Abstract
Source monitoring refers to cognitive processes involved in making attributions about the origins of memories, knowledge, and beliefs. One particular type of source monitoring with ample practical significance is reality monitoring, i.e., the ability to discriminate between internally vs. externally generated memories. Abundant evidence indicates that exposure to acute stress enhances declarative memory consolidation. To date, no study has looked at whether exposure to acute stress during the consolidation phase may promote reality monitoring performance. The authors examined this by administering cold pressor stress (CPS) or a control procedure to participants (N = 80) after they had either performed or only imagined performing simple motor acts, and assessing reality monitoring 24 h later. When compared with the control condition, CPS significantly elevated salivary free cortisol concentrations and enhanced reality monitoring. Stress-induced cortisol responses, however, were found not to be related to improved reality monitoring performance. Our findings are consistent with the view that post-learning stress hormone-related activity may modulate source memory consolidation.
Introduction
Because humans have a cognitive system that takes in information from a number of perceptual sources and that can itself internally generate information as well, one of the mind's most critical cognitive functions is discriminating the origin of information. Marcia K. Johnson (Citation1991, p. 180).
The ability to identify the source of a memory is critical to everyday life. Thus, recollecting episodic memories requires source attributions that are derived from specific phenomenal qualities of remembered autobiographical experiences. The cognitive processes involved in making attributions about the origins of memories traditionally are referred to as source monitoring (Johnson et al. Citation1993). According to Johnson and co-workers (Citation1993) (see also Johnson and Raye Citation1981), three major types of source monitoring can be distinguished: external source monitoring, internal source monitoring, and reality monitoring. External source monitoring refers to the ability to distinguish between memories of two externally derived sources (e.g., “did I see this on TV or did I read it in the paper?”). Internal source monitoring is said to occur when people have to discriminate between two internally generated memories (e.g., “is this a fantasy or did I dream this?”). Reality monitoring refers to processes by which people discriminate between memories derived from perception and those that were generated via thought, imagination, or fantasy (e.g., “did this really happen to me or is this just a fantasy?”).
Failures to remember the source of a memory (i.e., source monitoring errors) frequently occur in daily life and often have only minor consequences, such as when individuals doubt whether they really turned off the radio before leaving the house or whether they only imagined having done so. However, source monitoring errors may also have far-reaching consequences, as evidenced by mistaken eyewitness testimonies in which fragments of real experiences are accurately recalled, but are attributed to the wrong person, time, or location (Ross et al. Citation1994). Likewise, inaccuracies in reality monitoring can be profoundly disruptive, as is typically the case in the delusions and confabulations of schizophrenic individuals (Brébion et al. Citation2002, Maher Citation1988b; Stuss et al. Citation1978).
Accurate source monitoring is dependent on cognitive processes that initially bind features into complex memories and on processes that reactivate and evaluate such features (Johnson et al. Citation1993). Brain areas involved in these processes include the medial temporal regions, which are essential for binding and reactivation, and the frontal regions, notably lateral frontal regions, which are of special importance for strategic retrieval and/or evaluation of features of memories (Johnson et al. Citation1993; Moscovitch Citation1994). Evidence for the vital role of the prefrontal cortex (PFC) in source monitoring comes from clinical studies describing patients with frontal lobe damage (Janowsky et al. Citation1989; Schacter et al. Citation1984) and, more recently, neuroimaging studies (Dobbins et al. 2002, 2003; Mitchell et al. 2004a,b; Nolde et al. 1998; Ranganath et al. 2000; Raye et al. 2000; Rugg et al. 1999; Slotnick et al. 2003). On the basis of the evidence accumulated in these studies, Johnson and Raye (Citation1998, Citation2000) (see also Nolde et al. Citation1998) argued that the right PFC supports heuristic processing (e.g., item recognition), whereas the left PFC (possibly together with the right PFC) subserves source monitoring.
A plethora of research has shown that glucocorticoid (GC) secretion from the adrenal cortex during stressful episodes may modulate memory formation, consolidation, and retrieval (Buchanan and Lovallo Citation2001; de Quervain et al. Citation2000; for reviews, see Het et al. Citation2005, Lepage Citation2001). Specifically, memory facilitation may occur when GC receptors are moderately stimulated at the same time high affinity mineralocorticoid receptors are fully saturated. When GC receptors become extremely occupied under stressful circumstances, high GC concentrations may exert detrimental effects on memory (Abercrombie et al. Citation2003; Andreano and Cahill Citation2006). Furthermore, both noradrenergic activation of the basolateral amygdala (BLA) and the hippocampus seem to be required for GCs to impair retrieval (Roozendaal et al. Citation2004). Interestingly, animal research indicates that the PFC is a significant target for negative feedback actions of GCs and that chronic GC administration and behavioral stress can result in dendritic reorganization in the medial PFC (Charney Citation2004; Radley et al. Citation2004; Sanchez et al. Citation2000).
The effects of acute stress and GC elevations on episodic memory tests have been extensively investigated. Yet, so far, only one study has looked at whether acute stress and the subsequent GC responses affect PFC regulated source monitoring performance. In a recent study (Smeets et al. Citation2006), we demonstrated for the first time that exposure to acute psychosocial stress leads to enhanced source monitoring performance. In that study, we assigned healthy young men to either a psychosocial stress or a no-stress control condition. Next, they were given a source monitoring test developed to determine participants' ability to discriminate mere thoughts from actually verbalized thoughts. Thus, participants had to indicate whether they had really verbalized or only imagined answers to earlier presented questions. Relative to controls, participants who were stressed before the recognition aspect of the source monitoring test made fewer source monitoring errors, indicating that the retrieval of the source of a memory was enhanced by stress exposure.
In our previous work, we tested how stress affects people's source discrimination. Contrary to popular ideas about the amnestic and disorienting effects of acute stress (Kopelman Citation2002; Swihart et al. Citation1999), we found that stress contributes to optimal source monitoring. Of course, stress is known to have memory-enhancing effects when applied post-learning, i.e., during memory consolidation (Andreano and Cahill Citation2006; Cahill et al. Citation2003). The present study therefore was set out to investigate whether, in line with its effect on declarative memory tests (Andreano and Cahill Citation2006) and our previous research (Smeets et al. Citation2006), stress exposure would strengthen consolidation of the source of a memory. To the extent that reality monitoring and declarative memory share the same neurobiological underpinnings responsible for consolidating the (source) memory traces (e.g., cortisol), one would expect stressed participants to exhibit enhanced reality monitoring performance when compared with non-stressed control participants. The secondary aim of the present study was to determine whether stress-induced cortisol (i.e., the primary human GC) release is responsible for post-stress variation in reality monitoring performance. Therefore, salivary free cortisol responses to acute stress were assessed and related to post-stress measures of reality monitoring.
Aside from the fact that the present study differs from our previous work in that we now looked at the effect of consolidation stress on source memory instead of retrieval stress, a couple of other dissimilarities should be highlighted. First of all, while Smeets et al. (Citation2006) employed a psychosocial stress test, the present study used a physical stressor (see below). Secondly, Smeets et al. (Citation2006) used a source monitoring test specifically developed to determine participants' ability to discriminate mere thoughts from actually verbalized thoughts. In contrast, the present study employed an internal–external source (i.e., reality) monitoring test that focused on an individual's ability to discriminate between simple motor actions they had either actually performed or actions which they had merely imagined performing. Finally, as previous research obtained evidence for sex differences involving stress-induced cortisol effects on memory performance (Wolf et al. Citation2001) and our previous work relied on only a male sample, the current study included both male and female participants to investigate potential sex differences.
Materials and methods
Participants
Our sample consisted of 80 (32 men) healthy undergraduate students. Their mean age was 20.3 years (SD = 2.8). Participants were excluded from the study if they suffered from cardiovascular disease, endocrine or psychiatric disorder, or were on any kind of medication (including oral contraceptives). Test protocols were approved by the standing human subjects ethics committee of the Psychology Faculty of Maastricht University. All participants signed a written informed consent form and were given a small financial compensation (12.5 euro; approximately 15 US dollars) for completing the experiment.
Cold pressor stress
Stress was induced by exposing participants to cold pressor stress (CPS). The CPS is a widely used, low-risk technique in medical research to expose participants to painful stressors and is known to induce robust and reliable stress responses (Bohus et al. 2000; Cahill et al. 2003; Lovallo 1975; Mitchell et al. 2004a,b). As is typical in research employing CPS, participants were instructed to immerse their dominant arm up to the elbow in ice-cold (0–1°C) water for as long as possible with a maximum of 3 min. They were explicitly told that, as the procedure could be very uncomfortable, they could remove their arm from the ice-cold water at their own discretion without consequences. Participants who fully endured CPS were told to remove their arm after 3 min. In the control condition, participants were instructed to place their arm in warm (37–40°C) water until they were instructed to remove their arm. This instruction was given pseudo-randomly across participants after 1, 2, or 3 min following arm immersion. Arm immersion always occurred single-blind, that is, participants were not informed beforehand to which group they were assigned until immediately before arm immersion, even though they did know at the outset that they could be asked to put their arm in ice-cold water. Following CPS, all participants had to rest their arm covered by a blanket for 3 min. In line with Cahill et al. (Citation2003), participants were asked to rate the level of discomfort they experienced during water immersion. To this end, they first were asked to think back to the most intense physical pain they had ever experienced and rate this experience by appropriately marking a 0 to 10 scale (anchors: 0 = no pain or discomfort; 10 = the worst pain or discomfort imaginable). After this “calibration” scale, participants rated the peak level of discomfort they had experienced during the CPS on an analogue scale.
Reality monitoring test
The current study's action source monitoring test was based on Parks' (Citation1997) (see also Henquet et al. Citation2005; Smeets et al. Citation2006) study and modified according to Peters and co-workers (Citation2007b) to assess participants' ability to discriminate between simple motor actions they had actually performed or which they had merely imagined performing. Reality monitoring test materials included descriptions of simple motor actions that either had to be performed or imagined to be performed. The action items described simple non-intrusive acts like, for example, “break a toothpick in two pieces” and “open a newspaper”. The items were derived from previous experiments (Roediger Citation1998, CitationGoff and Roediger Citation1998; Hornstein and Mulligan Citation2004; Larøi et al. 2005). Action items were presented on a 38 cm computer screen using PowerPoint (Microsoft Corporation) with font type “Times New Roman”, font size 36.
In the first phase of the reality monitoring test, i.e., the source memory acquisition phase, participants were presented with 32 action source monitoring trials. On each trial, two actions were presented, with one action being located at the top half and the other at the bottom of the screen. After each pair of actions had been presented, participants were instructed to imagine performing the actions that had been presented on the screen. Preparation time varied between participants but never took longer than 10 s. When they indicated that they had imagined the actions, a blank screen appeared for 3 s. Next, an instruction appeared on the screen indicating “Do top” or “Do bottom”. Thus, participants had to imagine both actions, but actually performed only one of them. This procedure resulted in 16 performed actions as well as 16 covertly prepared but non-performed actions. Two independent lists were constructed such that each list included 32 actions that were exclusive to that particular list (i.e., without overlap in items between both lists), which were counterbalanced within and across groups. In both the lists, half of the actions that had to be performed were presented at the top, and the other half at the bottom of the screen. Of course, no indication whatsoever was given as to whether the performed action was the correct one. In case certain objects or materials were needed to perform the actions (e.g., toothpick, paper), the experimenter provided participants with objects/materials needed to carry out both actions after the imagination period. Immediately after the action had been completed, all objects were removed from view. The objects were hidden from the participants' view at all times, except when in use. An experimenter was present to monitor whether participants actually performed the actions. All participants were capable of performing the actions. The appendix lists the actions involved in the two lists.
Following the source memory acquisition phase and exposure to CPS or filler task, participants engaged in a number of unrelated filler tasks so that it was possible to obtain saliva cortisol samples 10 min as well as 20 min after the start of CPS. This constituted the final part of the first session of the experiment. After a 24 h retention interval, participants were given a surprise recognition and reality monitoring test. In this paper-and-pencil test, participants saw the 32 original action items, each paired with a new action item of the same content and form. For example: “break the toothpick in two pieces” was paired with “break the toothpick in three pieces”. Participants were asked to identify for each of the 32 pairs of old and new action items, the action they had seen before (i.e., the recognition memory aspect of the test). For action items classified as old, participants had to simultaneously indicate whether they had actually performed the action or only imagined having performed the action (i.e., the source memory aspect of the test).
Saliva sampling and biochemical analyses
Cortisol data were obtained with cotton Salivette (Sarstedt®, Etten-Leur, The Netherlands) devices. The saliva samples that were not centrifuged were stored at − 40° C immediately on collection. Salivary cortisol concentrations were determined in duplicate by direct radioimmunoassay (University of Liège, Belgium), including a competition reaction between 125iodohistamine-cortisol and anti-cortisol serum made against the 3-carboxymethyloxime–bovine serum albumin conjugate. After overnight incubation at 4° C of 50–μl of saliva, separation of free and antibody-bound 125iodohistamine-cortisol was performed via a conventional “second antibody” method. In order to reduce sources of variability, all three samples taken from each participant (see below) were analyzed in the same assay. Mean intra- and inter-assay coefficients of variation were less than 5.0 and 8.0%, respectively.
Design
Participants were randomly assigned to one of the two groups. Among them, 24 women and 16 men were exposed to the CPS serving as the consolidation stress group, whereas another 24 women and 16 men were assigned to a control group that included a warm water task (cf. supra). The two groups did not differ with respect to age or body mass index [both t(78) < 1.40; both p>0.17].
Procedure
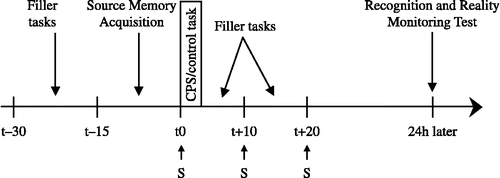
All participants were tested individually in experimental sessions run between 1 p.m. and 5 p.m. To allow for objective controlled cortisol sampling, all participants refrained from food, drinks, smoking, and heavy exercise at least one hour prior to the test phase. None of the participants reported to have violated these requirements. Upon arrival in the laboratory, participants were given a resting phase of 15 min during which they signed a consent form and completed a number of unrelated filler tasks. Subsequently, they performed the source memory acquisition. Participants then took part in either the CPS or the control task and, afterwards, engaged in filler tasks until a 20 min period that started with the onset of the CPS or control task had elapsed (cf. supra). Cortisol samples were obtained at three measurement points (i.e., t0, t+10, and t+20 min with reference to onset of the CPS or control task; also see ). Finally, participants were asked to return 24 h later to complete the key measure of interest, i.e., the recognition and reality monitoring test. To reduce the likelihood that participants would rehearse the actions they had performed, they were told that their source memory acquisition data would be analyzed and that their motor performance would be discussed the next day. Thus, no mention of an upcoming source memory test was made. During exit interviews, none of the participants indicated that they had expected a delayed source memory test. Acquisition and test sessions never exceeded 50 and 15 min, respectively. Participants were debriefed, paid, and thanked for their participation.
Figure 1 Sequence of filler tasks, cold pressor stress or control task, saliva sampling and performing memory tests. Notes: S, salivette; source memory acquisition (15 min); recognition and reality monitoring test (15 min; administered 24 h after source memory acquisition); CPS, cold pressor stress; filler tasks consisted of unrelated memory tasks.
Statistical analyses
Cortisol responses were analyzed using a 2 (group: consolidation stress vs. controls) × 3 (time: t0 vs. t+10 vs. t+20) analysis of variance (ANOVA), with the last factor being a repeated measure. When sphericity assumptions were violated, Greenhouse–Geisser corrected p-values are reported. For each participant individually, we computed a cortisol response (i.e., delta increase in cortisol) defined as peak cortisol concentration (t+10 or t+20) after the CPS or control task minus pre-stress cortisol concentration (t0). Delta responses were analyzed using an independent samples t-test. Additionally, a 2 (group: consolidation stress vs. controls) × 2 (sex: men vs. women) × 3 (time: t0 vs. t+10 vs. t+20) repeated measures ANOVA was conducted to check whether sex differences affected cortisol responses.
Performance on the recognition and reality monitoring test was analyzed as follows. First, a recognition memory score was calculated by summing the number of correctly identified old items divided by the number of items (i.e., proportion of correct recognition of memory items presented during the source memory acquisition phase). Secondly, a proportion of correct source hits was calculated reflecting the proportion of performed actions correctly reported as having been performed, conditionalized on correct memory recognition of the items. That is, source hits were only scored when the corresponding action items had been correctly recognized. Similarly, source false-alarm rates (i.e., the number of erroneous claims of having performed actions) were calculated conditionalized on correct memory recognition of the items. Hence, source false alarms reflect the proportion of imagined old action items that were correctly recognized as old, but mistakenly classified as having been performed rather than having been imagined. Finally, following the two-high threshold theory (Snodgrass and Corwin Citation1988; Corwin Citation1994), measures of accurate and biased discrimination between performed and imagined actions (i.e., a discrimination index and bias index, respectively) were determined. Thus, discrimination index (Pr) was defined as: Pr = [(number of source hits +0.5)/(number of source targets+1)] − [(number of source false alarms+0.5)/(number of source distracters+1)]. Bias index (Br) was defined as: Br = [(number of source false alarms+0.5)/(number of source distracters+1)]/(1 − Pr). For each of the recognition and reality monitoring test parameters, an independent samples (group: consolidation stress vs. controls) t-test was conducted. To evaluate potential sex differences in the recognition and reality monitoring test parameters, a series of 2 (group: consolidation stress vs. controls) × 2 (sex: men vs. women) univariate ANOVAs were conducted. Within the stress group, Spearman's ρ correlations (2-tailed) between delta increases in cortisol and reality monitoring indices were calculated. To evaluate whether cortisol in response to acute stress affects reality monitoring indices, low and high cortisol responder groups were defined by a median split (see below). Independent samples t-tests were then used to check whether low cortisol responders differed from high cortisol responders in their reality monitoring performance.
Alpha was set at 0.05 unless specified otherwise, and adjusted (Bonferroni) for multiple comparisons where necessary. In case of significance, independent samples t-tests are accompanied by Cohen's d as a measure of effect size while ANOVAs are supplemented with mean square error (MSE) and partial eta-squared (ηp2) values.
Results
Salivary cortisol stress responses
Pre-stress (i.e., t0) cortisol concentrations did not differ between CPS (M = 3.03; SE = 0.34) and control (M = 2.98; SE = 0.23) groups [t(78) = − 0.13; p = 0.90]. Participants rated CPS as more painful than the control task [t(78) = − 12.10; p < 0.001; d = 2.74], yet both groups did not differ regarding the time they kept their arm in ice-cold (CPS) or warm (control) water [t(78) = 0.80; p = 0.42].
As expected, the 2 (group: consolidation stress vs. controls) × 3 (time: t0 vs. t+10 vs. t+20) ANOVA yielded a significant Group × Time interaction [F(2,156) = 18.07; p < 0.001; MSE = 0.70; ηp2 = 0.19] and a significant main effect of time [F(2,156) = 3.59; p = 0.03; MSE = 0.70; ηp2 = 0.04], in the absence of a main effect of group [F(2,156) = 2.98; p = 0.09; MSE = 13.84; ηp2 = 0.04]. Increases (delta values) in salivary cortisol concentrations differed significantly between groups [t(78) = − 4.91; p < 0.001; d = 1.11], with means of 1.34 nmol/l (SE = 0.30) and − 0.15 nmol/l (SE = 0.06) for the CPS and control group, respectively. Individual variance in cortisol responses was typically high. Therefore, following Domes et al. (Citation2002) (see also Elzinga and Roelofs Citation2005; Smeets et al. Citation2006), a post hoc median split between low and high cortisol responders within the CPS group was conducted. This resulted in a group of 20 low cortisol responders (8 women), whose mean delta value was 0.36 (SE = 0.05) nmol/l, and a group of 20 high cortisol responders (12 women) whose mean increase was 2.33 (SE = 0.51) nmol/l [t(38) = − 3.82; p < 0.001; d = 1.24]. Low and high cortisol responders neither differed in their ratings of the painfulness of the CPS [t(38) = 1.32; p = 0.20] nor regarding the time they kept their arm in the ice-cold water [t(38) = 0.29; p = 0.78]. shows increases in cortisol concentrations throughout the experimental session for low and high cortisol responders and non-stressed controls.
Figure 2 Mean salivary free cortisol concentrations (nmol/l) for controls and high and low cortisol responders in the CPS group. Data points indicate cortisol concentrations throughout the session. Error bars represent standard error of mean (SE).
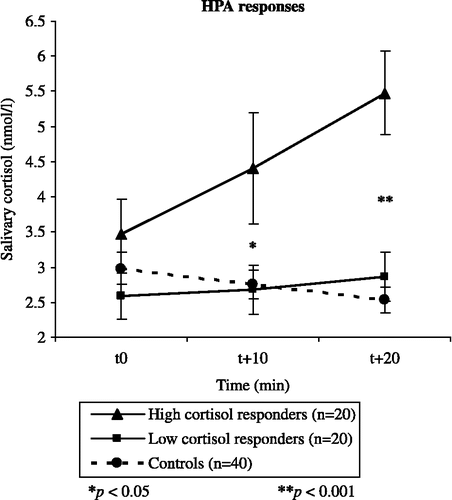
An ANOVA investigating sex differences in cortisol responses to CPS yielded a significant Group × Sex × Time interaction [F(2,152) = 5.31; p = 0.006; MSE = 0.66; ηp2 = 0.07]. Follow-up tests indicated that following CPS, women displayed a steep increase in salivary cortisol from t0 to t+10, while cortisol increases in response to CPS in men were most pronounced in the t+10 to t+20 interval.
Reality monitoring performance
Mean scores derived from the reality monitoring test for the CPS and control group can be found in . As can be seen, independent samples t-tests showed that participants in the CPS group outperformed controls with regard to proportion of correct source hits, proportion of source false alarms, discrimination index, and the recognition memory component of the reality monitoring test (all p < 0.001). Importantly, these effects were obtained without group differences in response bias (p = 0.31). Moreover, a series of univariate ANOVAs showed no influences of sex on any of the recognition and reality monitoring test parameters following CPS, with all main and interactive effects involving sex remaining non-significant (all F < 1.05; all p>0.31).
Table I. Reality monitoring performance (mean±SD) of participants who had immersed their hand in ice-cold (CPS group) or warm (controls) water during the memory consolidation phase.
To explore to what extent these overall group differences were carried by cortisol increases, we compared low and high cortisol responders. However, low and high cortisol responders did not differ with regard to proportion of correct source hits (0.89 vs. 0.91, respectively), proportion of source false alarms (0.06 vs. 0.06), recognition memory score (0.92 vs. 0.95), discrimination index (0.84 vs. 0.85), and response bias index (0.35 vs. 0.45), with all t < 1.61 and all p>0.12. Furthermore, within-stress group correlations between increases in salivary cortisol and source monitoring indices remained non-significant (all r < 0.08; all p>0.63).
Discussion
The findings of this study can be summarized as follows. To begin with, participants exposed to the stressor were better at discriminating between previously presented and new action descriptions (i.e., the recognition aspect of the reality monitoring test). Secondly, relative to non-stressed controls, stressed participants were more accurate at identifying enacted action items (i.e., source hits) and less frequently misclassified imagined action items as enacted (i.e., source false alarms). Thirdly, this resulted in the CPS group being better at discriminating between imagined and performed actions (i.e., discrimination index) than the control group. Importantly, these results were obtained without between-group differences in response bias. Finally, reality monitoring parameters did not differ between low and high cortisol responders and neither were there significant within-stress group correlations between the reality monitoring parameters and cortisol changes. Thus, stress leads to an optimisation of reality monitoring, but apparently, cortisol in itself cannot account for this effect.
Our findings closely replicate those of our previous study (Smeets et al. Citation2006) in which we for the first time demonstrated enhanced source monitoring performance following exposure to acute psychosocial stress. As in the present study, we found no relationship between cortisol responses and enhanced source monitoring in our previous study. There are, however, a number of differences between the present study's design and that of Smeets et al. (Citation2006) that deserve some attention. Smeets et al. (Citation2006) speculated that their finding of enhanced retrieval of source memory after stress exposure could be accounted for by increased cerebral blood flow to the PFC during psychological stress (Wang et al. Citation2005). However, most previous studies have shown that stress and stress hormones impair memory retrieval (de Quervain et al. 1998, 2000; Het et al. 2005). In the present study, then, a memory-enhancing effect of post-learning stress was noted when retrieval was delayed by 24 h after stress exposure. By implication, retrieval of source memory was accomplished under non-stressful conditions, which suggests that factors other than increased cerebral blood flow to the PFC are implicated in the present study's results. Also, while Smeets et al. (Citation2006) employed the Trier Social Stress Test (TSST; see Kirschbaum et al. Citation1993), the present study elicited stress by exposing participants to CPS. As cortisol responses to the TSST are generally much larger than those observed with other laboratory stressors (Dickerson and Kemeny Citation2004), one could speculate that these studies collectively suggest that the memory-enhancing effect of stress on source monitoring is driven by the sympatho-adrenal medullary axis (e.g., via the release of noradrenaline and adrenaline), perhaps in conjunction with hypothalamic-pituitary-adrenal influences (i.e., cortisol-related phenomena). Moreover, there is extensive evidence from animal (van Buskirk Citation1975) as well as human (Cahill and Alkire Citation2003) studies documenting that adrenaline modulates the consolidation of memory traces. Clearly, the contribution of the sympatho-adrenal medullary axis to the enhancing effects of stress on source discrimination needs further study (e.g., by evaluating salivary alpha amylase activity; Van Stegeren et al. Citation2006). Another difference between the present study and Smeets et al. (Citation2006) is that the latter study used a source monitoring test specifically developed to determine participants' ability to discriminate mere thoughts from actually verbalized thoughts. That is, participants had to indicate whether they had really verbalized or only imaged answers to earlier presented questions. In contrast, the present study studied individuals' ability to discriminate between simple motor actions that they had either actually performed or actions of which they had merely imagined performing them. Hence, the present study and our previous work collectively suggest that enhanced memory performance for source-related material following acute stress appears to be a phenomenon that generalizes across different types of source monitoring paradigms.
Interestingly, the present finding of enhanced memory for details (i.e., source information) to some extent is at variance with research suggesting that although stress and stress hormones may improve central aspects of memory, this may come at the expense of memory for details. For example, a study by Zorawski and Killcross (Citation2003) showed that while post-training injections of dexamethasone (a GC agonist) enhanced appetitive learning to a conditioned stimulus in rats, this also led to a decreased ability of the conditioned stimulus to elicit retrieval of information about the specific nature of the unconditioned stimulus. Additionally, it should be noted that the impact of stress and GCs may yield larger effects on memory performance when memory is assessed using recall tests than when recognition tests are being used. Indeed, a meta-analysis by Het et al. (Citation2005; p. 780) studying administered acute cortisol and memory performance concluded that for recognition memory “[…] the effect sizes were on average descriptively smaller—almost zero—than the effect sizes for free or cued recall performances. This finding may indicate that recognition memory performance is less suitable to uncover effects of cortisol on memory”. Nevertheless, in line with animal studies showing that stress and GCs may also affect recognition memory (Okuda et al. Citation2004), the current study obtained positive effects of stress on source memory using a source memory recognition test.
Another important issue raised by the present data is that the memory-enhancing effect of stress exposure on source monitoring does not seem to be restricted to the facilitation of source retrieval (Smeets et al. Citation2006), but also pertains to situations in which stress is applied post-learning (i.e., during consolidation). Our finding of enhanced consolidation of memory material is reminiscent of the work by Cahill et al. (Citation2003), who found that exposure to CPS during memory consolidation enhanced memory for emotionally arousing, but not neutral stimuli. Indeed, except for a study by Andreano and Cahill (Citation2006), previous studies have suggested that stress may enhance memory consolidation when the to-be remembered memory material is itself emotionally arousing (Buchanan and Lovallo Citation2001; Cahill et al. Citation2003). In contrast, our findings concur with those of Andreano and Cahill (Citation2006) in that they show that even relatively neutral memory materials (i.e., simple motor actions) may benefit from consolidation stress. One possible explanation would be that it is not the emotionality of the study material per se that affects stress-induced memory modulation, but rather the level of arousal produced by the study material at the time of encoding. As participants in the current study were not aware until the manipulation whether they would receive CPS or the control procedure, CPS alone thus may have produced arousal sufficient to permit stress-induced memory modulation.
Implicit in many studies showing enhanced memory consolidation due to stress exposure is the idea that GCs interact with adrenergic hormones and noradrenergic activation in the BLA to modulate consolidation (Wolf Citation2006). However, in the current study and in our previous work (Smeets et al. Citation2006), we failed to find an association between cortisol responses and source memory performance. One potential limitation of the current study is that although cortisol responses were comparable to those of other studies employing CPS (Petersen Citation2003, Citational'Absi and Petersen Citation2003; Andreano and Cahill Citation2006; Cahill et al. 2003), the range of the observed cortisol responses ( − 0.07 to +8.35 nmol/l) might have been too narrow to yield significant associations with the reality monitoring parameters. Another limitation of the present study is that it relied on only two post-stress measures of cortisol (i.e., t+10 and t+20). Hence, one could argue that although significant cortisol responses to CPS were observed, sensitivity to detect individual peak cortisol concentrations after CPS was low, which could account for the non-significant cortisol-reality monitoring correlations. Also noteworthy is that it has shown that menstrual cycle phase and the use of oral contraceptives may influence the magnitude of stress-induced cortisol responses (Kirschbaum et al. Citation1999). While women who were on oral contraceptives were excluded from participation, the present study did not attempt to control for potential differences in the menstrual cycle phase which may thus have affected the cortisol responses elicited by CPS.
It should also be noted that in the current study's reality monitoring test, sources were not exclusive in that all items were imagined with a subset being both imagined and executed. This partial redundancy of the sources may not be optimal for estimating the effects of stress because any facilitation of the binding of action-related context information may also be accompanied by facilitation of the binding of imagination-related content. Thus, even if cortisol facilitated the binding of context information during consolidation, the non-exclusivity of the conditions may partially mute the effect because benefits gained for some items with respect to action content may be offset to some extent by increased retrieval of imagination content. It should also be emphasized that new action items (i.e., distracters) in the recognition and reality monitoring test were highly similar to old action items (e.g., “break the toothpick in two pieces” vs. “break the toothpick in three pieces”), thereby rendering the recognition aspect of the reality monitoring test more akin to its source memory aspect. That is, the stronger the similarity between old and new action items, the more detailed information needs to be retrieved for correct recognition of the action items. This, of course, may limit the generalization of our findings showing enhanced recognition memory for old vs. new action items following CPS.
Because participants in the current study underwent CPS only after the source memory acquisition phase, their enhanced reality monitoring performance cannot be attributed to the CPS causing differences in attentional, tactile, or encoding processes between the two groups. Plainly, whether stress given before or during the encoding phase rather than during consolidation can affect reality monitoring performance therefore remains to be established. Also, it remains unclear whether higher concentrations of cortisol (e.g., through GC administration) would also result in enhanced reality monitoring performance, although our findings regarding the association between cortisol responses and reality monitoring indices would argue against this possibility.
In sum, to the best of our knowledge, our study is the first to show that exposure to CPS during memory consolidation results in enhanced long-term reality monitoring performance. Theories about the memory-enhancing effect of consolidation stress postulate that post-learning endogenous stress hormones interact with beta-adrenergic activity in the basolateral part of the amygdaloid complex in enhancing consolidation (McGaugh Citation2000; Roozendaal Citation2000). Thus, whether cortisol acts together with beta-adrenergic activity to enhance source monitoring processes, remains open to empirical testing. Future studies using functional neuroimaging techniques like, for example, positron emission tomography or functional magnetic resonance imaging during memory acquisition, consolidation, and retrieval under stressful circumstances, could shed further light on the precise neurobiological mechanisms behind these effects.
Acknowledgements
The authors would like to thank Dr José Sulon for conducting the cortisol analyses at the Université de Liège (Belgium). We would also like to thank the anonymous reviewers for their helpful comments on an earlier version of this manuscript.
References
- al'Absi M, Petersen KL. Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain 2003; 106: 285–295
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci 2003; 117: 505–516
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci 2006; 17: 466–470
- Bohus M, Limberger M, Ebner U, Glocker FX, Schwarz B, Wernz M, et al. Pain perception during self-reported distress and calmness in patients with borderline personality disorder and self-mutilating behavior. Psychiatry Res 2000; 95: 251–260
- Brébion G, Gorman JM, Amador X, Malaspina D, Sharif Z. Source monitoring impairments in schizophrenia: Characterization and associations with positive and negative symptomatology. Psychiatry Res 2002; 112: 27–39
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 2001; 26: 307–317
- Cahill L, Alkire M. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol Learn Mem 2003; 79: 194–198
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn Mem 2003; 10: 270–274
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am J Psychiatry 2004; 161: 195–216
- Corwin J. On measuring discrimination and response bias: Unequal numbers of targets and distractors and two classes of distractors. Neuropsychology 1994; 8: 110–117
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull 2004; 130: 355–391
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron 2002; 35: 989–996
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia 2003; 41: 318–333
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic-pituitary-adrenal axis reactivity to psychological stress and memory in middle-aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology 2002; 27: 843–853
- Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behav Neurosci 2005; 119: 98–103
- Goff LM, Roediger HL, III. Imagination inflation for action events: Repeated imaginings lead to illusory recollections. Mem Cogn 1998; 26: 20–33
- Gold PE, van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 1975; 13: 145–153
- Henquet C, Krabbendam L, Dautzenberg J, Jolles J, Merckelbach H. Confusing thoughts and speech: Source monitoring and psychosis. Psychiatry Res 2005; 133: 57–63
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 2005; 30: 771–784
- Hornstein SL, Mulligan NW. Memory for actions: Enactment and source memory. Psychon B Rev 2004; 11: 367–372
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia 1989; 27: 1043–1056
- Johnson MK. Reality monitoring: Evidence from confabulation in organic brain disease patients. Awareness of deficit after brain injury: Clinical and theoretical issues, GP Prigatano, DL Schacter. Oxford University Press, New York 1991; 176–197
- Johnson MK, Raye CL. Reality monitoring. Psychol Rev 1981; 88: 67–85
- Johnson MK, Raye CL. False memories and confabulation. Trends Cogn Sci 1998; 2: 137–145
- Johnson MK, Raye CL. Cognitive and brain mechanisms of false memories and beliefs. Memory, brain, and belief, DL Schacter, E Scarry. Harvard University Press, Cambridge, MA 2000; 35–86
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull 1993; 114: 3–28
- Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993; 28: 76–81
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamic-pituitary-adrenal axis. Psychosom Med 1999; 61: 154–162
- Kopelman MD. Disorders of memory. Brain 2002; 125: 2152–2190
- Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci 2006; 120: 217–223
- Larøi F, Collignon O, Van der Linden M. Source monitoring for actions in hallucination proneness. Cogn Neuropsychol 2005; 10: 105–123
- Lovallo W. The cold pressor test and autonomic function: A review and integration. Psychophysiology 1975; 12: 268–282
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: You can't live with it, can't live without it. Behav Brain Res 2001; 127: 137–158
- McGaugh JL. Memory: A century of consolidation. Science 2000; 287: 248–251
- Mitchell KJ, Johnson MK, Raye CL, Greene EJ. Prefrontal cortex activity associated with source monitoring in a working memory task. J Cogn Neurosci 2004a; 16: 921–934
- Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J Pain 2004b; 5: 233–237
- Moscovitch M. Cognitive resources and dual-task interference effects at retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology 1994; 8: 524–534
- Nolde SF, Johnson MK, D'Esposito M. Left prefrontal activation during episodic remembering: An event-related fMRI study. Neuroreport 1998; 9: 3509–3514
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA 2004; 101: 853–858
- Oltmanns TF, Maher BA. Delusional beliefs. Wiley, New York 1988
- Parks TE. False memories of having said the unsaid: Some new demonstrations. Appl Cogn Psychol 1997; 11: 485–494
- Peters M, Cima M, Smeets T, Jelicic M, Merckelbach H. Did I say that word or did you? Executive dysfunctions and susceptibility to leading questions influence memory functioning but not source attribution in schizophrenia. Cogn Neuropsychol 2007a; 12: 391–411
- Peters M, Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Confusing action and imagination: Action source monitoring in schizotypy. J Nerv Ment Dis 2007b; 195: 752–757
- de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 1998; 394: 787–790
- de Quervain DJF, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci 2000; 3: 313–314
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganizations in pyramidal neurons of the medial prefrontal cortex. Neuroscience 2004; 125: 1–6
- Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci 2000; 20(RC108)1–5
- Raye CL, Johnson MK, Mitchell KJ, Nolde SF, D'Esposito M. fMRI investigations of left and right PFC contributions to episodic remembering. Psychobiology 2000; 28: 197–206
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 2000; 25: 213–238
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci 2004; 24: 8161–8169
- Ross DF, Ceci SJ, Dunning D, Toglia MP. Unconscious transference and mistaken identity: When a witness misidentifies a familiar but innocent person. J Appl Psychol 1994; 79: 918–930
- Rugg MD, Fletcher PC, Chua PM-L, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: An fMRI study. Neuroimage 1999; 10: 520–529
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci 2000; 20: 4657–4668
- Schacter DL, Harbluk JL, McLachlan D. Retrieval without recollection. J Verb Learn Verb Behav 1984; 23: 593–611
- Slotnick SD, Moo LR, Segal JB, Hart J, Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res 2003; 17: 75–82
- Smeets T, Jelicic M, Merckelbach H, Peters M, Fett A, Taverniers J, et al. Enhanced memory performance on an internal–internal source monitoring task following acute psychosocial stress. Behav Neurosci 2006; 120: 1204–1210
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen 1988; 117: 34–50
- Stuss DT, Alexander MP, Lieberman A, Levine H. An extraordinary form of confabulation. Neurology 1978; 28: 1166–1172
- Swihart G, Yuille J, Porter S. The role of state-dependent memory in red-outs. Int J Law Psychiatry 1999; 22: 199–212
- Van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology 2006; 31: 137–141
- Vinogradov S, Willis-Shore J, Poole JH, Marten E, Ober BA, Shenaut GK. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. Am J Psychiatry 1997; 154: 1530–1537
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA 2005; 102: 17804–17809
- Wolf OT. HPA axis and memory. Best Pract Res Clin Endocrinol 2003; 17: 287–299
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 2001; 26: 711–720
- Zorawski M, Killcross S. Glucocorticoid receptor agonist enhances Pavlovian appetitive conditioning but disrupts outcome-specific associations. Behav Neurosci 2003; 117: 1453–1457
Appendix 1
Action items used in the source memory acquisition phase
Action Items List A
Toss a coin
Measure your index finger
Put a match in the matchbox
Write down the letter “B”
Pick up a pencil
Put your left hand in your left pocket
Touch your left shoulder with your right-hand thumb
Nod yes
Write down “7239”
Write down “name”
Point your finger at your mouth
Staple two sheets of paper together
Look at the ceiling
Tie your shoes
Stretch both of your arms simultaneously
Touch your thumb with your middle finger
Unscrew the cap of a bottle
Bounce a ball once
Perform a military salute
Fold out the blade of a Swiss Army knife
Write an address on an envelope
Break a toothpick in two pieces
Put the cap back onto a pen
Turn the pages of a book
Tear a sheet of paper in three pieces
Pick up a needle
Blow out a lighter
Play with an eraser
Wave hello
Scratch your knee
Blow your nose
Brush your hair
Action Items List B
Turn a page of paper 90°
Count the fingers of one hand
Touch your right shoulder with your left-hand thumb
Thread the eye of a needle
Cough twice
Put a letter in an envelope
Point your thumb upwards
Click a pen once
Light a match
Pull your lower lip
Cross your left leg with your right leg
Draw a circle in the air
Make a ball of paper
Raise your glass to say “cheers”
Turn around twice
Cut paper with a pair of scissors in three pieces
Make the gesture “come here”
Answer the phone
Open a newspaper
Make a cheering gesture
Put on a bracelet
Put a coin into a moneybox
Make a threatening gesture with your little finger
Wipe your mouth with a serviette
Sharpen a pencil
Throw a dice
Put a ring on your finger
Hit the table once
Close a case
Wind thread on to a reel
Blink twice with your eyes
Clap your hands twice
Appendix 2
New action items (i.e., distracters) used in the recognition and reality monitoring test
New Action Items List A
Roll a coin
Measure your middle finger
Take a match out of the matchbox
Write down the letter “P”
Lay a pencil down
Put your right hand in your right pocket
Touch your right shoulder with your left-hand thumb
Shake one's head
Write down “5914”
Write down “same”
Point your finger at your nose
Staple three sheets of paper together
Look at the floor
Untie your shoes
Stretch your left arm and afterwards your right arm
Touch your thumb with your index finger
Screw the cap of a bottle back on
Bounce a ball twice
Point at your forehead
Fold out the scissors of a Swiss Army knife
Write a number on an envelope
Break a toothpick in three pieces
Remove the cap from a pen
Close a book
Tear a sheet of paper in two pieces
Put down a needle
Use a lighter
Erase with an eraser
Wave goodbye
Scratch your head
Wipe your nose
Stroke your hair
New Action Items List B
Turn a page of paper 180°
Count the fingers of both hands
Touch your right elbow with your left-hand thumb
Unthread the eye of a needle
Cough thrice
Take a letter from an envelope
Point your thumb downwards
Click a pen twice
Blow out a match
Pull your upper lip
Cross your right leg with your left leg
Draw a rectangle in the air
Fold a piece of paper
Raise your glass to have a drink
Turn around once
Cut paper with a pair of scissors in two pieces
Make the gesture “go away”
Hang up the phone
Close a newspaper
Make a happy gesture
Put on a necklace
Take a coin from a moneybox
Make a threatening gesture with your index finger
Wipe your face with a serviette
Sharpen a set of pencils
Throw a couple of dices
Remove a ring from your finger
Hit the table twice
Open a case
Unwind thread from a reel
Blink once with your eyes
Clap your hands once