1. Introduction
Cantilevered bridge is a common replacement for patients who have a toothless mandible. The bridge is a cantilever fixed by 4–6 dental implants into interforaminal bone region. The excessive forces during biting can cause bone damage and resorption and even the breakage of dental implants and the bridge. The magnitude of the force is of a large variation between 400 and 1300 N (Koc et al. Citation2010). The bite force strongly depends on a sex factor and age (Zha and Ye Citation1994). The bite force has a dynamic character with large amount of cycles during life. This force character directly leads to a hypothesis whether the bridge fracture can be caused by fatigue damage. Despite the fact there are a lot of studies dealing with static analysis of biting force effect on implants, bone and bridge, the dynamical examination of excessive biting force has never been investigated and presents a potential explanation of bridge fracture.
2. Methods
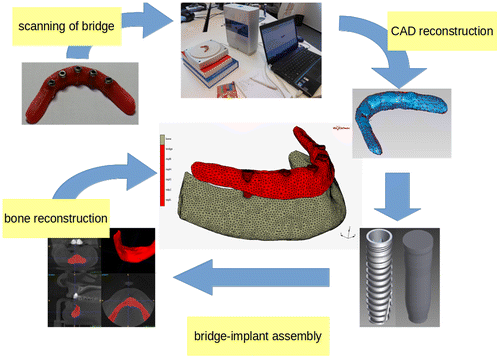
The computational model of the mandibular bone, cylindrical implants and bridge was created. The bone geometry and structural properties (density and Young’s modulus) were extracted from clinical CT data (Helgason et al. Citation2008, Yushkevich et al. Citation2006). The bridge geometry was obtained from scanned physical bridge geometry. The implant geometry was simplified to a cylindrical shape without threads. The implant-bridge interface permits only continuity of displacement without touch/separation. The implant-bone interface is modelled by surface-surface contact algorithm with Coulomb’s model of friction. The value of static friction coefficient was set to 0.3 (Rancourt et al. Citation1990). The geometry and displacement field were approximated by the finite elements with linear basis function on fourth node tetrahedral simplexes (MSC.Marc 2016). The fatigue limit cycle field was computed with linear Palmgren-Miner’s rule of cumulative damage. The limit cycle stress threshold was equal to yield stress of material. The average size of the element was 2 mm after adaptive mesh refinement to satisfy the relative error of stress below 5%. The mesh of whole assembly was created with CGAL library (The CGAL Project, Citation2016). The process of model building is shown in Figure . The material model of the bridge and implant was linear and elastic described by Young’s modulus, Poisson’s ratio, yield stress (Ti-6Al-7Nb E = 106 GPa, mu = 0.3 and Rm = 550 MPa) and S/N curve. The material model of the bone was linear elastic with locally isotropic Young’s modulus dependent on bone structural density.
The bone was fixed at the most distal surfaces by prescribing Dirichlet boundary conditions. The bite forces 500 and 1280 N were applied on the ends of bridge and parallel to implant axis. The dynamic biting force was formulated as 2700 bite cycles per day, in one year it is 106 cycles. The curve describing the dynamic cycling used to estimate the cumulative damage followed the asymmetric saw curve profile.
3. Results and Discussion
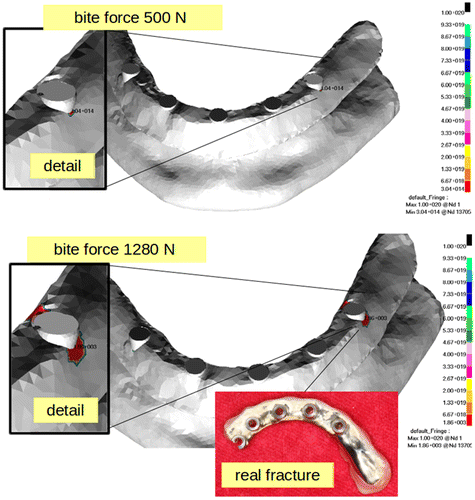
The maximal Von mises stress is located at the most distal implant on the right side with value 368 MPa in bridge. This value of stress was computed for 500 N of bite force. Once the bite force increase to 1280 N, the maximal stress value remains at the same location but its value was 736 MPa. The stress fields are shown in Figure . In the Figure the limit cycle up to bridge damage are shown. The number of cycles for bite force 500 N reaches value 314. For the bite force 1280 N the limit cycles number is 1860, which means that bridge fails in less than one day. The stress values for nominal biting force 500 N do not exceed the yield stress of the bridge’s material and also not exceed the limit cycles. These results indicate that the failure of bridge due to fatique damage cannot occur.
However increasing the biting force more than two times definitely leads to fatique damage and limit cycles that can be achieved during less than one day. Nevertheless the biting force 1280 N is an extremal value.
The location of stress (Figure ) at the bridge in the simulation model corresponds with those reported by surgeons from stomatologic clinic in Hradec Kralove (Czech republic). The averaged failure of the bridge was 5% with the same incidence. However the results supported the hypothesis that biting force can cause fatigue damage of the bridge, there are some drawbacks that needs to be resolved in the next study to obtain accurate computational model.
The current computational model does not take into account a patient’s individualities such as factors of bone structure and quality which directly influence the structural properties stress and strain fields. The bone quality influcences the implant’s stability and the overall surgery success. Another factor that influences the structural properties is the magnitude and direction of the biting force vector and its number of time cycles over the life. This variable has never been properly investigated with respect to bridge’s fatique and represents a chalenge for a scientific community.
4. Conclusion
The fatigue damage of the dental bridge due to excessive biting force must be taken as the serious failure factor of the dental bridge. The future study will include a sensitivity study of the model to bone material properties, magnitude and direction of biting force.
Acknowledgment
The research was financed from a grant SGS 2017-21196 of Technical University of Liberec - Textile Faculty, Czech Republic.
References
- Helgason B, Perilli E, Schileo E, Taddei F, Brynjolfsson S, Viceconti M. 2008. Mathematical relationships between bone density and mechanical properties: A literature review. Clinical Biomechanics. 23:135-146
- Koc D, Dogan A, Bek B. 2010. Bite Force and Influential Factors on Bite Force Measurements: A Literature Review. European Journal of Dentistry. 4:223–232.
- Rancourt D, Shirazi-Adl A, Drouin G, Paiement G. 1990. Friction properties of the interface between porous-surfaced metals and tibial cancellous bone. Journal of Biomedical Materials Research. 24:1503–1519.
- The CGAL Project. 2016. CGAL User and Reference manual. CGAL editorial Board
- Yushkevich PA, Piven J, Hazlett HC, Gimbel SR, Ho S, Gee JC, Gerig G. 2006. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage. 31:1116–1128.
- Zha Y, Ye D. 1994. Measurement of biting force of normal teeth at different ages. Journal of West China University of Medical Sciences. 25:414–417.

![Figure 2 Von mises stress at bridge [MPa]](/cms/asset/1c630634-5ffc-42b4-817a-60bfd01bfcf6/gcmb_a_1382882_f0002_oc.gif)