1. Introduction
Despite the high prevalence of diseases involving cardiomyocyte dysfunctions, the available options to study biomechanical properties of cardiomyocytes are limited, especially for human studies. Rat cardiomyocytes can be isolated from rat new-borns but display poor proliferation capacity in culture and require animal sacrifice. The aim of this work is to use induced pluripotent stem cells technology (iPSCs) to generate cardiomyocytes and assess biomechanical properties of those cells, including when submitted to different stresses like hypoxia.
2. Methods
2.1. iPS generation and culture
iPSCs were obtained by mRNA reprogramming from human foreskin fibroblasts and characterized, showing that they display all common features of human pluripotent stem cells (Steichen et al. Citation2014).
2.2. Differentiation of iPSCs into cardiomyocytes
iPSCs were differentiated into cardiomyocytes using a commercial kit (Fisher Scientific).
2.3. Cardiomyocyte characterization
Cells were fixed with PFA 4% for immunofluorescence analysis and a commercial kit was used to stain the cells (Molecular Probes).
3. Results and discussion
Human iPSCs were cultured, showing classical morphology with colonies containing small cells with high nucleo-cytoplasmic ratio. Human iPSCs were further plated for differentiation.
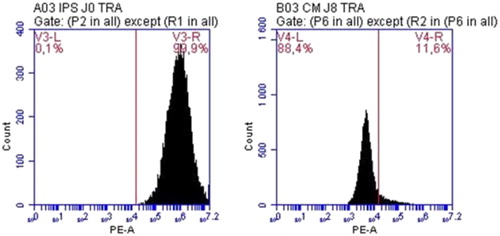
Using TRA-1-60 as a marker of human iPSCS, we showed that, compared to undifferentiated iPSC population expressing TRA-1-60 at more than 99%, differentiated cardiomyocytes loose this pluripotency marker rapidly within 8 days of differentiation ().
Figure 1. Quantification of TRA-1-60 positive cells by flow cytometry at Day 0 (left) and Day 8 (right) of differentiation.
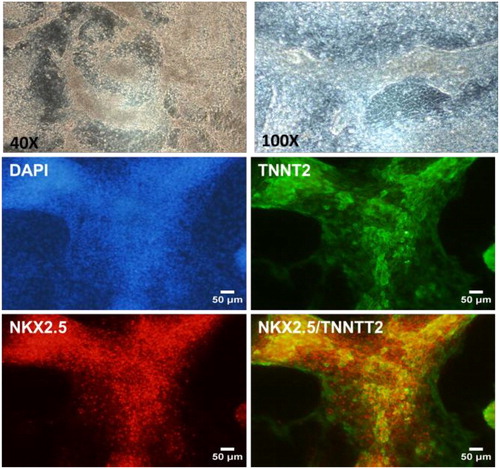
In addition, spontaneously beating cardiomyocytes appeared around Day 7–10 in the culture dishes, representing around 50% of the cells. They were fixed at Day 15 for further characterization by immunostaining. Our results showed that these cells co-express two markers of cardiomyocytes, Troponin T and NKX2.5 ().
4. Conclusion
Our work allow the generation of human cardiomyocytes displaying spontaneous beating and markers of human adult cardiomyocytes. We are working on improving the whole differentiation efficiency and purity by sorting the cells with magnetic sorting (using Troponine T). Then, we plan to study biomechanical properties of the differentiated cells at the single cell level using calcium transient, contraction force analysis as well as response to stretch or shear stress. One advantage of this technology is the possibility to control the iPSC genome and therefore the cardiomyocyte genome. This is possible either by generating patient-specific iPSCs, or by using genome editing technologies such as Crispr/Cas9 to induce specific mutations of interests. We are currently developing the technique to generate iPSCs deleted for genes of interests (potentially targets of injury following ischemia reperfusion) using Crispr/Cas9 system.
Acknowledgements
We are grateful to Anne Dubart-Kupperschmitt who kindly accepted to transfer the hiPSCs to our laboratory.
References
- Steichen C, Luce E, Maluenda J, Tosca L, Moreno-Gimeno I, Desterke C, Dianat N, Goulinet-Mainot S, Awan-Toor S, Burks D, et al. 2014. Messenger RNA-versus retrovirus-based induced pluripotent stem cell reprogramming strategies: analysis of genomic integrity. Stem Cells Transl Med. 3(6):686–691.