1. Introduction
Multiscale modeling is commonly used to assess in more detail the flow in a 3D region of interest, while the rest of the blood circulation is taken into account with reduced models (0D or 1D models). The latter constitute boundary conditions for the 3D part, and drive most of its dynamics. They are thus crucial for the model. Yet, their parameters are a priori unknown: they need to be identified in order to reflect blood flow measurements.
2. Methods
2.1. Multiscale models: Do we need patient-specific data?
For patient-specific predictions, multiscale models require tuning of the model parameters (typically the 0D part of the model) to individual patients. To this end, clinical measurements/data are required, and parameters are estimated such that the model reproduces the clinical data within the bounds of measurement errors and model fidelities. This work presents some strategies on how such parameter estimation may be performed given the type of clinical data available and the importance of the 3D part.
While it is clear that patient-specific measurements are important, some thought on when such data is indispensable is required. The utilities of haemodynamics models can be broadly classified into three categories: i) generic assessment of a novel medical device, for example a valve, or surgical/interventional procedure, for example creation of a shunt; ii) optimising the design of a medical device or surgical/interventional procedure to an individual patient for target hemodynamics indicators (pressure, flow rate, wall shear stress); and iii) studies where an overall analysis for a population is required.
2.2. How to include patient-specific data?
When patient-specific data needs to be integrated into the model (multiscale or purely 0D), strategies should be devised according to the available measurements, i.e., their implementation and computational complexity need to be coherent with the amount of information. Thus parameter identification can be done on purely 0D models, and for multiscale models, on loosely coupled 3D-0D models (meaning only a few 3D-0D simulations are required), or on stronglycoupled 3D-0D models (in the sense that each parameter identification simulation includes the 3D part).
Depending on the considered application, available measurements besides imaging data to build the 3D geometry vary. Direct or surrogate measurements of flow and/or pressure are considered:
phase-contrast magnetic resonance imaging provides flow rate over time on a surface and if the spatial resolution is fine enough even a velocity profile and its time evolution,
catheterization leads to pressure over time at a given location (direct or wedge as a surrogate),
Doppler ultrasound can provide maximum (in a small volume) velocity over time, typically interpreted as a flow rate over time assuming a certain flow profile on a surface or by direct integration of the machine.
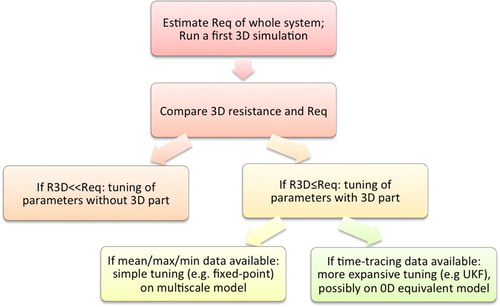
For most large vessels, their resistance is small compared to the overall system: in many pathophysiologies, almost all resistance to flow is due to downstream micro-vasculature. In such cases, if no major non-linearities, for example those arising due to stenoses, are estimated to exist in the 3D part of the multiscale model, then, for the purposes of parameter estimation, the 3D region may be neglected, thereby significantly reducing the computational cost of parameter estimation. The main idea of the different tuning options presented below is sketched in .
3. Results and discussion
In many pathophysiologies, particularly those involving aneurysms/stenoses or very complex anatomies, the 3D resistance may not be neglected. Measurements to be matched may be of two categories: i) cases where only mean values of key hemodynamic indicators such as pressure and flowrate are available; and ii) cases where full timevarying measurement curves are available.These two cases will be illustrated on patient-specific cases of single-ventricle pathophysiology (Arbia et al. Citation2015) and coarctation of the aorta (Pant et al. Citation2014), both complex congenital heart diseases.
An important aspect that requires attention is model validation. It serves two purposes:
when model validation is successful, it provides further confidence in the model results so that the unique insights provided by the model can be trusted; and
when model validation fails, it points to shortcomings in the coherence of the clinical data (Arbia et al. Citation2015) or in the model, thereby paving the way for further development and adaptation to account for important physical phenomenon. We will present two cases where validation led to model adaptation and significantly improved results are presented:
The need to take into account the surgical context (blood loss, infusion, …) for understanding of liver partial ablation (Audebert et al. Citation2017).
The need to take into account vascular adaptation to a change of flow (sensitivity to microvascular wall-shear stress) for prediction of multiple stenoses surgical planning (Yang et al. Citation2016).
4. Conclusions
We have presented the challenges in devising patientspecific model simulations, and in particular presented an overall strategy to choose a 0D parameter tuning method based on the type of available data and the importance of the 3D flow features compared to the overall system. When timetracings of hemodynamics data are available, the Unscented Kalman Filter has been shown to be a good option for such hemodynamics cases. Besides multiscale simulations, it has been successfully used 0D closed-loop model of the entire circulation in severe pathologies, including when pressure and flow rate data were acquired at different heart-rates (Pant et al. Citation2017). Although progress has been made in patientspecific hemodynamics simulations, to date large population studies with patient-specific hemodynamics data are lacking, and validation of such simulations remains a challenge. Both facts are due to the complexity of gathering patient-specific hemodynamics data, from multiple modalities, preoperatively or more often post-operatively to validate a prediction based on preoperative data and virtual surgery planning (Vignon-Clementel et al. Citation2010). Besides, circulation changes due to surgery, adaptation, growth or remodelling largely remain to be better understood and integrated into models.
References
- Arbia G, Corsini C, Baker C, Pennati G, Hsia T-Y, Vignon-Clementel I. 2015. Pulmonary hemodynamics simulations before stage 2 single ventricle surgery: patient-specific parameter identification and clinical data assessment. Cardiovasc Eng Tech. 6(3):268–280.
- Audebert C, Bekheit M, Bucur P, Vibert E, Vignon-Clementel I E. 2017. Partial hepatectomy hemodynamics changes: experimental data explained by closed-loop lumped modeling. J Biomech. 50:202–208.
- Pant S, Fabreges B, Gerbeau JF, VignonClementel I. 2014. A methodological paradigm for patient-specific multi-scale CFD simulations: from clinical measurements to parameter estimates for individual analysis. Int J Numer Meth Biomed Eng. 30:1614–1648.
- Pant S, Corsini C, Baker C, Hsia T-Y, Pennati G, Vignon-Clementel I. 2017. Inverse problems in reduced order models of cardiovascular haemodynamics: aspects of data-assimilation and heart-rate variability. J R Soc Interface. 14:20160513.
- Vignon-Clementel I, Marsden A and Feinstein J. 2010. A primer on computational simulation in congenital heart disease for the clinician. Prog Pediatr Cardiol. 30:3–13.
- Yang W, Feinstein J, Vignon-Clementel I. 2016. Adaptive outflow boundary conditions improve post-operative predictions after repair of peripheral pulmonary artery stenosis. Biomech Model Mechanobiol. 15(5):1345–1353.