1. Introduction
Post-operative liver failure (POLF) remains the leading cause of death after partial hepatectomy, the partial resection of the liver. Similarly, after liver transplantation (LT) of a whole or partial graft, POLF can occur. The high portal pressure and porto-caval gradient after surgery are major risk factors for POLF (Allard et al. Citation2013). Venous ischemia due to outflow blockage is one of the mechanisms of POLF (Kitajima et al. Citation2016).
The liver, due to its function of filtering blood, has a double inflow vascularization, both in venous and arterial feeding through the portal vein and hepatic artery, whereas the outflow to the vena cava is through the three hepatic veins. Since anatomical features of the later vary among the population as well as several surgical techniques of reconstruction during LT, numerous situations of vessel attachment are possible. It can be assumed that certain situations are favorable to a less disturbed blood flow with lower pressure drop.
Based on the team’s policy, many techniques of venous reconstructions are available but no hemodynamics study simulated the flow in such a setting to compare their efficiency.
This study aims to compare the three most common techniques in order to have a clearer understanding of their role in the alteration of the venous return process.
2. Methods
2.1. Simulation domain
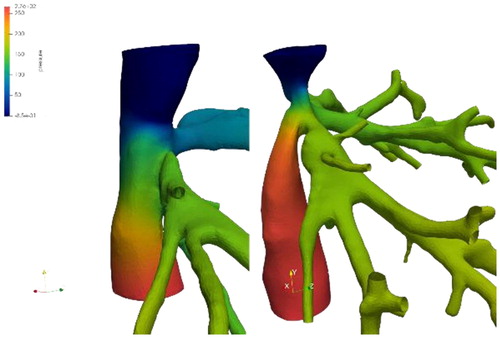
Two patients were included in the study, who underwent whole graft LT. For each one, two states are to be considered: the veins of the native liver before surgery and of the graft after transplantation. The lumen of the hepatic veins as well as the inferior vena cava were reconstructed from contrast-agent injected CT-scans with Myrian software (Intrasense) by a clinician.
The two liver grafts were connected to the venous system in two distinct ways: latero-lateral fashion [L-L] for one (the two veins are conserved and connected alongside each other) and termino-lateral for the other one (Piggy-back technique [PB]: the donor's vena cava is connected to the recipient’s one at a single upper end). In order to compare each case, virtual geometries were recreated to simulate for each patient the surgery they did not undergo. The surfaces have been modified to recreate a realistic situation. The modifications were performed under the supervision of a liver surgeon. In addition, a third type of “virtual” surgery was implemented: the vena cava replacement, where the retro-hepatic donor's vena cava is used instead of the native one (removed). We therefore have for each patient 4 configurations: a pre-surgery geometry and three post-surgery geometries, including a real one from CT-scans and two virtual ones. A total of 8 geometries were thus analyzed.
2.2. Flow simulation
The flow was considered laminar and Newtonian, within rigid walls. The Navier-Stokes equations were solved by finite element method with the FELISCE code. A flow rate was imposed at the entrance of the hepatic vein network and the vena cava as well as pressure at its exit. The values of the imposed flow rates came from PC-MRI measurements on healthy and sick patients. From these measurements and the literature, different hemodynamic scenarios were considered.
2.3. Post-processing
The main quantity examined for medical assessment was the pressure drop for the different approaches. Then, characterization metrics of parietal phenomena were used: wall shear stress and its variants. In order to visualize the stagnant flow areas, the Lagrangian coherent structures were visualized from the finite-time Lyapunov exponents field (Shadden et al. Citation2005), allowing the extraction of the underlying skeleton of the flow.
3. Results and discussion
We were able to simulate the venous return flow of the liver in different scenarios and compare various clinical strategies. In addition to virtual morphologies, several flow profiles (temporal variation, flow rate) characterizing the progression of fibrosis have been investigated. These simulations gave us a detailed mapping of the flow topology encountered at the site of hepatic veins joining the vena cava ().
Figure 1. Velocity streamlines (left) and pressure map (right) in the hepatic veins connecting to the vena cava.
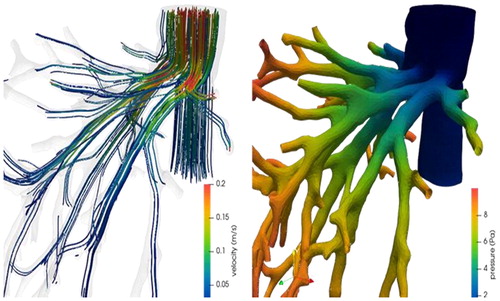
Even if the different pressure drops observed, in the order of the Pascal (), are far from those used as risk criteria in clinical practice (in the order of several millimeters of mercury - hundreds of Pascals), we can quantify the influence of each approach and their distance to the optimal preoperative conditions.
The flow structures differ depending on the type of venous connection, and between pre- and post-surgery geometries.
4. Conclusion
The quantification of flow metrics characterizing flow alteration is a challenging problem to overcome. Even more difficult is to find the metrics that can anticipate the occurrence of clinically significant problems. This study laid the foundations for a population study with patients where flow metrics will be confronted to surgery outcome. In addition, considering the limited literature on venous return after LT, this study allowed us to create a data-set of flow conditions in order to better understand the situation. Eventually, patient specific models could help the clinicians for choosing the best technique according to anatomical and hemodynamical considerations.
References
- Allard M-A, Adam R, Bucur P-O, Termos S, Cunha A S, Bismuth H, Castaing D, Vibert E. 2013. Posthepatectomy portal vein pressure predicts liver failure and mortality after major liver resection on noncirrhotic liver. Ann Surg. 258(5):822–830.
- Kitajima T, Kaido T, Iida T, Yagi S, Fujimoto Y, Ogawa K, Mori A, Okajima H, Imamine R, Shibata T, et al. 2016. Left lobe graft poses a potential risk of hepatic venous outflow obstruction in adult living donor liver transplantation. Liver Transpl. 22(6):785–795.
- Shadden SC, Lekien F, Marsden JE. 2005. Definition and properties of Lagrangian coherent structures from finite-time Lyapunov exponents in two-dimensional aperiodic flows. Phys D: Nonlin Phenomena. 212(3-4):271–304.