7 μm) were cut and mounted on silane-coated slides.
Immunohistochemistry
Sections were deparaffinized, rehydrated and subjected to antigen retrieval in a microwave oven for 12 min in 10 mM citrate buffer, pH 6.0. The slides were washed with phosphate-buffered saline (PBS) containing 0.4% Triton X-100 (SERVA, Germany) and then incubated in PBS containing 0.4% Triton X-100 and 10% normal rabbit serum (NRS; Dakocytomation, Denmark) for 1 h at room temperature (RT). Subsequently, slides were incubated overnight at 4°C with either an anti-GR (1:10 dilution) monoclonal antibody (kindly provided by Dr M. N. Alexis, National Research Institute, Athens, Greece) or an anti-MR (1:100 dilution) monoclonal antibody (1D5, kindly provided by Dr CE. Gomez-Sanchez, Division of Endocrinology, University of Mississippi Medical Center, Jackson, MS, USA [Gomez-Sanchez et al. Citation2006]) in PBS containing 0.4% Triton and 4% NRS. Following several rinses in PBS, slides were incubated for 1 h at RT with a biotinylated rabbit anti-mouse antibody (Dakocytomation) diluted 1:200 in PBS containing 2% NRS. Following several rinses in PBS, slides were exposed to the ABC reagent (Dakocytomation) for 60 min at RT. After incubation, slides were washed in PBS and stained with 3,3′-diaminobenzidine (DAB) solution (1.7 mM DAB, Sigma-Aldrich, St Louis, MO, USA) in Tris–HCl buffer 10 mM, pH 7.6, containing 0.03% H2O2 for 3–5 min at RT. Slides were then washed in water for 5 min, dehydrated, coverslipped with DePeX (SERVA, Heidelberg, Germany) and analyzed using a Nikon Eclipse E400 microscope. In each assay brain sections from all eight categories of rats (handled or non-handled, males and females of experimental cohorts I and II) were processed concurrently.
Cell counting
Brain area images were captured using a digital CCD color video-camera (TK-C1381, JVC, Japan) connected to an optical microscope, objective × 20, camera lens × 2.5 (Eclipse E400, Nikon, Japan). The image analysis program “Image Pro Plus” (Media Cybernetics, USA) was used for counting GR or MR immunopositive cells. Only cells with nuclear GR or MR immunostaining were counted. Small GR-stained cells, most probably glia, were not included in the quantification. Cell counting was performed independently and “blindly” by two investigators (variation between the two investigators was less than 5%). A “threshold” was set in the image analysis system, in order to include in the counting only cells stained above a certain, pre-set degree. This “threshold” was defined based on the non-specific background signal observed in the stratum radiatum of the hippocampus. The medio-lateral extent of the hippocampus to be examined was first defined according to the anatomical atlas of Paxinos and Watson Citation1986: Bregma position − 1.90 to − 2.12. Along the defined medio-lateral axis of the hippocampus, six sections spaced by 35 μm were quantified (one section counted for every five 7 μm sections cut), starting from a randomly selected section containing the brain area of interest. In each brain section, cells were counted in one optical field covering the CA2 area and in two different optical fields of the CA1 and CA3 areas. In the different brain areas, the number of positive cells per optical field of each rat was the average value calculated from the data for the optical fields of these areas in all brain sections evaluated.
Statistical analysis
The effects of “neonatal handling” and sex on the escape latency, distance swum and swimming speed during the learning trials in the MWM were assessed using a repeated measures two-way analysis of variance (ANOVA) with trials as the repeated measure (within subject factor) and “neonatal handling” and sex as independent factors (between subject factors). Accordingly, the effects of “neonatal handling” and sex on the time spent, distance swum and swimming speed in the Target and Opposite pool quadrants during the memory trial in the MWM were assessed using a repeated measures (quadrants) two-way ANOVA with “neonatal handling” and sex as the independent factors. When interactions between the independent factors or between an independent factor and a repeated factor were detected, Bonferroni post hoc tests were performed in order to identify specific differences.
Data on GR or MR immunopositive cells as well as on circulating corticosterone levels from both experiments (I and II) were analyzed by three way analysis of variance (ANOVA) with “neonatal handling”, sex and timing of stress exposure as independent factors. When interactions between the independent factors were detected, Bonferroni post hoc tests were performed in order to identify specific differences between groups. The level of statistical significance was set at 0.05. All tests were performed with the SPSS software (Release 10.0.1, SPSS, USA).
Photomicrograph production
High-resolution microscopic images were digitally captured using a digital CCD color video-camera (TK-C1381, JVC, Japan) on an optical microscope (Eclipse E400, Nikon, Japan) connected to a PC via an Image Pro Plus frame grabber (Image Pro Plus, Media Cybernetics, USA). Composite photomicrographs were prepared with Adobe Photoshop 7.0 (Adobe Systems, USA).
Results
Spatial learning
Two parameters of learning were examined during acquisition of the spatial task: latency and distance to locate the hidden platform. When the acute stress preceded the learning trials in the MWM (first animal cohort, Experiment I), a significant effect of trial was observed on both latency (F(7,35) = 13.801, p < 0.001; ) and distance (F(7,35) = 12.397, p < 0.001, not shown) as revealed by repeated measures (trials) two way ANOVA with handling and sex as the independent factors. The performance of all rats in the first learning trial was statistically different from their performance in all other trials (Bonferroni post hoc p < 0.002 for the comparison of learning trial 1 with each of the other learning trials, for both the data on latency and distance). Additionally, when the acute stress preceded the learning trials in the MWM (Experiment I) a significant handling × sex interaction in latency was also observed (F(1,35) = 4.469, p = 0.043; ). As revealed by further analysis, among males the learning performance of the handled was better than that of the non-handled (F(1,17) = 4.663, p = 0.045, repeated measures (trials) one way ANOVA with handling as independent factor) while no difference was observed between handled and non-handled females.
Figure 1 Effect of neonatal handling on learning (A, C) and memory (B, D) in the Morris water maze, with exposure to an acute restraint stress. In Experiment I (A, B), the adult rats were exposed to stress before training, and in Experiment II (C, D) before the memory trial. Note that the escape latency curves in (C) represent learning of handled and non-handled rats under basal conditions, i.e. in the absence of stress. Values are Means ± SEM. n = number of rats. Tr: trial. ♦ Effect of trial (p < 0.001); ⋄ Trial × Handling Interaction (p = 0.018); * Effect of handling (p = 0.02); † Handling × Sex Interaction (p = 0.042).
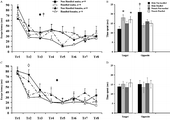
When rats were not stressed before the learning trials (second animal cohort, Experiment II) a significant effect of trial was again observed on both latency (F(7,35) = 24.858, p < 0.001; ) and distance (F(7,35) = 13.036, p < 0.001, not shown). Notably, a statistically significant trial × handling interaction in latency was also found (F(7,35) = 2.488, p = 0.018; ). Further analysis indicated that, this was due to a difference in the performance of non-handled and handled rats in the second learning trial: for handled animals of both sexes the latency in the first learning trial was statistically different from all other trials (Bonferroni post hoc p < 0.01 and p < 0.05 for male and female handled animals respectively). In contrast in the non-handled rats, latency in the first learning trial was not statistically different from the second, while both of them differed from the other learning trials (Bonferroni post hoc p < 0.05, for both male and female non-handled animals). Finally, the swimming speed of animals during spatial learning was not affected either by handling or sex in either of the two experimental cohorts.
Spatial memory
For the first animal cohort (Experiment I), statistical analysis revealed a significant quadrant × handling interaction for both time spent (F(1,35) = 8.037, p = 0.008; ) and distance covered (F(1,35) = 13.102, p = 0.001, not shown) in each quadrant. Further analysis revealed that handled rats of both sexes spent more time (F(1,35) = 5.936, p = 0.02) and swam longer distances (F(1,35) = 14.293, p = 0.001) in the target quadrant compared to the non-handled, while non-handled males spent more time (F(1,35) = 4.484, p = 0.042, Bonferroni post hoc p < 0.05) in the opposite quadrant. Finally, when swimming speed was examined no difference was found between the four animal groups.
In the second animal cohort (Experiment II), where stress was applied before the memory test, no animal group showed a statistically significant preference for any quadrant, in either the time spent () or the distance swum in any of them.
GR immunohistochemistry
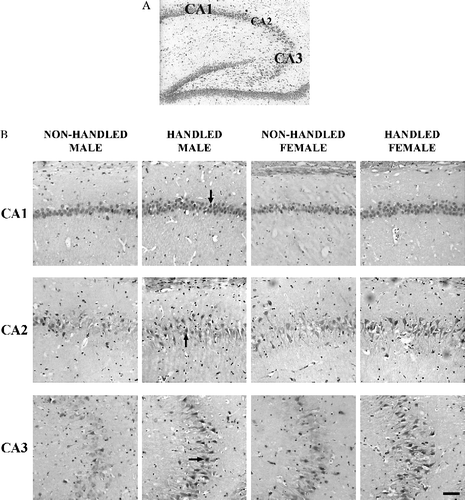
In all experimental groups, specific GR immunostaining was observed in the hippocampus of animals examined. In the CA1 area the signal was mainly nuclear, while in the CA2 and CA3 it was both cytoplasmic and nuclear. However, in all hippocampal areas, only cells with nuclear immunostaining, and characteristic neuronal morphology, were included in the counting.
Analysis of data using a three-way ANOVA with handling, sex and timing of acute stress as independent factors revealed that, in both experiments neonatally handled rats of either sex had significantly increased number of GR-immunopositive cells in the CA1 hippocampal area (F(1,47) = 22.698, p < 0.001) following the memory trial ( and 4A,). Interestingly, the timing of the acute stress (before the learning trials or prior to the memory trial in MWM) affected the number of GR-immunopositive cells in the CA2 and CA3 areas following the memory trial differently, since a handling × timing of acute stress interaction was observed (F(1,47) = 20.237, p < 0.001 for CA2; F(1,47) = 8.043, p < 0.007 for CA3). Further analysis indicated that, when the acute stress preceded the learning trials in the MWM (Experiment I), neonatally handled rats of both sexes had significantly increased numbers of GR-immunopositive cells in the CA2 and CA3 hippocampal areas (F(1,23) = 53.688, p < 0.001 for CA2; F(1,23) = 109.396, p < 0.001 for CA3) following the memory trial ( and ). In contrast, when the acute stress preceded the memory trial in the MWM (Experiment II), no difference was observed in the number of GR-immunopositive cells in the CA2 or CA3 areas between non-handled or handled, males or females ().
Figure 2 GR immunoreactivity in the hippocampus of adult rats, neonatally handled or non-handled, immediately after the memory trial in the Morris water maze. These rats (Experiment I) were exposed to stress before training, which occurred 24 h earlier. (A) Representative cresyl violet stained brain section, indicating the hippocampal areas in which GR immunopositive cells were counted. (B) Photomicrographs of GR immunopositive cells in the CA1, CA2 and CA3 areas of the hippocampus of male, female, handled and non-handled rats. The arrows point to cells with nuclear GR immunostaining, representative of those included in the quantification. Small immunopositive cells, most probably glia, were not included in the quantification. The scale bar corresponds to 60 μm.
MR immunohistochemistry
In all experimental groups, specific MR immunostaining was observed in the hippocampus of animals examined. In the CA1, CA2 and CA3 areas the signal was nuclear, and only cells with characteristic neuronal morphology, were included in the counting.
Statistical comparison of the data for each hippocampal subregion using a three-way ANOVA with handling, sex and timing of acute stress as independent factors indicated the existence of a significant handling × sex × timing of acute stress interaction on the number of MR immunopositive cells in both the CA1 and CA2 areas (F(1,47) = 4.963, p = 0.032 for CA1; F(1,47) = 5.161, p = 0.029 for CA2) while no effect was observed for the CA3 area ( and 4C,). More specifically, in Experiment I (acute stress before learning) the number of MR immunopositive cells in CA1 and CA2 of handled males was lower than that of non-handled males (F(1,11) = 12.354, p = 0.006 for CA1; F(1,11) = 6.410, p = 0.03 for CA2; and ). In contrast, in Experiment II (acute stress before memory trial in the MWM) the number of MR positive cells in the CA1 and CA2 areas was not statistically different between handled and non-handled males (). Non-handled males in Experiment II showed decreased number of MR positive cells in CA1 compared to the same group in Experiment I (F(1,11) = 8.474, p = 0.017); the respective comparison for the CA2 area showed a marginally significant difference (F(1,11) = 4.120, p = 0.07). Interestingly, no differences were observed in the number of MR immunoreactive cells in any of the hippocampal regions examined among females, handled or non-handled in either Experiment I or Experiment II.
Figure 3 Photomicrographs of MR immunopositive cells in the CA1, CA2 and CA3 areas of the hippocampus of male, female, handled and non-handled rats immediately after the memory trial in the Morris water maze. These rats (Experiment I) were exposed to stress before training, which occurred 24 h earlier. The arrows point to cells with nuclear MR immunostaining, representative of those included in the quantification. The scale bar corresponds to 60 μm.
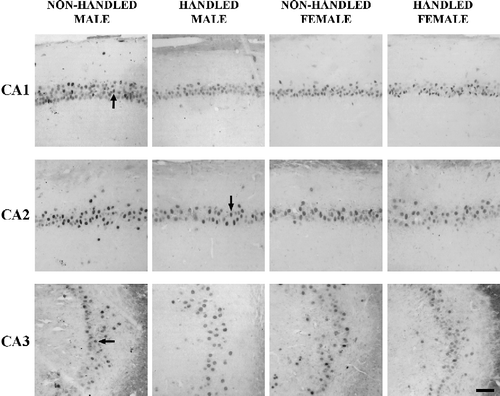
Figure 4 Number of GR (A and B) and MR (C and D) immunopositive cells in the hippocampus of adult rats, neonatally handled or non-handled, from Experiments I (A and C) and II (B and D), immediately after the memory trial in the Morris water maze. The rats were subjected to an acute restraint stress before either the training, which occurred 24 h earlier (A and C–Experiment I), or 90 min prior to the memory trial (B and D–Experiment II). Values are Means ± SEM. * Effect of handling (p < 0.05); § Handling × Timing of acute stress Interaction (p < 0.007); # Handling × Sex × Timing of acute stress Interaction (p < 0.05).

Plasma corticosterone concentrations
The concentrations of circulating corticosterone immediately following the memory trial in the MWM were not affected by neonatal handling in either of the two experimental animal cohorts. Yet, in both experiments females had higher levels of circulating corticosterone than males (F(1,71) = 43.303, p < 0.001) as revealed by a three-way ANOVA with handling, sex and timing of acute stress as independent factors (). Moreover, a statistically significant sex × timing of acute stress interaction was observed (F(1,71) = 12.048, p < 0.001); further analysis indicated that females (both handled and non-handled) showed higher levels of circulating corticosterone in Experiment II (approximately 90 min following the start of the acute restraint stress) compared to their corticosterone levels in Experiment I (24 h after the restraint stress), whereas males did not show any difference between the two experiments.
Table I. Plasma corticosterone concentrations (ng/ml) immediately following the memory trial in the Morris water maze.
In summary, we have shown that when non-handled rats are exposed to acute restraint stress prior to training (Experiment I) they learn the MWM task quicker, compared to their performance without stress. Notably, when acute stress precedes acquisition, male handled rats show an overall better learning performance, and both sexes of handled animals are less impaired in the subsequent memory trial, compared to the respective non-handled. In addition, the number of GR immunoreactive neurons is higher in all areas of the Ammon's horn of the handled rats, both male and female, during the memory recall. On the other hand, the number of MR immunopositive cells is higher in the CA1 and CA2 areas of the hippocampus of the non-handled males. When the same stressor is applied prior to the memory test (Experiment II), handling is not effective in preventing memory impairment, as all animal groups show a similar low performance in the recall trial of the MWM. In this case, the only difference in glucocorticoid receptors observed between handled and non-handled rats, during the memory test, is in the number of GR immunopositive cells in the CA1 area.
Discussion
The present study adds further support to the idea that the stress response, which is programmed by early experiences (Meaney et al. Citation1991b), can influence learning and memory, in a manner which depends on whether the exposure to stress precedes acquisition, consolidation or retrieval of information (Sandi et al. Citation1997; de Quervain et al. Citation1998; de Kloet et al. Citation1999; Joels et al. Citation2006). In our study, the early experience of neonatal handling interacted with the timing of stress exposure and gender to determine the final performance of adult rats on a spatial learning task. More specifically, our results show that exposure to an acute stress before training, resulted in an overall handling-induced advantage in the males (the handled males outperformed the non-handled). However, stress exposure prior to training also seemed to have a beneficial effect on the non-handled rats of both sexes, since their performance in the second trial of Experiment I was significantly improved compared to their performance in the same trial without prior stress (compare ,C). In the literature there are reports of similar results, showing that exposure to stress, which is mild (Pham et al. Citation1997), or within the context of learning (Sandi et al. Citation1997), can facilitate acquisition of the water maze task. When training was performed under basal conditions (Experiment II), handled rats of both sexes, which have a more efficient HPA axis reactivity and increased adaptability, learned the MWM task quicker than the non-handled. An improved performance of handled animals in another version of the MWM has also been reported by Huot et al. (Citation2002) and Fenoglio et al. (Citation2005). In addition, under basal conditions, handled animals are reported to be superior to the non-handled in a number of other cognitive tasks (Escorihuela et al. Citation1994; Beane et al. Citation2002; Pryce et al. Citation2003).
It should be noted that, although rats of all groups managed to learn the water maze task, they failed to show a clear spatial bias for the target quadrant, when tested in the probe trial. In Experiment I, it appeared that the stress, which preceded training, interfered with consolidation, leading to impaired recall 24 h later. Our findings are consistent with the extended literature reporting impairing effects of acute stress on memory performance (Diamond et al Citation1996; Klenerova et al. Citation2002, Citation2003; Shors and Miesegaes Citation2002; Conrad et al. Citation2004). In the aforementioned studies, as well as in our own, the stress used was out of the learning context, and it has been suggested that this can disrupt consolidation and subsequent recall (Joels et al. Citation2006). Nevertheless, a beneficial effect of handling was revealed as handled animals of both sexes spent more time in the target quadrant, compared to the non-handled.
In addition to the context, the timing of stress exposure seemed to be crucial for the final outcome on mnemonic performance. Exposure to stress around training can have either a facilitative (Pham et al. Citation1997; Sandi et al. Citation1997) or an impairing effect (Luine et al. Citation1994; Conrad et al. Citation1996; Kitraki et al. Citation2004) on memory, whereas stress prior to recall is usually detrimental (de Quervain et al. Citation1998; Stillman et al. Citation1998; Sida et al. Citation2003; Rashidy-Pour et al. Citation2004). Accordingly in our study, the performance of the rats in the memory test was also impaired when the acute restraint stress preceded the recall trial. Interestingly, the experience of early handling did not appear to influence the performance of the rats in this instance.
The effects of stress on learning and memory in the MWM observed in our study could be mediated by modulation of either information acquisition, consolidation, recall, or other cognitive processes such as attention to environmental cues. Relevant to this point is our previously reported finding that neonatal handling, which as shown herein influences performance in the MWM, affects the basal forebrain cholinergic system involved in the control of attentional processes (Pondiki et al. Citation2006).
Stress can affect spatial learning and memory processes by altering the levels of different molecules in the hippocampus, such as CRH and its receptors (CRH-R1 and CRH-R2) (Bale and Vale Citation2004; Chen et al. Citation2004, Citation2006) as well as glucocorticoid receptors type I (MRs) and II (GRs) (Sapolsky et al. Citation1984; Herman et al. Citation1995; Tritos et al. Citation1999; Karandrea et al. Citation2000, Citation2002; Kitraki et al. Citation2004). Furthermore, the circulating levels of glucocorticoids have been shown to play an important role in mnemonic processes (Oitzl and de Kloet Citation1992; Conrad et al. Citation1997; Pugh et al. Citation1997; de Kloet Citation1999). In our study, no differences in plasma corticosterone levels were detected between handled and non-handled rats of the same sex immediately after the probe trial, irrespective of whether the acute restraint stress had occurred 24 h (Experiment I), or 90 min (Experiment II) before. Thus alterations in corticosterone levels induced by the present experimental design do not seem to interfere or correlate with the observed learning or memory performance of the rats. The well-known sex differences in circulating corticosterone, according to which female rats have higher levels than males (Kitay Citation1961; Critchlow et al. Citation1963; Rhodes and Rubin Citation1999) was also documented in our study. Notably, in female animals irrespective of handling, plasma corticosterone levels were significantly increased after the MWM probe trial and 90 min following stress (Experiment II) compared to the levels observed after the probe in Experiment I (24 h after the restraint stress), probably reflecting a more reactive HPA axis in the females.
It is generally accepted that the basic cellular mechanism underlying the beneficial effects of neonatal handling on stress reactivity is the increased number of GRs in the hippocampus (Meaney et al. Citation1985a,Citationb, Citation1989, Citation1992; O'Donnell et al. Citation1994). In our study, we found increased number of GR-immunoreactive neurones in all Ammon's horn areas of the hippocampus of the handled rats, 24 h after the acute restraint stress and training in the MWM. The greater number of GR-containing neurones most probably represents the handling-induced basal state of the receptors. This finding could relate to the better performance of the handled rats compared to the non-handled, in the memory trial in the MWM, since it is well documented that GRs play an important role in memory consolidation and retrieval (Oitzl and de Kloet Citation1992; de Kloet et al. Citation1999; Oitzl et al. Citation2001). In contrast, in Experiment II, 90 min after the acute restraint stress, at which point the handled rats do not show an advantage over the non-handled in memory, the handling-induced alterations in number of hippocampal GR-containing neurone were no longer evident in the CA2 and CA3 areas. It appears that the acute restraint stress decreased GR protein levels selectively in the CA2 and CA3 areas of the handled rats. Down-regulation of GRs following acute stress has been reported previously (Liberzon et al. Citation1999; Paskitti et al. Citation2000, Filipovic et al. Citation2005) and the CA2 and CA3 area-specific change can be attributed to their specific roles, which are distinct from those of the CA1, and their increased plasticity towards stress-induced changes (Watanabe et al. Citation1992; Magarinos and McEwen Citation1995; Kole et al. Citation2004; Conrad Citation2006). This plasticity may be further increased in the hippocampus due to handling, as electrophysiological data also suggest (Tang and Zou Citation2002). Furthermore, the CA3 hippocampal area has been shown to play an important role in spatial pattern completion processes (Gold and Kesner Citation2005; Hunsaker et al. Citation2006), which are an integral part of the strategy employed to solve the MWM.
In the hippocampus of non-handled males exposed to stress prior to learning (Experiment I), more MR-immunoreactive neurones were observed in the CA1 and CA2 areas, compared to those of the handled. Such a stress-induced increase has been reported previously to occur in males following acute stress (Gesing et al. Citation2001) and may explain the preference of the male non-handled rats for the opposite quadrant during the memory test, as MRs participate in the selection of navigation strategy during the MWM test (Oitzl and de Kloet Citation1992).
Acknowledgements
The study was supported by an “IKY” (Hellenic state foundation for scholarships) scholarship to S.P. and by the special account for research of the University of Athens (Greece). We would like to thank Dr M. N. Alexis (National Research Institute, Athens, Greece) and Dr C.E. Gomez-Sanchez (Division of Endocrinology, University of Mississippi Medical Center, Jackson, Mississippi, USA) for kindly providing us with the anti-GR and anti-MR antibodies, respectively.
References
- Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviours. Ann Rev Pharmacol Toxicol 2004; 44: 525–557
- Beane ML, Cole MA, Spencer RL, Rudy JW. Neonatal handling enhances contextual fear conditioning and alters corticosterone stress responses in young rats. Horm Behav 2002; 41: 33–40
- Bhatnagar S, Meaney MJ. Hypothalamic–pituitary–adrenal function in chronic intermittently cold-stressed neonatally handled and non handled rats. J Neuroendocrinol 1995; 7: 97–108
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 2003; 79: 359–371
- Chen Y, Brunson KL, Adelmann G, Bender RA, Frotscher M, Baram TZ. Hippocampal corticotropin releasing hormone: Pre- and postsynaptic location and release by stress. Neuroscience 2004; 126: 533–540
- Chen Y, Fenoglio KA, Dube CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatr 2006; 11: 992–1002
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus?. Behav Cogn Neurosci Rev 2006; 5: 41–60
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 1996; 110: 1321–1334
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacol Biochem Behav 2004; 78: 569–579
- Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS. The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Res 1997; 759: 76–83
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol 1963; 205: 807–815
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys?. Trends Neurosci 1999; 22: 422–426
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998; 394: 787–790
- Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: Relevance to electrophysiological studies of hippocampal function. Behav Neurosci 1996; 110: 661–672
- Escorihuela RM, Tobena A, Fernandez-Teruel A. Environmental enrichment reverses the detrimental action of early inconsistent stimulation and increases the beneficial effects of postnatal handling on shuttlebox learning in adult rats. Behav Brain Res 1994; 61: 169–173
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology 2005; 146: 4090–4096
- Fernandez-Teruel A, Escorihuela RM, Castellano B, Gonzalez B, Tobena A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: Focus on the Roman rat lines. Behav Genet 1997; 27: 513–526
- Ferre P, Nunez JF, Garcia E, Tobena A, Escorihuela RM, Fernandez-Teruel A. Postnatal handling reduces anxiety as measured by emotionality rating and hyponeophagia tests in female rats. Pharmacol Biochem Behav 1995; 51: 199–203
- Filipovic D, Gavrilovic L, Dronjak S, Radojcic MB. Brain glucocorticoid receptor and heat shock protein 70 levels in rats exposed to acute, chronic or combined stress. Neuropsychobiology 2005; 51: 107–114
- Gesing A, Bilang-Bleuel A, Droste SK, Linthorst AC, Holsboer F, Reul JM. Psychological stress increases hippocampal mineralocorticoid receptor levels: Involvement of corticotropin-releasing hormone. J Neurosci 2001; 21: 4822–4829
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus 2005; 15: 808–814
- Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 2006; 147: 1343–1348
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 1995; 61: 180–190
- Hunsaker MR, Thorup JA, Welch T, Kesner RP. The role of CA3 and CA1 in the acquisition of an object-trace-place paired-associate task. Behav Neurosci 2006; 120: 1252–1256
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res 2002; 950: 52–63
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work?. Trends Cogn Sci 2006; 10: 152–158
- Karandrea D, Kittas C, Kitraki E. Contribution of sex and cellular context in the regulation of brain corticosteroid receptors following restraint stress. Neuroendocrinology 2000; 71: 343–353
- Karandrea D, Kittas C, Kitraki E. Forced swimming differentially affects male and female brain corticosteroid receptors. Neuroendocrinology 2002; 75: 217–226
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology 1961; 68: 818–824
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience 2004; 125: 47–55
- Klenerova V, Jurcovicova J, Kaminsky O, Sida P, Krejci I, Hlinak Z, Hynie S. Combined restraint and cold stress in rats: Effects on memory processing in passive avoidance task and on plasma levels of ACTH and corticosterone. Behav Brain Res 2003; 142: 143–149
- Klenerova V, Kaminsky O, Sida P, Krejci I, Hlinak Z, Hynie S. Impaired passive avoidance acquisition in Sprague-Dawley and Lewis rats after restraint and cold stress. Behav Brain Res 2002; 136: 21–29
- Kole MH, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience 2004; 125: 337–347
- Levine S. Infantile experience and resistance to physiological stress. Science 1957; 126: 405
- Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: Relevance to post-traumatic stress disorder. J Neuroendocrinol 1999; 11: 11–17
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997; 277: 1659–1662
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639: 167–170
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1995; 69: 89–98
- Meaney MJ, Aitken DH, Bhatnagar S, Sapolsky RM. Postnatal handling attenuates certain neuroendocrine, anatomical, and cognitive dysfunctions associated with aging in female rats. Neurobiol Aging 1991a; 12: 31–38
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Sapolsky RM. The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog Neuropsychopharmacol Biol Psychiatry 1985a; 9: 731–734
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav Neurosci 1985b; 99: 765–770
- Meaney MJ, Aitken DH, Sharma S, Viau V. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology 1992; 55: 204–213
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 1988; 239: 766–768
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology 1989; 50: 597–604
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev Neurosci 1996; 18: 49–72
- Meaney MJ, Mitchell JB, Aitken DH, Bhatnagar S, Bodnoff SR, Iny LJ, Sarrieau A. The effects of neonatal handling on the development of the adrenocortical response to stress: Implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology 1991b; 16: 85–103
- O'Donnell D, Larocque S, Seckl JR, Meaney MJ. Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Brain Res Mol Brain Res 1994; 26: 242–248
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci 1992; 106: 62–71
- Oitzl MS, Reichardt HM, Joels M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci USA 2001; 98: 12790–12795
- Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: Time-course analysis. Brain Res Mol Brain Res 2000; 80: 142–152
- Paxinos G, Watson C. The rat brain in stereotaxic co-ordinates 2nd ed. San Diego: Acadamic Press 1986
- Pham TM, Soderstrom S, Henriksson BG, Mohammed AH. Effects of neonatal stimulation on later cognitive function and hippocampal nerve growth factor. Behav Brain Res 1997; 86: 113–120
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 1993; 18: 195–200
- Pondiki S, Stamatakis A, Fragkouli A, Philippidis H, Stylianopoulou F. Effects of neonatal handling on the basal forebrain cholinergic system of adult male and female rats. Neuroscience 2006; 142: 305–314
- Pryce CR, Bettschen D, Nanz-Bahr NI, Feldon J. Comparison of the effects of early handling and early deprivation on conditioned stimulus, context, and spatial learning and memory in adult rats. Behav Neurosci 2003; 117: 883–893
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci 1997; 111: 503–511
- Rashidy-Pour A, Sadeghi H, Taherain AA, Vafaei AA, Fathollahi Y. The effects of acute restraint stress and dexamethasone on retrieval of long-term memory in rats: An interaction with opiate system. Behav Brain Res 2004; 154: 193–198
- Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: A selective review. Brain Res Brain Res Rev 1999; 30: 135–152
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci 1997; 9: 637–642
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology 1984; 114: 287–292
- Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci USA 2002; 99: 13955–13960
- Sida P, Koupilova M, Hynie S, Klenerova V. Effects of two types of restraint stress on the learned behaviour in rats. Acta Medica (Hradec Kralove) 2003; 46: 153–156
- Stillman MJ, Shukitt-Hale B, Levy A, Lieberman HR. Spatial memory under acute cold and restraint stress. Physiol Behav 1998; 64: 605–609
- Tang AC, Zou B. Neonatal exposure to novelty enhances long-term potentiation in CA1 of the rat hippocampus. Hippocampus 2002; 12: 398–404
- Tritos N, Kitraki E, Philippidis H, Stylianopoulou F. Neurotransmitter modulation of glucocorticoid receptor mRNA levels in the rat hippocampus. Neuroendocrinology 1999; 69: 324–330
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: Correlation with stress-induced corticosterone secretion. J Neurosci 1997; 17: 2626–2636
- Vallee M, Mayo W, Maccari S, Le Moal M, Simon H. Long-term effects of prenatal stress and handling on metabolic parameters: Relationship to corticosterone secretion response. Brain Res 1996; 712: 287–292
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 1992; 588: 341–345
- Wong R, Jamieson JL. Infantile handling and the facilitation of discrimination and reversal learning. Q J Exp Psychol 1968; 20: 197–199