1. Introduction
Cardiovascular diseases are the leading cause of death worldwide, but their aetiology is debated and the therapeutic approaches, as well as the diagnosis are still based on the definition of risk parameters mainly evaluated on the own experience of clinicians, and, furthermore they still have a high percentage of failure (Kelly and Fuster Citation2010). In this framework, computational models have been recently proved useful to provide novel insights into aortic biomechanics (Gasser Citation2018), allowing to furnish helpful indications towards more effective clinical assessment. With the aiming of understanding physiological and pathological processes to improve diagnosis and therapy, the multiscale structurally-motivated approach for tissue constitutive description, recently proposed by some of the authors (Morin et al. Citation2018), has been integrated in a computational procedure. Such a multiscale structural approach has been proved to be effective for describing the non-affine kinematics of collagen fibers on simple case studies, but it has never been employed in finite element simulations allowing the simulation of real experimental or clinical cases. In order to allow for the use of this refined constitutive description towards fully-personalized clinical applications, an integrated computational procedure for analyses of arterial segments is presented. In detail, the anisotropic and non-linear elastic mechanical response of arterial tissue is computationally treated allowing the mechanical analyses of arterial segments via a nonlinear finite-element formulation.
2. Methods
The methodology for developing 3 D computational analysis of the mechanical behaviour of arterial tissues is herein briefly summarized.
2.1. Constitutive modeling
The arterial tissue is regarded as a continuum body and, neglecting damage and viscous effects, the constitutive response is modelled via a multi-scale approach taking into account the non-affine fiber kinematics, addressed via the Eshelby's inclusion problems theory (Morin et al. Citation2018).
The soft tissues are micro-heterogeneous materials, with different fiber networks (e.g., collagen, elastin) as prominent load-bearing components. Accordingly, a simplified representative volume element (RVE) of a hundred-micrometer size made of arterial tissue (see ), which hosts different relatively, variously oriented, fiber-like inclusions (with their orientation characterized by the Euler angles θ and φ as seen on ), as well as a soft, linear elastic matrix in between is considered.
Figure 1. (a) Representative volume element of arterial tissue; (b) definition of the fiber orientation.
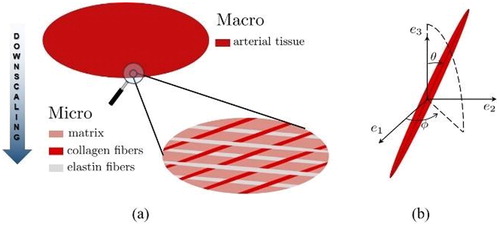
It is important to highlight that the values of model parameters employed for numerical simulations straight describe histological and biochemical properties (e.g., fiber angle distribution, volume fraction of collagen fibers) and are chosen in agreement with experimental data (Krasny et al. Citation2018).
2.2. Numerical modeling
In order to treat the non-linearity associated to the mechanical response of the tissue, an incremental numerical strategy is adopted. In the framework of an updated-Lagrangian approach, the computational strategy is developed via an incremental formulation. In particular, by subdividing the loading and/or stretch process in small enough steps, the computational method is developed by considering a series of subsequent mechanical elastic problems, solved via the finite element method (FEM) imposing increments of the load/stretch until the final value of the step. A schematic flowchart of the numerical scheme is represented in .
Figure 2. Flowchart of the solution algorithm based on a finite element implementation. LRS: local reference system; FEM: finite element method.
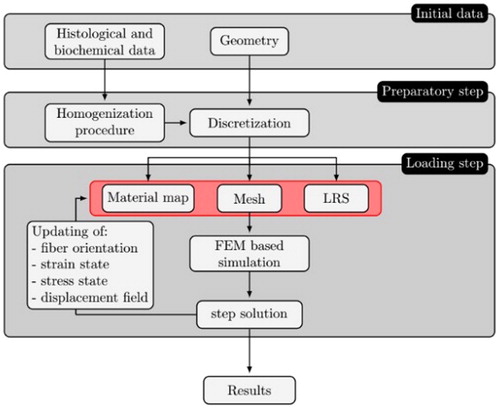
In detail, in the framework of the adopted incremental approach, the solution algorithm is based on the stepby-step updating of: (i) geometry; (ii) local reference system; (iii) material properties.
In particular, the geometry and the local reference system are updated through the displacement field obtained from the step solution of FEM simulation and the material properties of each element are updated through the updated homogenized RVE, obtaining also the updated orientation of the collagen fibers at the end of the step.
3. Results and discussion
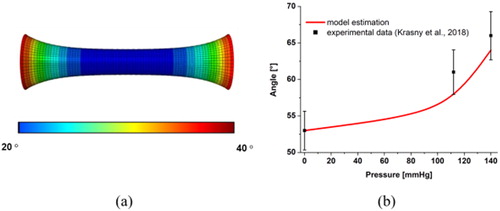
The results of many experimental mechanical tests have been compared with numerical results obtained with finite element simulation to validate the model. Reproducing the experimental analysis of (Krasny et al. Citation2018), a cylindrical segment of rabbit carotid is considered addressing two kinds of mechanical tests: a uniaxial stretch at constant pressure and an inflation test at constant stretch. As an example of the performed micromechanical analysis, a cylindrical domain 0.15 mm thick, 8 mm long and having an internal diameter of 1.3 mm is considered in . In order to prove the effectiveness of the proposed approach, two collagen family fibers are considered with the initial orientation defined, respectively, from the couple of Euler angle (φ1, θ1) = (90°, 30°), (φ2, θ2) = (90°, 130°) with respect to the local orthonormal reference system (ê1, ê2, ê3) that denote respectively the normal, the circumferential and the tangential direction in the cylindrical domain. shows the orientation of θ1 angle of the fibers, which is in agreement with the experimental value (range 20° - 25°) found in (Krasny et al. Citation2018).
Figure 3. Exemplary results: numerical simulation of an inflation test at constant uniaxial stretch λ, on a cylindrical arterial segment. (a) Spatial distributions of θ1 angle of the fibers at stretch λ = 1.8 and pressure P = 20 mmHg. (b) Evolution of θ1 angle during the inflation test at stretch λ = 1.3.
4. Conclusions
This work presents a novel computational model that captures the complexity of the load-induced microstructural morphological changes. Furthermore, the proposed framework, providing data about the stress/strain patterns at the different length scales of the arterial tissue, can open to clarify the aetiology of the aneurysms and the patho-physiological remodeling mechanisms and could be used in clinical applications.
Additional information
Funding
References
- Gasser TC. 2018. The biomechanical rupture risk assessment of abdominal aortic aneurysms method and clinical relevance. In: Biomedical technology. Cham: Springer; p. 233–253.
- Kelly BB, Fuster V. editors. 2010. Promoting cardiovascular health in the developing world: a critical challenge to achieve global health. Washington (DC): National Academies Press.
- Krasny W, Magoariec H, Morin C, Avril S. 2018. Kinematics of collagen fibers in carotid arteries under tension-inflation loading. J Mech Behav Biomed Mater. 77:718–726.
- Morin C, Avril S, Hellmich C. 2018. Non-affine fiber kinematics in arterial mechanics: a continuum micromechanical investigation. Z Angew Math Mech. 98(12):2101–2121.
