1. Introduction
Liver tissue engineering aims at the reconstruction in vitro of a tissue mimicking as much as possible the organ functions. For the liver, the challenge is very high since this organ is in charge of more than 500 functions in a healthy organism, coming from the presence of several cell types, complex 3D organisation.
Several approaches are currently under investigation at the organ scale (Legallais et al. Citation2018) or at the tissue scale, using in this case mostly microfluidic devices (Kimura et al. Citation2018).
In these reconstruction attempts, biomechanics should not be forgotten: indeed, liver is a highly perfused organ with both venous and arterial blood, with a significant influence of local pressure and oxygen content on cell activities. In addition, this organ is very soft and most of its related diseases results in an alteration of its mechanical properties.
In this article, we propose several bioinspired approaches at different scales to offer to the cells the ‘best’ biomechanical environment. This means that both artificial matrix and bioreactor can be designed to meet physiological conditions encountered in the native liver.
2. Methods
2.1. Cell culture
Several cell types were employed the experimental studies: C3A cell line from ATTC or Hepa-RG from Biopredic for proof of concept and primary cells from rat or human origin for more physiological studies. Cell viability and basic performances (glucose consumption, albumin synthesis, biotransformation activities) were assessed at different time points to evaluate cell response.
2.2. Matrices
Liver cells were entrapped in alginate, a natural linear polysaccharide with mannuronate (M) and guluronate (G) residues arranged as blocks of similar and alternating units. After gelation or reticulation, these blocks rearrange themselves to form a more or less porous structure, allowing easy mass transfer of nutrients and oxygen. Additional collagen coating allows cell adhesion to the scaffold. Absence of coating can be promoted to allow cells to aggregates and form spheroids in the porous structure.
2.2. Bioreactors
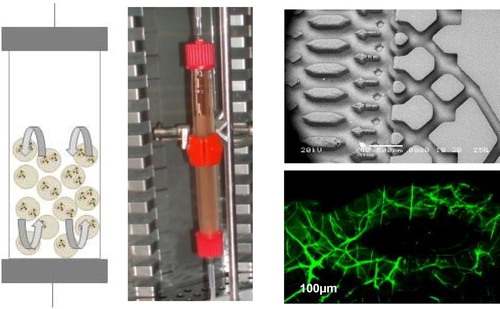
At the microscale level, we use microstructured biochips in which alginate was reticulated. Perfusion was then achieved under flow rates from 10 to 25 µl/min. For extracorporeal liver support, alginate beads hosting cells were placed in fluidized bed bioreactors, whose size can be tuned according to the application (animal or human size) ().
3. Results and discussion
3.1. Liver on chip
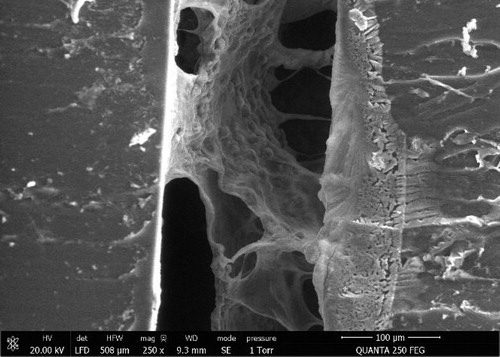
Alginate was successfully reticulated in the biochips. According to the concentration, elastic modulus varied from 1 to 10 kPa, covering a range from healthy liver to early grades of cirrhosis. Within an intermediate range of 2–3 kPa, we observed that cells successfully reorganized in 3D structure filling alginate pores (). Perfusion was possible with limited pressure drops and cells’ functions could be maintained for several days.
3.2. Cell encapsulation in alginate beads
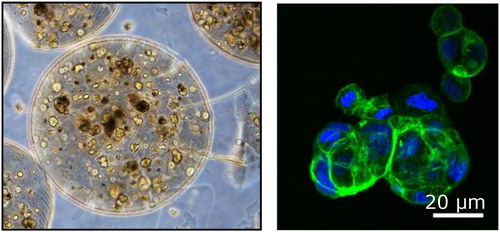
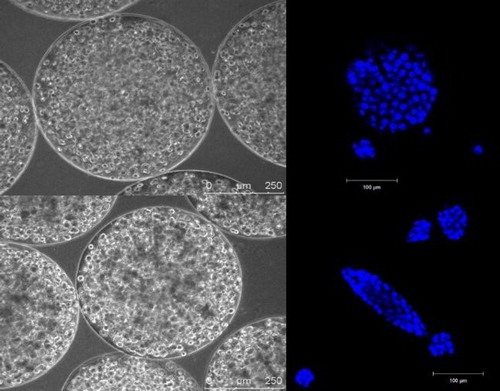
In alginate beads, Hepa-RG cells self reorganized as spheroids and showed significant differentiation in the absence of differentiation factors classically used in 2D culture (). In other conditions (different matrix’s stiffness), C3A cells could be ‘pushed’ to reorganize in filaments that may better mimic liver organisation (). It is still not clear whether these different types of 3D culture alter or not the cell’s metabolic responses.
4. Conclusions
These comparative studies demonstrate the importance of considering both mechanical properties of the surrounding artificial matrix and perfusion conditions to reproduce in vitro major liver functions.
Applications of liver tissue engineering can be found either in organ supply as extracorporeal treatment or in toxicology studies for drug screening.
Acknowledgements
The authors would like to acknowledge the French PIA ‘RHU Ilite Innovations in tissue liver engineering’ ANR-16-RHUS-0005.
References
- Legallais C, Kim D, Mihaila SM, Mihajlovic M, Figliuzzi M, Bonandrini B, Salerno S. Yousef Yengej FA, Rookmaaker MB, Romero NS, et al. 2018. Bioengineering organs for blood detoxification. Adv Healthc Mater. 19:e1800430.
- Kimura H, Sakai Y, Fujii T. 2018. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 33(1):43–48.