 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.1. Introduction
In the last century, most of the bone remodelling models available in the literature were based on mechanical strain energy and the interdependence between the bone mineral density and the applied mechanical loads. More recently, trying to optimize the knowledge of the links between the mechanical forces and the biological reactions (Stoltz et al. Citation2018), a number of mechanobiological models were developed (George et al. Citation2018, Citation2019). Some models try to take into account the bone cells activity both at the local (Lemaire et al. Citation2011) and at the global (Frame et al. Citation2018) scales. However, bridging between these two scales remains challenging (Sheidaei et al. Citation2019; Bagherian et al. Citation2020). The next step in bone remodelling is to integrate biological processes that are at the origin of the bone tissue evolutions such as the capillary growth (Bednarczyk and Lekszycki Citation2016), the nutrients supply, or the cells migration (Allena and Maini Citation2014). In the present work, we describe the bone remodelling phenomenon at a macroscopic scale based on a direct relation between the mechanical strain and the cellular activity. Our main objective is to show that reverse effects (Zhang and Zheng Citation2011; Maïmoun et al. Citation2016) of the mechanical strains on bone mineral density can be modeled, more precisely that a decrease of the bone density as a function of the cellular activity can be due to the overloading of the structure.
2. Method
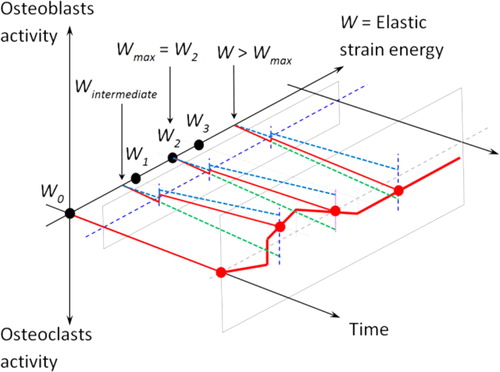
The herenew proposed model is based on a strain energy density (SED) approach accounting for external mechanical load leading to a macroscopic bone density change at the continuous scale. The main assumption is that the cellular activity is proportional to the intensity of the mechanical load, but with a predefined scenario. We define such a cellular activity as a quantity of bone formation (osteoblasts)/resorption (osteoclasts) as a function of time and mechanical energy. The combination of these two dependencies is presented in . As we are working at the continuous macroscopic scale, we define a continuous representative volume (RVE) that is large enough to include both active osteoblasts and osteoclasts. Thus, the total bone density is the sum of the osteoclasts and osteoblasts activities within the given RVE.
Figure 1. Global schematic of the mechano-regulatory model or cellular activity as a function of time and energy.
We applied this framework to a simplified geometry which is assumed to represent a femur diaphysis under simple tension. Since we assume a constant distribution of the stresses through the thickness of the femur diaphysis, we propose to study a simplified 2D rectangular beam of length L = 50mm and height H = 20mm.
The materials parameters are defined by the Young modulus of the cortical bone E = 20 GPa and Poisson ratio ν = 0.3. The applied mechanical load is equivalent to a human body weight F = 400 N and an initial bone density is taken as 0.5. Several scenarios were studied corresponding to different values of the external force. The model was implemented within the Software Comsol Multiphysics.
3. Results and discussion
The proposed model hypotheses are based on the fact that osteoblasts have higher initial kinematics than osteoclasts but a lower maximum value leading to the theoretical bone density variation as proposed in . shows the obtained numerical results. When the load is applied, osteoclasts and osteoblasts activities develop. The osteoblasts’ activity maximum value (corresponding to the mechanical energy W1) is reached first, followed by the osteoclast’s activity maximum value (corresponding to the mechanical energy W3). As the bone density is the sum of both cellular activities, bone density will vary accordingly.
Figure 2. Numerical results obtained for the osteoblasts and osteoclasts activity as well as bone density as function of the mechanical energy.
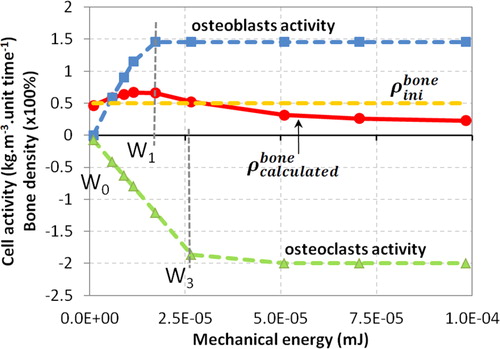
Several constant load intensities are applied on the bone (represented by the symbols at different energy levels on ) from homeostasis up to overloading the structure. The bone mineral density starts by increasing up to an energy threshold where osteoclasts take over leading to a bone degradation scenario.
4. Conclusion
With this new mechanoregulatory model based on cellular activity, the bone degradation observed experimentally when mechanically overloading the structure can be predicted leading to a more realistic bone density behavior under medium and high mechanical load. This could help define better practices in exercise training activity.
Acknowledgement
The authors would like to thank the CNRS for its financial support through the DEFI CNRS Mécanobiologie to carry out the experiments at the origin of this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allena R, Maini PK. 2014. Reaction-diffusion finite element model of lateral line primordium migration to explore cell leadership. Bull Math Biol. 76(12):3028–3050.
- Bagherian A, Baghani M, George D, Rémond Y, Chappard C, Patlazhan S, Baniassadi M. 2020. A novel numerical model for the prediction of patient dependent bone density loss in microgravity based on micro-CT images. Cont Mech Thermodyn. 32:927–943.
- Bednarczyk E, Lekszycki T. 2016. A novel mathematical model for growth of capillaries and nutrient supply with application to prediction of osteophyte onset. ZAMP. 67:94.
- Frame J, Rohan PY, Corté L, Allena R. 2018. Optimal bone structure is dependent on the interplay between mechanics and cellular activities. Mech Res Com. 92:43–48.
- George D, Allena R, Rémond Y. 2018. A multiphysics stimulus for continuum mechanics bone remodelling. Math Mech Compl Sys. 6(4):307–319.
- George D, Allena R, Rémond Y. 2019. Integrating molecular and cellular kinetics into a coupled continuum mechanobiological stimulus for bone reconstruction. Continuum Mech Thermodyn. 31(3):725–740.
- Lemaire T, Capiez-Lernout E, Kaiser J, Naili S, Sansalone V. 2011. What is the importance of multiphysical phenomena in bone remodelling signals expression? A multiscale perspective. J Mech Behav Biomed Mater. 4(6):909–920.
- Maïmoun L, Paris F, Coste O, Sultan C. 2016. Sport intensif et troubles du cycle chez la jeune femme: retentissement sur la masse osseuse. Gyn Obst Fert. 44(11):659–663.
- Sheidaei A, Kazempour M, Hasanabadi A, Nosouhi F, Pithioux M, Baniassadi M, Rémond Y, George D. 2019. Influence of bone microstructure distribution on developed mechanical energy for bone remodelling using a statistical reconstruction method. Math Mech Sol. 24(10):3027–3041.
- Stoltz JF, Magdalou J, George D, Chen Y, Li Y, de Isla N, He X, Rémond Y. 2018. Influence of mechanical forces on bone: introduction to mechanobiology and mechanical adaptation concept. J Cell Immunother. 4(1):10–12.
- Zhang C, Zheng L. 2011. Research on the changes and its mechanism of bone mass density of overtraining female rats. Chin J Rehab Med. 26(6):555–560.
