 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
Theoretical and numerical models of the mechanical behavior of the annulus fibrosus (AF) are of primary importance to understand the development of pathologies, to propose early diagnostic tools and to evaluate possible treatment strategies for the intervertebral disc (IVD). Identifying a complete set of parameters from a structure-based finite constitutive model of the AF provides direct input for meaningful IVD models. They are in turn a crucial prerequisite for the understanding of physiological and pathological IVD and provide resources for tissue engineering such as the development of innovative prosthesis (Noailly et al. Citation2005).
Spatial variations of the collagen fiber angle from the outer to the inner periphery as well as from the anterior to the posterior quadrant of the AF have been densely reported (Cassidy et al. Citation1989; Holzapfel et al. Citation2005): the collagen bundles are oriented at about ±30° in the outer periphery to about ±45° in the inner periphery to the transverse plane. This structural heterogeneity generates a strong anisotropic and an inhomogenous stress-strain behavior (Skaggs et al. Citation1994; Elliot and Setton Citation2001) but data for the corresponding transverse radial and vertical strains are almost not available. Very few authors combine quasistatic conditions and present original results on the transverse behavior of AF samples (Acaroglu et al. Citation1995). Unfortunately, no inverse analysis was done to identify mechanical parameters. These deficits in simultaneously acquiring the force in the direction of traction and the strains in all three directions combined to a hyperelastic modeling suggest the need for further studies to determine a complete set of material and structural parameters for the AF in quasi-static conditions. The completeness of the proposed characterization method should enable us to identify the variations of mechanical and structural parameters of the AF.
2. Methods
2.1. Experimental procedure
The traction specimens were tested under displacement control by incrementally applying 0.1 mm displacement eight times. The samples were held at each strain level for 2 min for the first step to 20 minutes for the last step, to allow the circumferential stresses to relax to a steady-state value in order to realize a multi-relaxation protocol. The traction force, the displacement and 2 series of images (in two perpendicular directions) were acquired simultaneously at 0.5 Hz. The force was measured with a miniature S Beam load cell (Futek Inc., model 10 N LSB210, Irvine, USA).
2.2. Constitutive behavior
The anisotropic nonlinear behavior of the annulus fibrosus was modeled by a hyperelastic constitutive model split into a purely isotropic part ψm, which describes the ground matrix, and an additional anisotropic part ψf, which describes the contribution of the collagen fibers. The constitutive behavior of the isotropic matrix part was described by a compressible Neo-Hookean energy function:
The contribution of the collagen fibers was taken into account by the following strain energy density (Gasser et al. Citation2005):
2.3. Material parameter identification procedure
Numerical traction specimens were created with specific dimensions of each experimental samples. Due to the symmetry planes, only a quarter of each numerical specimen was considered.
We used three experimental datasets to identify the material and structural parameters: (a) the force in the direction of traction Fexpϑ, (b) the dilatation in the radial direction
and (c) the dilatation in the vertical direction
Two cost functions were used to investigate the influence of the account of the dilatations in the identified material parameters:
and
With n, the number of data points for each experiment.
3. Results and discussion
Remarkably, we have noticed that some of the samples, for stretching values λϑ < 1.05, experienced an increase of the transverse dilatations (for 16 samples) and
(for 18 samples) (see for one example).
Figure 1. Comparison of model predictions for two different cost functions (A. Stress in the direction of traction, B. Vertical dilatation, C. Radial dilatation and D. Volume change).
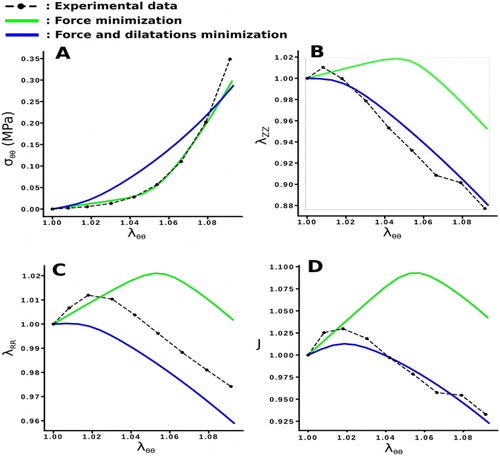
shows an example of the increase of the ability of the numerical traction specimen to fit the experimental transverse dilatations of the sample. If the cost function f1 is used, the transverse dilatations are well described by the model but not the stress, whereas if the used cost function is f2, the stress in the tensile direction is well estimated, but not the transverse dilatations.
shows an increase of the ability of the model to predict the transverse dilatation behaviors by 16.02% (p < 0.001) on λZ, by 7.42% (p < 0.05) on λR and by 7.61% (p < 0.01) on J, when taking into account the transverse dilatations in the objective function f1, while the ability of the model to predict the circumferential stress only decreases by 4.48% (p < 0.001).
Table 1. Comparison of function minimization on the average normalized root mean square error (NRMSE)+/standard deviation.
All parameters show no significant differences between the exterior and interior specimens (p > 0.05) except the fiber angle which is significantly lower in the exterior part (26.33° ± 4.78°) than in the interior part (33.52° ± 6.5°, p < 0.05) for the force and dilatations minimization (function f1), corresponding to an increase by 1.27 times. As for the minimization on both force and dilatations, the minimization on force (function f2) shows a significant difference only on the fiber angle which is lower in the exterior part (26.03° ± 4.24°) than in the interior part (37.29° ± 4.65°, p < 0.05).
4. Conclusions
The results of this study are particularly relevant for the modeling of both the heterogeneous and the compressible behavior of the annulus fibrosus. The use of the transverse dilatation in the objective function showed a significant improvement in the ability of our model to predict the transverse swelling behavior of the samples.
Acknowledgements
We thank Gille Camp, Stéphan Devic and Patrice Valorge who provided expertise that greatly assisted the research.
References
- Acaroglu ER, Latridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum AM. 1995. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 20(24):2690–2701.
- Cassidy JJ, Hiltner A, Baer E. 1989. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 23(1):75–88.
- Elliot DM, Setton LA. 2001. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: experimental measurement and material model predictions. J Biomech Eng. 123:256–263.
- Gasser TC, Ogden RW, Holzapfel GA. 2005. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface. 3:15–35.
- Holzapfel G, Schulze-Bauer CAJ, Feigl G, Regitnig P. 2005. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech Model Mechanobiol. 3(3):125–140.
- Noailly J, Lacroix D, Planell J. A. 2005. Finite element study of a novel intervertebral disc substitute. Spine. 30(20):2257–2264.
- Skaggs DL, Weidenbaum M, Latridis JC, Ratcliffe A, Mow VC. 1994. Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine. 19(12):1310–1319.
