1. Introduction
Surgery planning in cerebral palsy is a complex task given the number of surgical procedures that can be combined in the same operation. Decision-making is usually based on physical examination and 3-D clinical gait analysis, but the surgery outcome remains difficult to predict. Some decision-making tools have recently been developed to predict surgery outcome (Desailly et al., Citation2013; Hicks et al., Citation2011; Reinbolt et al., Citation2011), but most of these predictions are whether qualitative (‘good’ and ‘bad’ outcomes), or adapted to unique surgical procedures or gait patterns, which makes difficult to imagine how the patient would walk after the surgery. Galarraga et al. (Citation2017) predicts postoperative kinematics for several surgical combinations, but the contribution of each surgical procedure is unclear.
The objective of this study is to simulate the effect of surgery on gait of children with cerebral palsy in order to predict the most likely outcome and the probable contribution of each considered surgical procedure.
2. Methods
Data of 134 (268 limbs) operated children with cerebral palsy that had clinical gait analysis and physical examination before and after surgery were considered. Preoperative kinematics and physical examination data were projected into a lower-dimensional space using principal component analysis (PCA) (Jolliffe, Citation2002). Then a non-trainable ensemble method was applied in order to learn a mathematical relation between postoperative kinematics and dimensionality-reduced preoperative data given the surgery.
Ensemble based machine learning (Polikar, Citation2006) consists in utilizing several models or experts, whose outputs are then combined in order to give an optimal solution to the problem. In this case, an ‘expert’ model was trained for each surgical procedure (Figure ) using multiple linear regression and nonlinear regression with feedforward neural networks (Bishop, Citation2006). Nine surgical procedures categories were considered according to their functional goal: bony hip, muscle hip, rectus femoris, hamstring lengthening, patella lowering, femoral distal osteotomy, bony shank, muscle ankle/foot and bony foot.
Figure 1. Diagram of fusion model for 3 surgical procedures by weigthed average. MA, MB and MC correspond to the trained models for surgical procedures A, B and C respectively.
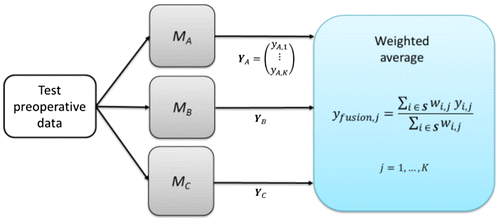
For each surgical procedure i, a model Mi was trained with data of all limbs that had procedure i in their surgery independently of the other surgical procedures that were performed in the same operation. The combination rule of the outputs is the weighted average of the outputs of the models associated to the considered surgical procedures (Figure ), where the weights correspond to the statistical significance of the procedures on the kinematic variables. The statistical significance was computed by performing an independence test on the pre-post kinematic variations of limbs that had a specific procedure and those who did not have the same procedure.
Performance was evaluated with a leave-one-out testing procedure in order to compute the error of all the patients in the database without bias. The prediction error is the root mean-squared error (RMSE) between the prediction and the real postoperative kinematics.
The performance of the proposed method was compared to the performance of the PCA + ML predictor in Galarraga et al. (Citation2017) on the same database and to a naive predictor Mean-P, which for every test case predicts the average postoperative over all available patients in the database.
3. Results and discussion
The system output shows the preoperative kinematics and the prediction according to the considered surgery, and the postoperative kinematics if available (the tested surgery must match to the actual performed surgery, Figure ). The system also shows the probable contribution of each considered surgical procedure.
Figure 2. Prediction for a random limb (knee flexion) and contribution of each surgical model. Green dotted line corresponds to the preoperative curve, blue dash-dotted line is the prediction and red dashed line is the postoperative curve. The black line with the grey band corresponds to the standard gait signal (mean ± 2 standard deviations). Lines with markers correspond to contributions surgical procedures (5 procedures in this example).
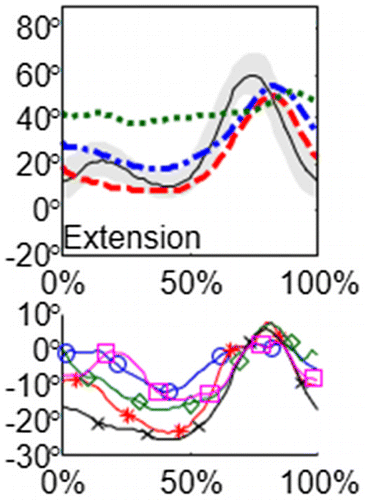
The prediction errors of the proposed method are 3.7° and 10.0° depending on the considered kinematic angle and are smaller than the errors of the other considered prediction methods for all the kinematic angles (Table ). In addition, there are significant differences between the postoperative average predictor (Mean-P) and the errors of the proposed method.
Table 1. Average prediction error (RMS) over all test limbs per kinematic angle and prediction method. *Significant difference (p < 0.05) with respect to Mean-P.
4. Conclusions
The proposed system gives a quantitative estimation of the postoperative kinematics considering large numbers of surgical combinations and gait patterns in cerebral palsy. The performance is slightly better to the average postoperative over all patients with significant differences for all nine considered kinematic angles. The system also shows the most likely contribution of each considered surgical procedure in the global surgery outcome.
The system provides a tool for showing the most likely surgery outcome to the patient, clinicians and the patient’s family. This allows better comprehension and discussion of both the treatment itself and its outcome. This could also motivate the patient to pursue a certain surgical treatment. However, while not externally validated, the usage of the proposed system should be limited to patients that are similar to those in the training base.
Acknowledgements
This work is part of the SIM-PC2 project funded by Région Ile de France, Fondation Ellen Poidatz and Fondation Bettencourt Schueller. The authors are grateful to the medical and technical staff of Fondation Ellen Poidatz for useful discussions and for recording the data used for this work.
References
- Bishop CM. 2006. Pattern Recognition and Machine Learning. 2nd edition. Springer-Verlag. New York.
- Desailly E, Sebsadji A, Yepremian D, Djemal K, Hoppenot P, Khouri N. 2013. Supervised classification of the effect of hamstrings lengthening in cerebral palsy children after single event multilevel surgery. Gait & Posture. 38(S1):39.
- Galarraga CO, Vigneron V, Dorizzi B, Khouri N, Desailly E. 2017. Predicting postoperative surgery in cerebral palsy. Gait & Posture. 52:45–51.
- Hicks JL, Delp SL, Schwartz MH. 2011. Can biomechanical variables predict improvement in crouch gait? Gait & Posture. 34(2):197–201.
- Jolliffe I. 2002. Principal Component Analysis. Springer Series in Statistics. Springer-Verlag. New York.
- Polikar R. 2006. Ensemble based systems in decision making. Circuits and Systems Magazine, IEEE. 6(3):21–45.
- Reinbolt JA, Fox MD, Schwartz MH, Delp SL. 2011. Predicting outcomes of rectus femoris transfer surgery. Gait & Posture. 30(1):100–105.
