1. Introduction
Long bone fracture constitutes a common reason for medical consultation within veterinary orthopedic services (Miller et al. Citation1998; Kumar et al. Citation2007). Canine bone fracture repair differs from the human case in the sense that (1) the physiological characteristics and morphology of the injured bones in animals vary considerably (Palierne et al. Citation2006), (2) the animal is not able to limit its activity during the post-operative period, leading to premature overloading, and (3) the surgeon is confronted to cost limitations concerning orthopedic material. There is a lack of studies assessing the effect of different treatment types on the biomechanical properties of a reconstructed bone, which may partly explain the frequent associated complications in the field of canine fracture repair (Dvořák et al. Citation2000; Jackson & Pacchiana Citation2004). In this work, we evaluate the effect of such orthopedic reconstructions on the behavior of canine humeri in compression, using a combined experimental and numerical approach.
2. Methods
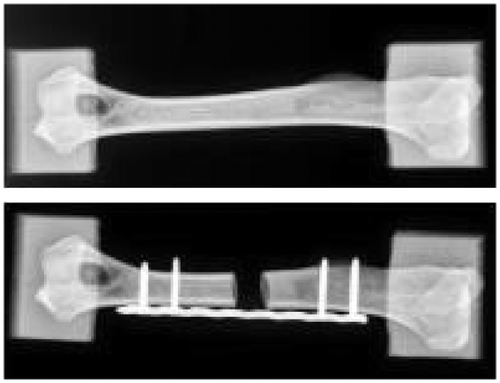
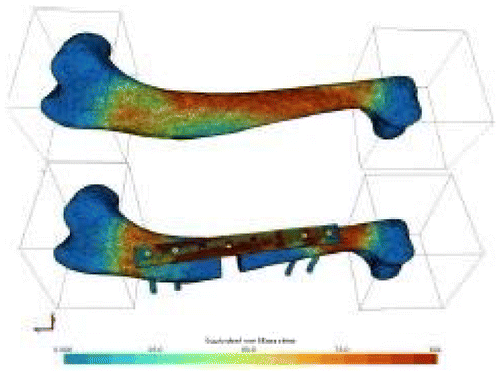
Ten canine humeri were harvested from adult dogs. Soft tissues were carefully removed and samples were wrapped in saline-soaked sponges and stored at -20°C. The bone epiphyses were embedded into 60x60x60 mm3 moulds made of polymeric resin (Laurent et al. Citation2016) (Figure ) to properly prescribe the compressive loading. The same method was used to create the resin moulds for each humerus. The origins of the medial and lateral collateral ligaments were taken as anatomical landmarks to define a reference axis. Two surfaces of the distal resin block had to be parallel to the anatomical reference axis. After creating the distal resin block, the second mould was realized, using custom made jig, in order to be perfectly aligned with the first one. The embedded samples were CT- scanned, before being tested in compression within the elastic range by applying an axial displacement of 1mm at a speed of 0.01 mms-1 after a preload of 160 N. Tool displacement and force were recorded. A bone defect of 10 mm was then created at the midpoint between the two resin moulds, and a Locking Compression Plate (LCP) associated with four screws were then used to repair this defect (Figure ). The same protocol was applied numerically using a subject-specific FE approach detailed and validated previously (Laurent et al. Citation2016). For each sample, user interaction was only needed for the image segmentation step. Briefly, the geometry of both bone and resin were reconstructed from CT-scans, and density-dependent transversely isotropic elasto-plastic properties were assigned to bone, without any distinction between cortical and trabecular bones. Resin moulds were modeled as single linear elastic deformable hexahedra linked with bone epiphyses using spring elements. The value of the spring stiffness was chosen from a previous sensitivity study. The resin elastic modulus was evaluated from preliminary experiments and fixed at 900 MPa. Compression tool was modeled as rigid surfaces of 80*80 mm2, in rigid-deformable contact with the resin blocks. Coulomb’s friction law was used, therefore a sensibility study was conducted to determine the accurate dynamic and static friction coefficients, as well as the tangent normal penalties. Compression tests were firstly simulated and compared with experimental data. A bone defect was then generated. Bone was automatically drilled using Boolean operations, and as a first step approximated geometries of plate and screws were automatically generated (Figure ). The second step was to model the exact external geometry of the LCP issued from Computer-Aided Design (CAD). Results were compared with the case of an idealized LCP geometry, to evaluate the influence of the plate model. Similarly, screws were modeled either from CAD or idealized as simple cylinders. The stainless steel orthopedic material was considered elasto-plastic, and tie contact was considered between plate and screws and between screws and bone. Compression tests of repaired bones were simulated and compared to the associated experimental force-displacement curves.
3. Results and discussion
The behavior of intact bone in compression was satisfyingly predicted by the FE simulations, with less than 30% of error on predicted stiffness. The decrease in stiffness due to bone repair was accurately predicted, as well as the buckling behavior of LCP. A significant difference was observed between simplified or accurate descriptions of plate geometry, indicating the need for proper modelling of the plate.
In comparison with reported studies concerning subject-specific FE modeling of human bone, the current study constitutes a first combined experimental and numerical analysis of both intact and repaired bones conducted on the same samples. A second originality of the current study lies in the fully automated generation of orthopedic material from intact bone geometry, allowing to operate predictive surgery as a mid-term objective. Nevertheless, further steps should be firstly implemented. Automatic generation of subject-specific bone geometries in order to save time is a crucial stage. Finally, the impact of implants on bone failure and remodeling should be taken into account. With this in mind, a canine bone rupture criterion is being firstly developed. Secondly, in order to discriminate trabecular and cortical bones, and to take into account post-surgery bone remodeling, continuum damage variables will be added as reported recently in our team (Mengoni & Ponthot Citation2015).
4. Conclusions
The present study reports a validated numerical approach capable of predicting the stiffness decrease due to bone repair using orthopedic devices. Our team is now actively working on characterizing the influence of different orthopedic configurations (plate location and type, number of screws) on the global properties of canine long bone. Therefore, this study may constitute a milestone towards the development of computational tools for predictive orthopedic surgery.
References
- Dvořák M, Nečas A, Zatloukal J. 2000. Complications of Long Bone Fracture Healing in Dogs: Functional and Radiological Criteria for their Assessment. Acta Vet Brno. 69:107–114.
- Jackson LC, Pacchiana PD. 2004. Common complications of fracture repair. Clin Tech Small Anim Pract. 19:168–179.
- Kumar K, Mogha IV, Aithal HP, Kinjavdekar P, Amarpal Singh GR, Pawde AM, Kushwaha RB. 2007. Occurrence and pattern of long bone fractures in growing dogs with normal and osteopenic bones. J Vet Med A Physiol Pathol Clin Med. 54:484–490.
- Laurent CP, Böhme B, Mengoni M, d’Otreppe V, Balligand M, Ponthot J-P. 2016. Prediction of the mechanical response of canine humerus to three-point bending using subject-specific finite element modelling. Proc Inst Mech Eng [H]. 230:639–649.
- Mengoni M, Ponthot JP. 2015. A generic anisotropic continuum damage model integration scheme adaptable to both ductile damage and biological damage-like situations. Int J Plast. 66:46–70.
- Miller CW, Sumner-Smith G, Sheridan C, Pennock PW. 1998. Using the Unger system to classify 386 long bone fractures in dogs. J Small Anim Pract. 39:390–393.
- Palierne S, Asimus E, Mathon D, Meynaud-Collard P, Autefage A. 2006. Geometric analysis of the proximal femur in a diverse sample of dogs. Res Vet Sci. 80:243–252.