Abstract
Human–wildlife conflict is a leading cause of adult mortality for large carnivores worldwide. Train collision is the primary cause of mortality for threatened grizzly bears (Ursus arctos) in Banff National Park. We investigated the use of stable isotope analysis as a tool for identifying bears that use the railway in Banff. Rail-associated bears had higher δ15N and δ34S values than bears sampled away from the rail, but similar δ13C values. Because elevated δ15N values are indicative of higher animal protein consumption, rail-associated bears likely preyed on ungulates that foraged along the rail or scavenged on train-killed animals. The higher δ34S values in bear hair could have resulted from bears consuming sulfur pellets spilled on the rail or through the uptake of sulfur in the plants bears or animals consumed. Similar δ13C values suggest that the two types of bears had generally similar plant-based diets. Results from this study suggest that stable isotopes analysis could be used as a non-invasive, affordable, and efficient technique to identify and monitor bears that forage on the railway in Banff and potentially other transportation corridors worldwide.
1. Introduction
Large-bodied carnivores typically range over large areas [Citation1], exhibit slow population growth rates [Citation2], and occur at low population densities [Citation3]. All of these factors increase their susceptibility to extirpation, particularly in areas with increasing anthropogenic impacts [Citation4]. Consequently, human–carnivore conflict is often the leading cause of adult mortality for large carnivores, even in protected areas [Citation5]. Preventing or mitigating human–wildlife conflict is dependent on a mechanistic and behavior-based understanding of the human–wildlife interface [Citation6]. In particular, it is important to identify the individuals within a population that exhibit behavior(s) that increase their risk of human-induced mortality and target the associated individuals and specific behavior(s) that engender conflict with the appropriate monitoring and management practices. Traditional methods for monitoring such animals through time generally rely on capture and telemetry, but these techniques can be invasive, costly, and time-consuming [Citation7]. Meanwhile, the rate of human–wildlife conflict is increasing worldwide [Citation8], creating an imperative to identify novel methods that are non-invasive, inexpensive, and efficient at identifying conflict-prone individuals.
Human–carnivore conflict occurs frequently along the transportation corridor in Banff National Park (hereafter, Banff) [Citation9]. Carnivores are killed in Banff by automobiles on the Trans-Canada Highway (TCH) [Citation10,Citation11] and by trains on the Canadian Pacific Railway (CPR; ) [Citation12]. Grizzly bears (Ursus arctos) have attracted particular concern because the local population reproduces slowly and has one of the lowest densities in North America [Citation13]. High rates of mortality caused by collisions with automobiles on the TCH have been reduced by the installation of exclusionary fences [Citation10] and wildlife crossing structures [Citation9]. Unfortunately, the rate of collisions with trains on the CPR is now the leading cause of grizzly bear mortality in Banff [Citation14]. No one knows what change in bear or train behavior has caused this increase, but identifying conflict-prone individuals could make it possible to mitigate this source of mortality.
The most prevalent hypotheses for the increase in train-caused mortality of grizzly bears in Banff National Park apply to transportation systems everywhere. Many species of animals are attracted to roads for the foraging and travel opportunities they provide, while lacking the evolutionary heritage to identify vehicles as mortality threats. The high rate of attraction to roads across taxonomic groups contributes to the role of mortality as potentially being the most detrimental impact of all road effects [Citation15–17]. Although there has been much less research on the effects of railways on wild animal population, it is clear that grizzly bears in Banff use the rail both for travel and food [Citation12,Citation18]. Grizzly bears that use the railway in Banff forage on plants and a variety of human-derived foods including train-killed animals and deposits of agricultural products being carried by the rail (hereafter, grains) [Citation12,Citation18,Citation19], including wheat (Triticum aestivum), barley (Hordeum vulgare), canola (Brassica spp.), lentils (Lens culinaris), peas (Pisum sativum), and corn (Zea mays) [Citation12,Citation20]. In addition to grains, researchers and park staff frequently observe sulfur pellets along the tracks and occasionally in bear scats (J. Hopkins, personal observation). Grains and sulfur pellets are deposited on the rail during train derailments, leaked from grain hopper cars, or blown off cars after being spilled on their exteriors during loading [Citation19–22]. Not all grizzlies in the Mountain Parks appear to forage on the rail (Hal Morrison, personal communication), but those that do are presumed to be more susceptible to train-caused mortality [Citation21,Citation22].
Grizzly bears that are at risk of being killed by trains on the CPR in Banff epitomize the kind of human–wildlife conflict for which it would be helpful to have a non-invasive, inexpensive, and efficient method for identifying conflict-prone individuals. The goal of this study was to determine if stable isotope ratios could be used for such a purpose. Stable isotopes ratios have been used in past studies to identify individuals that forage on human-derived foods [Citation23–25]. For instance, black bears (Ursus americanus) that foraged on meat-rich, human foods [Citation25] or animals [Citation26] exhibited higher nitrogen isotope ratios (15N/14N, expressed as δ15N values) than bears that primarily foraged on plants. Similarly, individuals that foraged on C4 plants such as corn from agricultural fields or corn-derived products, which include processed foods or corn-fed livestock [Citation27,Citation28], had higher carbon isotope ratios (13C/12C, expressed as δ13C values) than individuals that forage on C3 plants exclusively [Citation23,Citation25].
We hypothesized that the isotopic composition of hair for bears killed on or captured near the rail (hereafter, rail-associated bears) in Banff would reflect the anthropogenic foods they likely forage on. Our hypothesis predicted: (1) higher δ15N values for rail-associated bears due to preying on ungulates that foraged along the rail or scavenging on rail-killed animals; (2) higher δ34S values for rail-associated bears due to ingesting sulfur pellets directly (e.g. when lapping up grains) or indirectly (e.g. via plants that grew along the rail in sulfur-enriched soil or via animals that consumed those plants or pellets); and (3) different δ13C values for rail-associated bears due to foraging on different plants (e.g. growing along the ballast) or train-spilled agricultural products (e.g. corn elevating δ13C in their tissues). We tested these predictions by comparing the isotope values in hair of rail-associated bears to those of bears sampled away from the rail throughout the park.
2. Materials and methods
2.1. Study area
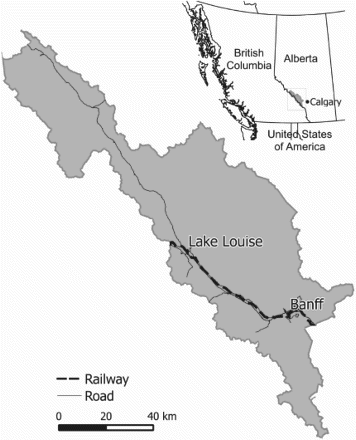
Banff National Park (51.2°N, 115.5°W; 6,641 km2) is located approximately 150 km west of Calgary, Alberta () in the Canadian Rocky Mountains. Banff receives more than three million visitors each year. The park contains rugged mountains with elevations ranging from 1000 to 3500 m with a climate characterized by long, cold winters, and short, cool summers [Citation29]. Montane and subalpine ecoregions in Banff are dominated by lodgepole pine (Pinus contorta), Douglas-fir (Pseudotsuga menziesii), white spruce (Picea glauca), Englemann spruce (Picea englemannii), trembling aspen (Populus tremuloides), and natural grasslands. Primary grizzly bear foods include sweetvetch roots (Hedysarum spp.), bearberries (Arctostaphylos uva-ursi), graminoids (Family), horsetails (Equisetum spp.), buffalo berries (Shepherdia Canadensis), and other fruits (Vaccinium spp.) [Citation30].
In addition to grizzly bears, Banff hosts a diversity of medium- to large-bodied mammals including black bears, wolves (Canis lupus), coyotes (Canis latrans), foxes (Vulpes vulpes), cougars (Puma concolor), lynx (Lynx canadensis), wolverines (Gulo gulo), moose (Alces alces), elk (Cervus canadensis), mule deer (Odocoileus hemionus), white-tailed deer (O. virginianus), bighorn sheep (Ovis canadensis), and mountain goats (Oreamnos americanus). Most of these species have been killed on the TCH and the CPR (∼82 km) over the past 20 years in Banff [Citation12].
2.2. Sampling
We used isotope values from three different samples of bear hair collected in Banff in 2000–2012. We collected hair samples from independent grizzly bears killed on the tracks in 2000–2011 (n=9) and those captured adjacent to the railway in 2012 (n=9) (). We also used hair samples that were collected away from the rail at wildlife crossing structures, hair traps, and bear rubs in 2006–2008 (see Sawaya et al. [Citation31] for detailed descriptions of sampling methods).
Table 1. Isotope values (δ13C, δ15N, δ34S) for bears killed and captured on the railway in Banff National Park, 2000–2012.
Parks Canada personnel collected hair from grizzly bears killed on and captured near the rail. Park personnel visited each mortality site and collected an envelope of hair from each dead bear. Some personnel noted that the stomachs of train-killed bears contained grain (Parks Canada, unpublished data). Parks Canada personnel also collected hair from immobilized bears captured near the railway. Park personnel captured grizzly bears using a combination of free-range darting and culvert traps using protocols approved by Parks Canada Animal Care Committee and in accordance with guidelines approved by the American Society of Mammalogists [Citation32]. The capture team immobilized bears using low impact, slow-inject darts containing 3 mg/kg of tiletamine-zolazepam and 2 mg/kg of xylazine. Once immobilized, the capture team recorded standard morphological measurements for each bear, fit it with a GPS radio-collar, injected 0.2 mg/kg of atipamizol to reverse the effects of xylazine, and then released the bear. We sub-sampled >2 guard hairs of equal length from each of these samples. We included only those bears in our samples that had been independent of their mothers for more than one year as nursing may enrich 15N and deplete 13C in the tissues of young bears [Citation33] (). Because grizzly bears molt during spring and summer months, we assumed full-length guard hairs collected from captured bears in spring 2012 were grown and contained isotopes derived from bear foods consumed in 2011 [Citation34,Citation35].
Recent studies collected hair samples in 2006–2008 to estimate the demographic rates of grizzly bears in Banff [Citation31] and to evaluate population connectivity using genetic data [Citation36,Citation37]. We identified bears for isotope analysis (n=115) by querying unpublished data from Sawaya et al. [Citation31]. First, we sorted individuals by year and by hair availability. Next, we queried samples by season. We used only guard hairs that were of full and equal length and included only those samples collected in May–August because samples collected in September onward were likely grown that year. We considered individuals that were recaptured in successive years to be independent samples to acknowledge potential differences in diet among years.
2.3. Sample preparation and stable isotope analysis
We rinsed hairs with 2:1 chloroform–methanol solution to remove surface oils and then air-dried the samples for stable isotope analysis (SIA). We conducted SIA at University of Alberta (hereafter, U Alberta Lab) and University of Ottawa (hereafter, U Ottawa Lab). The U Alberta Lab weighed single hairs into tin capsules (4 mm×6 mm; #D1006 from Elemental Microanalysis, Okehampton, UK) using a Starorius CP2P micro-analytical balance. The U Alberta Lab analyzed samples for their carbon (13C/12C) and nitrogen (15N/14N) isotope compositions by continuous flow methods using a Costech 4010 EAS Elemental Analyzer (Costech Analytical Technologies Inc., Valencia, USA) coupled to a ThermoFinnigan Conflo III and ThermoFinnigan Delta+Advantage stable isotope mass spectrometer (Thermo-Scientific, Waltham, USA). The U Ottawa Lab analyzed samples for their sulfur (34S/32S) isotope compositions by loading the encapsulated hairs into a Vario Micro Cube (Elementar Americas, Mt. Laurel, USA), coupled to a DeltaXP IRMS (Thermo-Scientific).
Stable isotope ratios are expressed in delta (δ) notation as parts per thousand or per mil (‰). SIA results are reported and defined as
where jX is the heavier isotope (13C, 15N, 34S), and iX the lighter isotope (12C, 14N, 32S) in the sample (numerator) and international measurement standard (denominator) [Citation38]. Reference standards are V-PDB for carbon (± 0.2 ‰), atmospheric N2 for nitrogen (±0.2 ‰), and Vienna Cañon Diablo Troilite for δ34S (± 0.2 ‰) [Citation39–41].
We corrected δ13C values for the Suess effect, the global decrease in 13C in Earth's atmospheric CO2 due to fossil fuel burning over the past 150 years [Citation42]. Following Chamberlain et al. [Citation43], we applied a time-dependent correction of –0.022 ‰ per year through 2011 for all hair samples except those collected in 2012.
2.4. Statistical analyzes
We calculated intra-sample error by conducting isotope analysis on multiple hairs of equal length within samples. This error (δ15N:
; δ34S:
; δ13C:
; Table S1) accounts for the inherent isotopic variation among hairs within a sample and acknowledges the degree of precision afforded by conducting SIA (Table S1). We conducted our analyzes described below using mean isotope values for a minimum of two hairs from each killed and captured bear and one hair from each hair-snared bear.
We tested for differences in isotope values between bears killed on and captured near the rail, between male and female bears, and between rail-associated bears and those sampled away from the rail throughout the park. We used t-tests to assess for differences in δ34S because these isotope values follow a normal distribution. We also used Mann–Whitney, Wilcoxon tests to test for differences in both δ13C and δ15N because these isotope values follow non-normal distributions. We used R (2.15.1) to conduct all significance tests and set α=0.05.
3. Results
We conducted univariate analyses to identify differences in isotope values for the two samples of rail-associated bears and for the two sexes in both types of samples. As expected, we found that the 18 grizzly bears killed on or captured near the railway had similar isotope values (δ15N: W=24, P=0.16; δ34S: t=1.37, P=0.19; δ13C: W=55, P=0.21; ), suggesting they had similar diets. Likewise, isotope values were similar for male and female bears for rail-associated samples (δ15N: W=55.5, P=0.07; δ34S: t=0.29, P=0.78; δ13C: W=49.5, P=0.21; ) and hair-snared samples ,
, W=1423.5, P=0.256;
,
, t=−0.63, P=0.53;
,
, W=1704.5, P=0.65; Table S2). Accordingly, we aggregated rail-associated bears into a single group.
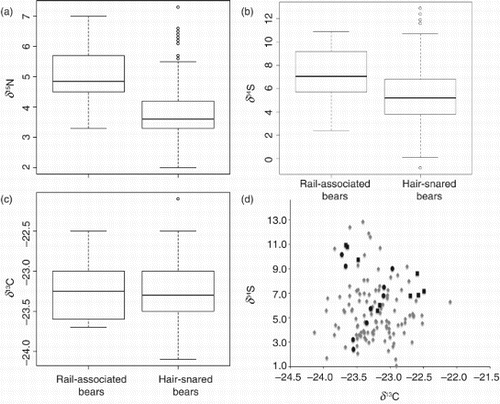
When we compared the isotope values for rail-associated bears and those sampled away from the rail, we found significant differences for both δ15N and δ34S values ((a) and 2(b)) but no difference in δ13C values ((c)). Mean values of δ15N were 1.2 ‰ greater for rail-associated bears relative to hair-snared bears (Tables 1 and S2; W=1700.0, P=<0.001). Likewise, the values of δ34S were 1.9 ‰ greater for rail-associated bears (Tables 1 and S2; W=1490.0, P=0.003). By contrast, mean values of δ13C were effectively equal (W=992, P=0.78) for the two groups of bears (Tables 1 and S2).
Figure 2. Boxplots depicting nitrogen (δ15N; Panel a), sulfur (δ34S; Panel b), and carbon (δ13C; Panel c) isotope values derived from the hair of grizzly bears killed (black squares) or captured (black circles) on the railway (defined as rail-associated bears; Panel d) and those sampled via hair-snare (grey diamonds; Panel d) in Banff National Park, Alberta, Canada, 2000–2012.
4. Discussion
Rail-associated mortality of grizzly bears has increased dramatically in Banff National Park. Evidence from this study suggests that it is plausible that SIA of hair can be used as a reliable tool to reveal the rail-based foraging behavior of grizzly bears in Banff. Our results demonstrated that rail-associated bears had different isotope values than bears sampled away from the rail, supporting the hypothesis that hair samples could be used to identify individuals that forage on the rail and are thereby at risk of being killed by trains. Rail-associated bears in our study had higher δ15N and δ 34S values than hair-snared bears, but similar δ13C values. We found no differences in isotope values between bears that were killed on or captured near the rail or between the two sexes, suggesting that the nitrogen and sulfur isotopes contained in hair are robust measures for detecting independent grizzly bears that forage on the rail in Banff.
Higher δ15N values measured in the hair of rail-associated bears are indicative of diets that were higher in animal protein [Citation26,Citation44,Citation45]. Grizzly bears in Banff are considered to be protein-limited [Citation13]; although they periodically feed on ungulates, ants are a more consistent source of animal protein [Citation30]. It is plausible that rail-associated bears had access to more ants along the rail in the Bow Valley, but it is more likely that higher animal protein in the diets of rail-associated bears was due to preying on ungulates or scavenging on rail-killed animals. Dozens of animals are killed on the rail each year in Banff (Parks Canada, unpublished data), and many large carcasses are found and removed by park staff. Transplanted carcasses are deposited in nearby restricted areas where they are available to carnivores, and many bears that use the rail are also known to feed at these sites (D. Garrow, Parks Canada, personal communication). Whether feeding on carrion on the railway or at carcass dumps, bears that used the transportation corridor in Banff likely obtained substantial protein subsidies by feeding on animals killed by trains, thus elevating δ15N values in their hair [Citation26]. Even though δ15N values for rail-associated bears are significantly higher than hair-snared bears, the actual difference between groups might be greater if we included dependent bears that nursed during the previous year in our rail-associated sample or excluded these bears from our sample of hair-snared bears [Citation33].
Rail-associated bears also had higher δ34S values than hair-snared bears, indicating that some bears may have fed directly on sulfur pellets or foraged on plants or animals that assimilated 34S into their tissues. Sulfur isotopes may prove to be a particularly informative predictor of railway use by bears and other animals in the area because 34S occurs in relatively low abundance in bear foods; one exception occurs in whitebark pine seeds [Citation35], but these are not common in the mountain parks (J. Whittington, personal observation).
Although it appears that δ34S is a clear indicator of rail-associated foraging for grizzly bears in Banff ((b)), it is important to determine the source(s) of 34S in Banff and the foraging behavior(s) bears use to acquire it. As reflected by the historic attraction of railway passengers to Banff afforded by the Sulfur Mountain hot springs, 34S may occur at elevated levels naturally in and around the rail corridor. In addition, soil adjacent to the rail was augmented with 34S for decades when railway firemen shoveled the spent coal from steam engines onto the ground adjacent to the rail (K. Roberge, personal communication). Modern combustion engines fueled with diesel or gasoline emit sulfur dioxide, which could also result in heightened δ34S values in the vegetation [Citation46] parallel to the TCH and CPR in Banff. Whether from geological or anthropogenic origins of historic or recent eras, plants growing in those areas may have elevated δ34S values, which might then be consumed by bears directly or indirectly through the animals they feed on. Finally, some observers in Banff have detected sulfur pellets in the scats of bears, which may be consumed intentionally or incidentally when bears target other products on the rail. Much further work will be needed to estimate the proportion of sulfur isotopes emanating from these potential sources.
The lack of difference in the carbon isotopes of rail-associated and hair-snared bears ((c)) was contrary to our prediction and surprising because corn, a C4 plant that is relatively high in 13C, is transported on the railway. Moreover, some rail-associated bears in Banff have been known to consume other sources of anthropogenic foods, which have resulted in higher δ13C values of hair for black bears elsewhere [Citation23,Citation47]. We may have failed to detect a difference because none exists, which would be expected if corn were rarely deposited on the tracks, or if a tendency to consume other forms of anthropogenic food is uncommon in the rail-associated bears. Without these influences, similar δ13C values would be expected as grizzly bears in Banff have homogeneous plant-based diets [Citation30]. Although it was beyond the scope of this study, integrating carbon isotopes with other isotopes could refine the differences in plant-based diets in rail-associated bears ([Citation26,Citation45,Citation47]; (d)).
Further work that integrates all pair-wise combinations of the three isotope measures and behavioral data may reveal additional information about the diets of individuals that associate with the rail in Banff and other transportation systems. In other contexts, the combined signals of multiple isotope values have revealed intra-population niche variation [Citation48–50] and dietary specialization of anthropogenic foods [Citation23–25]. Evaluating these differences more comprehensively may make it possible to distinguish between rail-associated bears that forage heavily on grains and those that forage on other foods while traveling along the rail. In subsequent analyses, greater differentiation of rail-associated bears that were known to specialize on grain may reveal δ13C to be an informative predictor of this behavior (as suggested in (d)), in turn supporting more specific management intervention.
5. Conclusion
Results from this study demonstrate that stable isotopes in the hair of grizzly bears can distinguish between individual bears that were known to be associated with the railway in Banff and those without a known association. Because stable isotopes are ubiquitous in the environment [Citation51] and their analyses are standardized [Citation52], this result has important implications for wildlife conservation in other jurisdictions around the world where train collisions and other transportation effects threaten populations. The non-invasive and inexpensive attributes of this method make it particularly well suited for the developing nations where rail-based transportation is prevalent, but lack of funding precludes more expensive methods for identifying at-risk behaviors, populations, and individuals. Once vulnerable individuals are identified, managers could analyze their hair repeatedly through time to monitor their behavior and to determine the success of mitigation efforts, adjusting their interventions subsequently within a framework of adaptive management. Such a behavior-based approach to management could increase the proportions over time of both behaviors and individuals that coexist most sustainably with the burgeoning human population.
Acknowledgements
We are grateful to Parks Canada for providing hair samples, to A. Harms, P. Middlestat, and W. Abdi for assistance with SIA, and to C. McCallum and P. Gilhooly for administrative assistance.
Funding
This work was funded with grants from the Joint Initiative for Grizzly Bear Conservation administered by Canadian Pacific Railway and Parks Canada Agency, and by a Collaborative Research Development Grant from the Natural Science and Engineering Research Council of Canada.
Notes
† Contribution to the Special Issue “Stable Isotopes in Mammals”.
References
- McNab BK. Bioenergetics and the determination of home range size. Am Nat. 1963;97:133–140. doi: 10.1086/282264
- Fenchel T. Intrinsic rate of natural increase: the relationship with body size. Oecologia. 1974;14:317–326. doi: 10.1007/BF00384576
- Damuth J. Population density and body size in mammals. Nature. 1981;290:699–700. doi: 10.1038/290699a0
- Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-Emonds ORP, Sechrest W, Orme CDL, Purvis A. Multiple causes of high extinction risk in large mammal species. Science. 2005;309:1239–1241. doi: 10.1126/science.1116030
- Woodroffe R. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126
- Treves A, Karanth KU. Human–carnivore conflict and perspectives on carnivore management worldwide. Conserv Biol. 2003;17:1491–1499. doi: 10.1111/j.1523-1739.2003.00059.x
- Long RA, MacKay P, Ray J, Zielinski W, editors. Noninvasive survey methods for carnivores. Washington, DC: Island Press; 2008.
- Madden F. Creating coexistence between humans and wildlife: global perspectives on local efforts to address human–wildlife conflict. Hum Dimens Wildl. 2004;9:247–257. doi: 10.1080/10871200490505675
- Clevenger AP, Waltho N. Performance indices to identify attributes of highway crossing structures facilitating movement of large mammals. Biol Conserv. 2005;121:453–464. doi: 10.1016/j.biocon.2004.04.025
- Clevenger AP, Chruszcz B, Gunson KE. Highway mitigation fencing reduces wildlife–vehicle collisions. Wildl Soc Bull. 2001;29:646–653
- Clevenger AP, Chruszcz B, Gunson KE. Spatial patterns and factors influencing small vertebrate fauna road-kill aggregations. Biol Conserv. 2003;109:15–26. doi: 10.1016/S0006-3207(02)00127-1
- Dorsey BP. Factors affecting bear and ungulate mortalities along the Canadian Pacific railroad through Banff and Yoho national parks [thesis]. Bozeman (MT): Montana State University; 2011.
- Garshelis DL, Gibeau ML, Herrero S. Grizzly bear demographics in and around Banff National Park and Kananaskis country, Alberta. J Wildl Manage. 2005;69:277–297. doi: 10.2193/0022-541X(2005)069<0277:GBDIAA>2.0.CO;2
- Bertch B, Gibeau ML. Grizzly bear monitoring in and around the Mountain National Parks: mortalities and bear/human encounters 1980–2009. Parks Canada Report; Lake Louise, Alberta; 2010. p. 1–28.
- Forman RT, Alexander LE. Roads and their major ecological effects. Annu Rev Ecol Syst. 1998;29:207–231. doi: 10.1146/annurev.ecolsys.29.1.207
- Seiler A. Ecological effects of roads: a review. Introductory research essay No. 9. Department of Conservation Biology, SLU Uppsala; 2001. p. 1–40.
- Donaldson A, Bennett AF, Victoria P. Ecological effects of roads: implications for the internal fragmentation of Australian parks and reserves. Melbourne, Vic: Parks Victoria Technical Series 12; 2004.
- Gibeau M, Heuer K. Effects of transportation corridors on large carnivores in the Bow River Valley, Alberta. In: Evink GL, Garrett P, Ziegler D, Berry J, editors. Trends in addressing transportation related wildlife mortality. FL-ER-58-96. Tallahassee: State of Florida Department of Transportation; 1996. p. 67–79.
- Wells P, Woods J, Bridgewater G, Morrison H. Wildlife mortalities on railways: monitoring methods and mitigation strategies. Unpublished Report: Parks Canada; Revelstoke, British Columbia; 1999. p. 85–88.
- Dorsey BP. Monitoring of train spilled grain on the Canadian Pacific rail line through Banff and Yoho National Parks (2008 to 2010). Report to Parks Canada; Lake Louise, Alberta; 2011. p. 1–16.
- Huber D, Kusak J, Frkovic A. Traffic kills of brown bears in Gorski kotar, Croatia. Ursus. 1998;10:167–171.
- Waller JS, Servheen C. Effects of transportation infrastructure on grizzly bears in northwestern Montana. J Wildl Manage. 2005;69:985–1000. doi: 10.2193/0022-541X(2005)069[0985:EOTIOG]2.0.CO;2
- Mizukami R, Goto M, Izumiyama S, Hayashi H, Yoh M. Estimation of feeding history by measuring carbon and nitrogen stable isotope ratios in hair of Asiatic black bears. Ursus. 2005;16:93–101. doi: 10.2192/1537-6176(2005)016[0093:EOFHBM]2.0.CO;2
- Newsome SD, Ralls K, Job CVH, Fogel ML, Cypher BL. Stable isotopes evaluate exploitation of anthropogenic foods by the endangered San Joaquin kit fox (Vulpes macrotis mutica). J Mammal. 2010;91:1313–1321. doi: 10.1644/09-MAMM-A-362.1
- Hopkins JB III, Koch PL, Schwartz CC, Ferguson JM, Greenleaf SS, Kalinowski ST. Stable isotopes to detect food-conditioned bears and to evaluate human–bear management. J Wildl Manage. 2012;76:703–713. doi: 10.1002/jwmg.318
- Mowat G, Heard D. Major components of grizzly bear diet across North America. Can J Zool. 2006;84:473–489. doi: 10.1139/z06-016
- Koch PL. Isotopic study of the biology of modern and fossil vertebrates. Stable isotopes in ecology and environmental science. Boston, MA: Blackwell Publishing; 2007. p. 99–154.
- Chesson L, Podlesak D, Thompson A, Cerling T, Ehleringer J. Variation of hydrogen, carbon, nitrogen, and oxygen stable isotope ratios in an American diet: fast food meals. J Agric Food Chem. 2008;56:4084–4091. doi: 10.1021/jf0733618
- Holland WD, Coen GM. Ecological land classification of Banff and Jasper National Parks. Volume 1: summary. Publication M-83–2. Edmonton: Alberta Institute of Pedology; 1983.
- Hamer D, Herrero S. Grizzly bear food and habitat in the front ranges of Banff National Park, Alberta. In: Zager P, editor. Bears: their biology and management. International Bear Association. 1987;7:199–213.
- Sawaya MA, Stetz JB, Clevenger AP, Gibeau ML, Kalinowski ST. Estimating grizzly and black bear population abundance and trend in Banff National Park using noninvasive genetic sampling. PLoS ONE. 2012;7:e34777. doi: 10.1371/journal.pone.0034777
- Sikes RS, Gannon WL. Guidelines of the American society of mammalogists for the use of wild mammals in research. J Mammal. 2011;92:235–253. doi: 10.1644/10-MAMM-F-355.1
- Polischuk S, Hobson K, Ramsay M. Use of stable-carbon and -nitrogen isotopes to assess weaning and fasting in female polar bears and their cubs. Can J Zool. 2001;79:499–511. doi: 10.1139/z01-007
- Fortin JK, Schwartz CC, Gunther KA, Teisberg JE, Haroldson MA, Evans MA, Robbins CT. Dietary adjustability of grizzly bears and American black bears in Yellowstone National Park. J Wildl Manage. 2012;77:270–281. doi: 10.1002/jwmg.483
- Felicetti L, Schwartz C, Rye R, Haroldson M. Use of sulfur and nitrogen stable isotopes to determine the importance of whitebark pine nuts to Yellowstone grizzly bears. Can J Zool. 2003;81:763–770. doi: 10.1139/z03-054
- Sawaya MA, Clevenger AP, Kalinowski ST. Demographic connectivity for Ursid populations at wildlife crossing structures in Banff National Park. Conserv Biol. 2013;27:721–730. doi: 10.1111/cobi.12075
- Sawaya MA, Clevenger AP, Kalinowski ST. Genetic connectivity for two bear species at wildlife crossing structures in Banff National Park. Proc R Soc B–Biol Sci. 2014;281:20131705. doi: 10.1098/rspb.2013.1705
- Bond AL, Hobson KA. Reporting stable-isotope ratios in ecology: recommended terminology, guidelines and best practices. Waterbirds. 2012;35:324–331. doi: 10.1675/063.035.0213
- Mariotti A. Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature. 1983;303:685–687. doi: 10.1038/303685a0
- Coplen TB. Reporting of stable hydrogen, carbon, and oxygen isotopic abundances. Pure Appl Chem. 1994;66:273–273. doi: 10.1351/pac199466020273
- Krouse HR, Coplen TB. Reporting of relative sulfur isotope-ratio data. Pure Appl Chem. 1997;69:293–295. doi: 10.1351/pac199769020293
- Francey RJ, Allison CE, Etheridge DM, Trudinger CM, Enting IG, Leuenberger M, Langenfelds RL, Michel E, Steele LP. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B. 1999;51:170–193. doi: 10.1034/j.1600-0889.1999.t01-1-00005.x
- Chamberlain C, Waldbauer J, Fox-Dobbs K, Newsome S, Koch P, Smith D, Church M, Chamberlain S, Sorenson K, Risebrough R. Pleistocene to recent dietary shifts in California condors. Proc Natl Acad Sci USA. 2005;102:16707–16711. doi: 10.1073/pnas.0508529102
- Hilderbrand G, Farley S, Robbins C, Hanley T, Titus K, Servheen C. Use of stable isotopes to determine diets of living and extinct bears. Can J Zool. 1996;74:2080–2088. doi: 10.1139/z96-236
- Hobson K, McLellan B, Woods J. Using stable carbon (δ13C) and nitrogen (δ15N) isotopes to infer trophic relationships among black and grizzly bears in the upper Columbia River basin, British Columbia. Can J Zool. 2000;78:1332–1339. doi: 10.1139/cjz-78-8-1332
- Case JW, Krouse HR. Variations in sulphur content and stable sulphur isotope composition of vegetation near a SO2 source at Fox Creek, Alberta, Canada. Oecologia. 1980;44:248–257. doi: 10.1007/BF00572687
- Hopkins JB III, Koch PL, Ferguson JM, Kalinowski ST. The changing anthropogenic diets of American black bears over the past century in Yosemite National Park. Front Ecol Environ. 2014;12:107–114. doi: 10.1890/130276
- Araujo MS, Bolnick DI, Machado G, Giaretta AA, Reis SF. Using δ13C stable isotopes to quantify individual-level diet variation. Oecologia. 2007;152:643–654. doi: 10.1007/s00442-007-0687-1
- Layman C, Quattrochi J, Peyer C, Allgeier J, Suding K. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol Lett. 2007;10:937–944. doi: 10.1111/j.1461-0248.2007.01087.x
- Quevedo M, Svanbäck R, Eklöv P. Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology. 2009;90:2263–2274. doi: 10.1890/07-1580.1
- Michener R, Lajtha K. Stable isotopes in ecology and environmental science. Malden, MA: Wiley; 2008.
- Coplen TB. Guidelines and recommended terms for expression of stable–isotope–ratio and gas–ratio measurement results. Rapid Commun Mass Spectrom. 2011;25:2538–2560.